Abstract
BACKGROUND
Endometriosis is a gynecological condition that is characterized by extreme abdominal pain and also decreased fertility. Regulatory T cells (Tregs) have immunosuppressive activity critical for embryonic implantation and likewise the acceptance of tissue engraftment. Utilizing the induced non-human primate (Papio anubis) model of endometriosis, we hypothesize that endometriosis decreases the peripheral and endomet rial Treg profile, whereas ectopic lesions have increased Treg localization.
METHODS
Peripheral blood and endometrium were obtained throughout the menstrual cycle prior to and after induction of disease. Animals were randomly assigned to control (n = 7) or diseased (n = 16) treatment groups. Endometriosis was induced by i.p. injection of autologous menstrual tissue for 2 consecutive months during menses. Peripheral blood and endometrial tissue were collected at d9-11PO at 1, 3, 6, 9, 12 and 15 months post-induction of disease for fluorescence-activated cell sorting, quantitative RT–PCR and immunohistochemistry. Ectopic lesions were excised at 1 and 6 months post-inoculation and also harvested at necropsy (15 months) and processed for RNA of IHC. Identification of Tregs through analysis of FOXP3 expression was conducted utlilizing several methodologies. Differences were determined by non-parametric statistical analysis between all treatment groups and time points.
RESULTS
In control animals, the proportion of peripheral natural Tregs (nTregs) was reduced (P < 0.05) during the mid- and late secretory stages of the menstrual cycle compared with menses. The induction of disease decreased peripheral Treg expression at early time points (P < 0.05) and this remained low throughout the time course, compared with the pre-inoculatory level of an individual. FOXP3 gene expression and Treg populations were also decreased in the eutopic endometrium (P < 0.05) compared with control animals, whereas these parameters were increased in ectopic lesions (P < 0.05), compared with the eutopic endometrium.
CONCLUSIONS
Our data suggest that a reduction in peripheral Tregs may be a causative factor for endometriosis-associated infertility, while the increase in ectopic Treg expression may aid lesion development. Furthermore, endometriosis appears to disrupt Treg recruitment in both eutopic and ectopic endometrium.
Keywords: endometriosis, regulatory T cells, primate, fertility, pathogenesis
Introduction
Endometriosis is classified pathologically as the existence of endometrium fragments in the abdominal cavity (Sampson, 1925). The growth of ectopic endometrium is stimulated by estrogen and normally suppressed by progesterone (Banu et al., 2008; Hirota et al., 2008; Monckedieck et al., 2009), although the theory of progesterone-resistant ectopic growth has been validated in some cases of endometriosis (Igarashi et al., 2005; Bulun et al., 2006; Burney et al., 2007; Tranguch et al., 2007; Fazleabas, 2010). The primary clinical symptoms of endometriosis are infertility related to endometrial dysregulation (Sherwin et al., 2008; Jones et al., 2009; Umezawa et al., 2009); poor oocyte development (Barnhart et al., 2002); anatomic distortion of the Fallopian tubes and intense abdominal pain from ectopic lesion growth (Medina and Lebovic, 2009; Wang et al., 2009). Although this disease has been recognized for over a century, the pathophysiology of ectopic lesion development is still unclear (Meyer, 1919). A major limitation to understanding the pathophysiology is the time from the initiation of the disease until the onset of symptoms, and differential diagnosis takes on average of 8–11 years (Arruda et al., 2003). Recently endometriosis has been investigated as a reproductive inflammatory immune disorder because of its negative effect on fertility status and the high degree of pain associated with this disease (Sinaii et al., 2002; Cakmak et al., 2009; Weiss et al., 2009; Berbic and Fraser, 2011; Maybin et al., 2011; Osuga et al., 2011).
The peritoneal cavity of patients with endometriosis has been well characterized as a proinflammatory environment. Peritoneal fluid from patients with endometriosis has elevated levels of inflammatory cytokines which is believed to result from improper clearance of ectopic fragments (Hou et al., 2009; Kyama et al., 2009; Kuroda et al., 2010). Additionally, the immune cell profile of the peritoneal cavity, eutopic endometrium and lymph nodes of patients with endometriosis has an enhanced proinflammatory phenotype indicated by the Th1/Th2 cell ratio, macrophage activation and also natural killer cell activity (Tran et al., 2009; Wang et al., 2010a; Hey-Cunningham et al., 2011; Mier-Cabrera et al., 2011; Podgaec et al., 2011; Sikora et al., 2011). The immunological shift caused by peritoneal implantation of endometrium fragments has prompted the development of immune-based therapeutic targets with variable success in the inhibition of ectopic lesion development (Altan et al., 2010; Krikun et al., 2010; Lv et al., 2010). More recently, subfertility and ectopic lesion growth were attributed to dysregulation of immunosuppressor cells known as regulatory T cells (Tregs; Christodoulakos et al., 2007; Berbic et al., 2010).
Tregs are derived from the CD4 lineage of T cells and are produced naturally in the thymus (nTregs) and characterized by their expression of both interleukin (IL)-10 receptor (CD25+) and the forkhead transcription factor (Foxp3+). Additionally, CD4+ T cells can adapt to become Tregs through the induction of Foxp3 by the cytokine microenvironment in target tissues (Th3:CD4+CD25−Foxp3+) and (Tr1:CD4+CD25+Foxp3−; Bluestone and Abbas, 2003; Sakaguchi et al., 2010). Activated nTregs suppress the responses of effector T cells indirectly by inhibiting the dendritic cells or other antigen presenting cells (APCs) from triggering effector T cell proliferation (Tang et al., 2006). Both natural Tregs (nTreg) and adaptive Tregs can confer immune tolerance through the production of the anti-inflammatory cytokines IL-10 and transforming growth factor-β which then inhibit the activation of T helper cells (Th; Bach and Chatenoud, 2001; von Boehmer, 2005). Migration of peripheral Tregs to target tissues is stimulated through the chemokine ligand/receptor CXCL12/CXCR4 (Zou et al., 2004). In the reproductive system, Tregs are critical for tolerance of the maternal fetal allograph (Aluvihare et al., 2004) and patients with recurrent miscarriage have decreased peripheral and endometrial Treg expression (Sasaki et al., 2004; Yang et al., 2008; Basta et al., 2011). Tregs are also critical for tumor growth by providing an immunologically protective microenvironment promoting tumor metastasis (Bergmann et al., 2007; Strauss et al., 2007; Wang et al., 2012). The importance of Tregs for embryonic implantation and tumor growth validate the investigation of Treg expression during the pathogenesis of endometriosis.
The baboon (Papio anubis) is an excellent model for the study of biological and pathological gynecological conditions. Unlike rodents and mice, baboons have a menstrual cycle similar to humans both in terms of duration and endometrial remodeling. In addition, baboons also develop spontaneous endometriosis with ectopic lesions resembling those of women (Merrill, 1968; Folse and Stout, 1978). Endometriosis can also be induced by injection of menstrual effluent into the pelvic cavity (D'Hooghe, 1997; Fazleabas et al., 2002) allowing investigators to study disease progression from the initial onset of the disease. We hypothesize that Tregs are decreased in the eutopic endometrium resulting in reduced fertility and are increased in ectopic endometrium allowing for growth and maintenance of lesions. Using this animal model of induced endometriosis in olive baboons (P. anubis), the aims of this study were to (i) determine the expression and localization of Tregs in the eutopic endometrium throughout the disease pathogenesis during the window of uterine receptivity; (ii) determine if Tregs promote and maintain the ectopic endometrial growth and (iii) determine if surgical removal of lesions restores Treg expression in the eutopic endometrium and inhibits that expression in the ectopic endometrium.
Materials and Methods
Animal procedures and induction of endometriosis
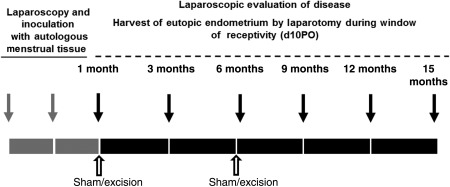
Twenty-three animals were included in this study and randomly assigned to the following groups (controls: 1-month treatment, n = 3; 6-month treatment, n = 4; diseased: 1-month sham, n = 4; 1-month excision, n = 4; 6-month sham, n = 4 and 6-month excision, n = 4). All animals were of reproductive age, ranged in weight from 15 to 20 kg and were confirmed disease free by laparoscopic viewing of the abdominal cavity prior to inoculation. Endometriosis was induced experimentally in olive baboons by laparoscopic i.p. inoculation of menstrual endometrium on Day 2 of two consecutive menstrual cycles, where Day 1 was the first day of menses (Fig. 1). Control animals received laparoscopic injection of warm saline. Details of the inoculation procedure and tissue collection have been previously described (Fazleabas et al., 2002). Animals were determined to have induced endometriosis upon the visualization of multiple ectopic lesions (red, brown, blue and white) and adhesions on the surface of peritoneal organs. The staging of disease was 2 or 3, according to the American Society for Reproductive Medicine guidelines (Medicine, 1997). Animals could receive a total of five invasive (laparotomy) surgeries according to our Institutional Animal Care and Use Committee (IACUC) protocol, which justified the need for two control surgical groups. The surgical procedure timeline began the first month following the second inoculation and described as follows: control (-free animals) received surgery at 1, 3, 6, 9 and 15 months (n = 3; surgical controls for 1 month groups) or 1, 3, 6, 12 and 15 months (n = 4; surgical controls for 6 month groups); 1 month groups received surgery at 1, 3, 6, 9 and 15 months post-inoculation; 6 month groups received surgery at 6, 9, 12 and 15 months post-inoculation. Operational procedures (sham or excisional) were performed on animal groups at 1 or 6 months post-inoculation; therefore animals receiving a 1-month (operational) time point were compared with their 6- and 15-month (post-excisional) time points and animals receiving a 6-month (operational) time point were compared with their 15-month (post-excisional) time point (Fig. 1). Surgical and sham procedures were performed through the introduction of two operational ports into the abdominal wall, lateral of the pelvic midline. Lesions were removed by cauterization or curettage procedures. Animals were housed in the animal care facility at the University of Illinois, Chicago, USA, and all studies were approved by the University of Illinois IACUC.
Figure 1.
Schematic of induction of endometriosis and surgical procedures in non-human primates (baboon). Gray arrows indicate the time points of i.p. inoculation using autlogous menstrual tissue. Black arrows indicate laparoscopy and endometriectomy surgical time points and peripheral blood collection during the window of receptivity d9-11PO (post-ovulatory). White arrows indicate operational procedure (sham or excisional) time points. All animals were euthanized at 15 months following disease induction.
Analysis of lymphocyte preparations using a fluorescence-activated cell sorter
Peripheral blood was collected in heparinized tubes during: mense, late proliferative (d9-12 post-mense), mid-secretory (surgical time point) or late secretory stages of the menstrual cycle. Peripheral mononuclear cells were isolated by the standard Ficoll-paque method. Briefly blood was diluted with an equal volume of sterile phosphate-buffered saline (PBS) and gently layered over the Ficoll paque solution. Samples were centrifuged for 40 min at 400g at 20°C and the lymphocyte layer (buffy coat) was isolated and further washed with PBS. Lymphocytes were treated with heat-inactivated human AB serum to block Fc receptor binding and then hemocytometer counts were performed. Lymphocytes were stained directly for T cell surface antigens using: FITC-CD4 (550628; BD Pharmingen, Franklin Lakes, NJ, USA), APC-CD25 (17-0259; eBioscience, San Diego, CA, USA) and PE-FOXp3 (12-4776; eBioscience) according to manufacturer recommendations. Lymphocyte cell populations were sorted using Facscan and Cellquest software (Becton Dickinson, Franklin Lakes, NJ, USA). Populations were gated initially on CD4 fluorescent intensity and CD4+ subpopulations were identified by CD25 and Foxp3 fluorescent intensity.
Immunohistochemical localization of Tregs
Baboon endometrial, lesion and femoral lymph tissues were fixed in 10% neutral-buffered formalin for 24 h. Tissues were then processed and embedded in paraffin blocks. Tissue blocks were sectioned at 5 μm and mounted on poly-L-lysine coated slides. Antigen retrieval was performed by boiling sections in Target Retrieval Solution (pH 9.0; S2367; Dako, Carpentaria, CA, USA) for 40min. Non-specific binding was blocked with 5% normal goat serum in PBS containing 1% bovine serum albumin (BSA) for 20 min. Slides were then incubated with 10 μg/ml rat monoclonal antibody anti-human Foxp3 (14-4776; eBioscinece) in 1% BSA in PBS at 4°C for 2 h. Non-specific rat immunoglobulin (Ig)G (10 μg/ml) in 1% BSA in PBS was used as a negative control. Femoral lymph node tissue was used as a positive control. Slides were washed three times for 5 min each in PBS then incubated with biotinylated horse anti-rat IgG (Vector Labs, Burlingame, CA, USA) diluted 1:200 with 1% BSA in PBS for 60 min at room temperature. Indirect detection of Foxp3 protein was performed by incubation of sections for 45 min with avidin–biotinylated peroxidase complex, reacted with 0.2 mg/ml Metal-3, 3′-diaminobenzidine (Sigma, St. Louis, MO, USA) in Tris–HCl buffer, pH 7.6, for 3 min and finally counterstained with hematoxylin.
Stained slides were digitally scanned using a Nanozoomer 2.0 HT (Hamamatsu, Japan) and these digital images were used for quantification of Foxp3 staining intensity. Staining intensity was calculated by counting the number of cells stained positive for Foxp3 per total area of section. Staining intensity was then normalized to 10 mm2 for all tissues stained.
RNA isolation and quantitative RT–PCR
Total RNA was extracted from tissue using TRIzol™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Total RNA (1 μg) was used in 20-μl volume reverse transcription reactions using high-capacity reverse transcription kit (4368814; Applied Biosystems, Atlanta, GA, USA). Synthesized cDNA was then used for real-time PCR analysis. Real-time PCR analyses were performed in 10-μl volumes containing 1X TaqMan® Universal PCR Master Mix No AmpErase® UNG (4324018, Applied Biosystems), diluted cDNA and RNase-free water. Primer probe sets used for CXCL12 (Hs00171022_m1) for Foxp3 (Hs01085834_m1) amplification were purchased from Applied Biosystems. Histone 3.3 primers (Forward: GGCGCTCCGTGAAATTAGAC; Reverse: CGCTGGAAGGGAAGTTTGC) and probe (CGCTGGAAGGGAAGTTTGC) were designed for use as an internal control. Minor groove binding probes were labeled with fluorescent reporter dye FAM™ to detect PCR products directly. Real-time PCR amplification and detection were performed in MicroAmp optical 384-well reaction plates using the ABI Prism 7000 sequence detection system. Amplification conditions included 10 min at 95°C, then 40 thermal cycles of denaturing for 15 s at 95°C and annealing/extension for 1 min at 60°C. Relative fold induction levels were calculated using the comparative CT method for separate tube amplification. Fold induction was expressed relative to H3.3 endogenous control gene after normalization for background signal.
Scoring ectopic lesions
At each surgical time point, the entire peritoneal cavity was imaged by laparoscopy and the following lesion information was collected: lesion location, color and operational procedure (excised: yes/no). Lesion dynamics were recorded for each surgical time point to allow for lesion tracking throughout disease progression. After the terminal procedure (at 15 months) each animal had their lesion dynamics evaluated by: (i) the total number of lesions found at the 15-month procedure, (ii) number of new lesions at 15 months, (iii) the total number of lesions which had developed over the entire time frame and (iv) the number of lesions that re-grew after the excisional procedure (1 month or 6 months). The percentage of sustained lesions, percentage of lesions regressed and the percentage of lesion regrowth was calculated for each animal with induced disease.
 |
Statistical analysis
An analysis of variance model to evaluate the experimental variability between all treatment groups was used to determine differences in experimental end-points. For all end-points, the difference between treatment groups was analyzed using post-hoc orthogonal comparisons to determine the statistical significance for each treatment. Specifically, data from the fluorescence-activated cell sorter (% of CD4+ histology), immunohistochemistry (Foxp3-positive cells) and for gene expression studies [the difference between the threshold cycle of Foxp3 and H3.3 (ΔCt)] served as our experimental end-points. Orthogonal contrasts statements were written that measured for differences in treatment groups (control versus diseased; sham versus excisional) over the time course of disease progression. For FACS analysis, the proportion of each Treg type was compared with either menses (Fig. 2) or normalized to individual pre-inoculatory levels and then compared across time points (Fig. 3). For gene expression studies, threshold cycle was defined as the cycle number where all transcripts are in the linear phase of amplification. The difference between CXCL12, Foxp3 and H3.3 was then normalized to control animal expression and expressed as a relative fold difference. For immunohistochemistry, the total number of positive cells was normalized to the area of 10 mm2. For both qRT–PCR and immunohistochemistry, the significance was determined through comparison of all treatment groups across surgical time points. For lesion dynamics, comparisons between surgical treatment and time points were evaluated. For all analyses, a P < 0.05 was considered statistically significant. SAS (Cary, NC, USA) statistical software was used to perform all statistical testing.
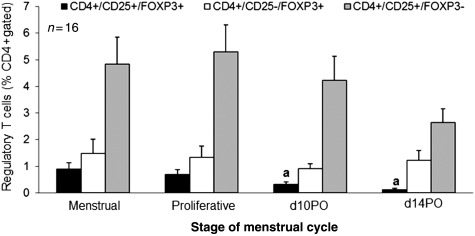
Figure 2.
Peripheral Tregs in the baboon menstrual cycle. Peripheal blood was collected at the indicated stages of the menstrual cycle and analyzed for natural (black) and adaptive [white (Th3) and gray (Tr1)] regulatory T cell populations. Percentage histology is shown for all CD4+-gated cells. Significance of time points compared with mense levels is indicated by different letters. n = 23, P ≤ 0.05, error bars indicate SEM.
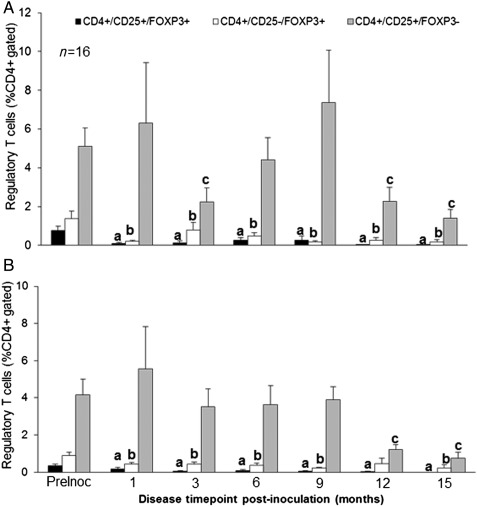
Figure 3.
Peripheral Treg populations following disease induction in baboon. Peripheral blood samples were collected during the (A) proliferative, (B) mid-secretory phase of the menstrual cycle at each surgical time point post-inoculation of the disease. Percentage histology is shown for all CD4+-gated cells. All animals were normalized to their pre-inoculatory levels. Significance is indicated by different letters from preinoculatory measurements for all Treg sub-types. n = 16, P < 0.05, error bars indicate SEM.
Results
Peripheral Treg cell populations are altered by induction of endometriosis
Analysis of nTreg (CD4+/CD25+/Foxp3+) and adaptive Treg (CD4+/CD25+/Foxp3− or CD4+/CD25−/Foxp3+) populations showed that the most prevalent Treg population was Tr1 (CD4+/CD25+/Foxp3−) at 2.5–5%, followed by the Th3 subpopulation at 1.5% and nTregs were the least abundant at 0.25–1% of all CD4+ cells (Fig. 2). nTregs were further reduced in the peripheral blood collected during the secretory stage (mid- and late) compared with other stages, 1% compared with 0.25% (P < 0.05). This reduction was not apparent in the inducible Treg populations.
We then compared Treg populations in the peripheral circulation following the induction of endometriosis to determine if the presence of disease altered Treg populations throughout the disease progression. The induction of disease significantly reduced all peripheral Treg populations during both the proliferative and mid-secretory stages of the menstrual cycle ((Fig. 3A and B), n = 16, P < 0.05)). Natural and Th3 cell populations were reduced as early as 1 month post-inoculation and remained low at each following surgical time point. The Tr1 cell population showed a transient reduction during the proliferative stage (only reduced at 3, 12 and 15 months) but was not reduced until later time points (12 and 15 months) during the mid-secretory stage of the cycle. The reduction of peripheral Treg populations was not altered by operational (sham versus excisional) procedures (data not shown). These results indicate that the induction of peritoneal endometriosis results in a significant reduction of Tregs throughout the pathogenesis of disease induction. These results also suggest that nTregs and Th3 cells are more susceptible to disease status than Tr1 cells.
Foxp3 and CXCL12 expression in eutopic endometrium
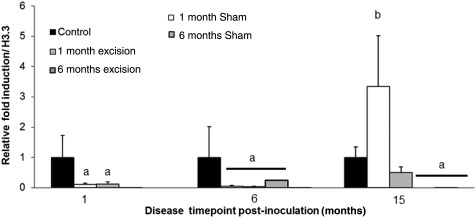
Our next investigation examined if the reduction of peripheral Treg cell populations upon disease induction was similarly evident in the eutopic and ectopic endometria. In addition, we examined if excisional removal of all lesions at early (1 month) or later time points (6 months) of disease pathogenesis would alter the localization of Treg cells in both eutopic and ectopic tissues at post-excisional time points. Through quantitative analysis of the Foxp3 transcript, we determined that disease induction significantly reduces Tregs in the eutopic endometrium following disease inoculation. Comparison of Foxp3 transcript levels prior to operational surgeries and post-operational surgeries indicated that excisional procedures did not alleviate the negative effects of disease on Treg expression. There was no significant difference in Foxp3 transcript levels between control groups of animals, therefore these animals were combined into one control treatment group for these analyses. At 1 and 6 months of disease, both 1 month operational groups (sham or excisional) had similar reduction in Foxp3 transcript levels compared with control animals (Fig. 4, P < 0.05, n = 7 controls, n = 8 1 month operational)), but at 15 months Foxp3 transcript levels were similar to control. Foxp3 transcript levels were also reduced in animals receiving an operational procedure at 6 month and these levels remained reduced at their 15-month time point (P < 0.05, n = 7 controls, n = 8 6 month operational)). Together these data indicate that the endometrium undergoes transient changes in Foxp3 transcript expression regardless of whether a sham or excision operation is performed. Additionally, we did not find any change in the chemokine CXCL12 expression in the eutopic endometrium either throughout the menstrual cycle or disease progression (data not shown). To validate our Foxp3 genetic profile, we also performed immunohistochemistry to identify Foxp3+ cells in the eutopic endometrial tissues from all animal treatment groups. The localization of Foxp3+ cells in the eutopic endometrium parallels the Foxp3 transcript data. Animals in the control groups showed no differences in localization of Foxp3+ cells but diseased animals at 1 and 6 months post-inoculation had significantly reduced Treg localization compared with control animals (Fig. 5A and B versus D–E and H). At 15 months post-inoculation, animals receiving either a sham or excisional operation at 1 month had similar Treg localization, while animals receiving a sham or excisional operation at 6 months still had reduced Treg localization, compared with control animals (Fig. 5C versus F and I).
Figure 4.
Foxp3 quantitative RT–PCR of baboon eutopic endometrium. Foxp3 transcipt levels were measured in the eutopic endometrium of control animals or diseased animals undergoing sham or excisional operations at 1 or 6 months. Transcript levels were measured at 1, 6 and 15 months post-inoculation in each animal. Transcript values were normalized to endogenous H3.3 expression and expressed as a relative fold induction over control eutopic endometrial Foxp3 expression. Significance is indicated by different letters compared with controls. n = 7 (controls), n = 4/group for diseased animals, P < 0.05, error bars indicate SEM.
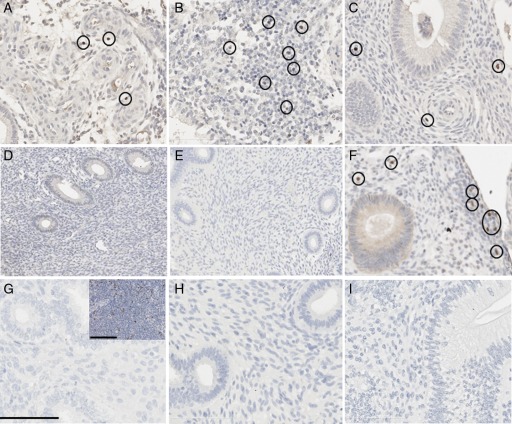
Figure 5.
Foxp3+ cell localization in the baboon eutopic endometrium. Eutopic endometrial biopsies from 1 month (A, D), 6 months (B, E, H) and 15 months (C, F, I) were analyzed for control animals (A–C), animals receiving a 1-month operational procedure (D–F) and animals receiving a 6-month operational procedure (H & I). G, IgG isotype control; inset, positive control lymph tissue. Foxp3+ cells are circled. Scale bar = 100 μm.
Foxp3 expression in ectopic endometrium and its role for lesion invasion
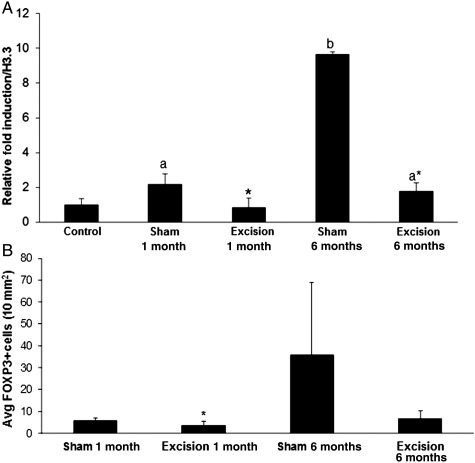
Ectopic lesions from animals that had an excisional 1-month operational procedure had the lowest Foxp3 transcript levels and lowest number of Foxp3-positive cells (Fig. 6A and B) among all diseased animal treatment groups (P < 0.05). Lesions from animals that had a 1-month sham procedure had 2-fold elevated Foxp3 transcript levels and elevated Foxp3+-positive cells, compared with the excisional control group (P < 0.05). Lesions from animals that had an excisional procedure at 6 months post-inoculation also had 2-fold elevated Foxp3 transcript levels, similar to the 1-month sham treatment group (P < 0.05). Overall, lesions from animals that had a sham 6-month operational procedure had the highest (9.5-fold) Foxp3 transcript level (P < 0.05) and highest number of Foxp3+-positive cells (P = 0.057). These findings indicate that early surgical intervention, whether excisional or sham procedures, can reduce the suppressive immune cell infiltration into the ectopic lesion. At later time points post-inoculation, once the disease has been established, only excisional removal of the lesions maintains a reduced immunosuppressive lesion environment. Together these findings suggest that Tregs may govern ectopic lesion survival and regression.
Figure 6.
Foxp3 transcript levels (A) and Foxp3+ cells (B) in baboon ectopic endometrium. Ectopic lesions were collected at 15 months post-inoculation and Foxp3 transcript levels were determined through qRT–PCR (A) and Foxp3+ cells were labeled through immunohistochemistry (B). (A) Transcript levels were normalized to endogenous control H3.3 expression and expressed as a relative fold induction over control eutopic endometrial Foxp3 expression. (B) The number of Foxp3+ cells was averaged and then normalized to a 10-mm2 area. Significance of animal operational treatments versus controls is indicated by different letters. Significance between sham versus excision treatment within each time point is indicated by asterisk (*). n = 7 (controls), n = 4 animals/group and at least two lesions/animal for diseased animals, P < 0.05, error bars indicate SEM.
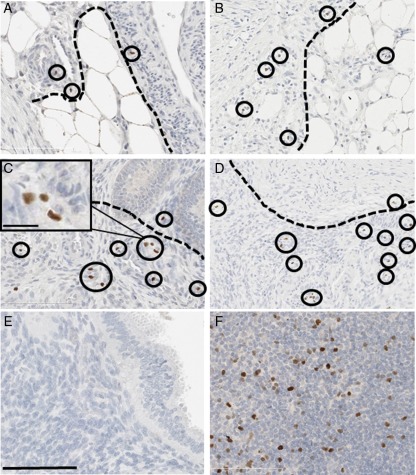
To address the role of Tregs in lesion survival and regression, we analyzed the localization of Tregs in highly invasive lesions and mapped lesion dynamics among our animal treatment groups. We found that Foxp3+ cells were abundantly localized along the mesenchymal/endometrial border in highly invasive lesions (Fig. 7A–D). This abundant localization was evident in lesions invading adipose tissue of the abdominal wall (Fig. 7A and B) and also lesions invading the peritoneal lining of abdominal organs (Fig. 7C and D). We then determined if an excisional procedure could enhance lesion regression of all ectopic lesion sites. We found that lesion regression (or turnover) occurred in every animal treatment group, however, the percentage of lesions that regressed was significantly higher in animals receiving an excisional procedure at 6 months than at 1 month or the relative sham controls (Table I; P < 0.05). We did not detect any statistically significant difference in the rate of lesion regrowth after the excisional procedures (P = 0.65). Therefore, infiltration of Tregs to the site of ectopic lesion implantation is important for maintenance of the lesion and surgical removal of lesions can alter the rate of lesion regression throughout disease.
Figure 7.
Foxp3+ cells in baboon ectopic endometrium. (A, B), Blue lesions invading the peritoneal wall. (C, D) Blue lesions invading peritoneal organs. (E) IgG isotype control. (F) Positive control lymph tissue. Foxp3+ cells are circled and the mesenchymal/endometrial border is indicated by a dashed line. Scale bar = 100 µm.
Table I.
Summary of lesion dynamics for each surgical group.
| % Lesions sustained | % Lesions regressed | % Lesions regrowth | |
|---|---|---|---|
| 1-month sham | 36.23 ± 4.96 | 63.78 ± 4.96 | NA |
| 1-month excision | 25.18 ± 8.59 | 74.82 ± 8.59 | 67.08 ± 16.12 |
| 6-month sham | 40.28 ± 7.56 | 59.72 ± 7.56 | NA |
| 6-month excision | 19.32 ± 4.54** | 80.68 ± 4.54** | 39.76 ± 19.56 |
The proportion of lesion regression, sustainance and regrowth was analyzed and compared among the surgical treatment groups and time points. Values are expressed as a proportion of total lesions documented (regression and sustainance) or as a proportion of total lesions excised (regrowth). Significance is indicated by asterisks (**) as compared with sham treatment group within each time point. No difference was found between time points within treatment groups. SEM is reported as ±value.
Discussion
Immunological regulation of reproductive function and tumorigenesis is well known; however, the dysregulation of Tregs in both eutopic and ectopic tissues highlights the complexity of a single treatment to combat the many faces this disease presents. Endometriosis is characterized by dysregulation of the eutopic endometrium resulting in decreased embryonic implantation success (Lessey, 2002; Kao et al., 2003; Giudice, 2004; Tomassetti et al., 2006; Tranguch et al., 2007; Jackson et al., 2009; Luo et al., 2011) and decreased responsiveness to ovarian steroid hormones (Attia et al., 2000; Bulun et al., 2006; Jackson et al., 2007; Banu et al., 2008; Aghajanova et al., 2009; Braundmeier et al., 2010). Ectopic endometrial fragments have increased estrogen responsiveness and invasive properties (Banu et al., 2008; Yu et al., 2008; Attar et al., 2009; Trukhacheva et al., 2009; Braundmeier et al., 2010; Wang et al., 2010b), which are crucial for ectopic lesion development. Eutopic and ectopic tissues share an alteration of the immunological phenotype compared with a healthy endometrium (Berbic et al., 2010; Wang et al., 2010a; Mier-Cabrera et al., 2011; Osuga et al., 2011; Podgaec et al., 2011; Sikora et al., 2011). Of greater concern is that this immunological alteration is not only targeted to endometrial tissues (eutopic and ectopic) but also systemically, based on findings that patients with endometriosis are more susceptible to the development of autoimmune disorders (Inagaki et al., 2011; Nielsen et al., 2011; Sundqvist et al., 2011). Our study investigated both peripheral and endometrial-specific alterations of Tregs throughout the progression of endometriosis. In addition, we investigated Treg expression and localization in ectopic endometrial fragments and if excisional removal of lesions altered their immunological tolerance.
Adaptive Treg populations were expressed most abundantly in the peripheral circulation of baboons but their migration was not hormonally regulated (i.e. did not change during the cycle). In comparison nTregs were the least abundant population of Tregs and there was a further decrease in abundance during the secretory stage of the menstrual cycle. Ovarian hormones estrogen and progesterone can stimulate the proliferation of nTregs (Polanczyk et al., 2006; Prieto and Rosenstein, 2006; Lee et al., 2011), although we did not find this in our animals. We hypothesized that the decreased abundance of nTregs during the secretory (receptive) stage of the menstrual cycle could be related to their migration from the peripheral circulation to the uterine endometrial lining, to prepare the endometrium in the event of embryo implantation. Chemotaxis of peripheral Tregs is directed by CXCL12 (SDF-1, stromal derived factor-1) signaling through its receptor CXCR4 (Zou et al., 2004). The regulation of CXCL12 by ovarian steroid hormones in the endometrium and peripheral circulation is variable (Kitaya et al., 2004; Elsheikh et al., 2011; Laird et al., 2011) and we did not detect any ovarian hormonal regulation of CXCL12 transcript in the endometrium of our cycling control or diseased animals (data not shown). Additionally, there are reports that the percentage of endometrial Foxp3-positive cells changes with the cycle stage (Arruvito et al., 2007; Berbic et al., 2010), consequently it is unclear what is driving the peripheral decrease in nTreg abundance. Future investigations into cyclical changes in endometrial Treg populations are required to determine how ovarian steroid hormones effect Treg recruitment to the uterus.
The induction of endometriosis resulted in a dramatic and rapid decrease in both nTregs and adaptive Tregs in the peripheral circulation and endometrium. This reduction was most evident in nTregs and Th3 adaptive Tregs by the reduction of Foxp3-positive cells in the peripheral circulation and secretory endometrium but also by the reduction of Foxp3 transcript in the secretory endometrium. These data contradict previous reports in women (Berbic et al., 2010); we believe that the use of each animal as its own internal control prior to disease induction in our study supports the validity of our results; this approach is impossible in human studies. Also unlike in human studies, we were able to investigate the changes in Foxp3-positive cells and transcript levels across the pathophysiology of the disease and found that following disease induction (1-month post-inoculation) Tregs were reduced and this reduction was maintained throughout the remainder of the study (15 months). These data support endometriosis as an autoimmune condition that stably results in systemic reduction in Treg populations throughout the time course of the disease (reviewed in Chavele and Ehrenstein, 2011) The systemic and uterine-specific reduction of Tregs explains why low Treg expression is associated with poor fertility prognosis (Sasaki et al., 2004; Saito et al., 2005; Yang et al., 2008; Mjosberg et al., 2009).
Excisional removal of ectopic endometrial fragments has improved fertility success in both spontaneous and IVF-assisted pregnancies (Abbott et al., 2003; Littman et al., 2005; Opøien et al., 2011) but how surgical removal directly increases fecundity of patients remains unknown. We found that an early operational procedure (1-month post-inoculation), whether excisional or sham, resulted in a recovery of Foxp3 transcipt levels and Foxp3-positive cells at 15 months post-inoculation. Because this recovery was not immediate we do feel that the examination of the Foxp3 profile at later time points (18 months or greater) in our 6-month operational animals would result in the same conclusion as those animals receiving operational surgery at the 1 month. These data suggest that any disturbance of the peritoneal environment during surgical manipulation, whether excision of lesions occurs or not, could potentially give the immune system a chance to rebound from a proinflammatory to a normal immunological environment.
The pathogenesis of ectopic lesion establishment, growth and maintenance is relatively unclear; however, this process can be modeled after the establishment and growth of tumors in the abdominal cavity. Although ectopic endometrial fragments are HLA-compatible tissues, the implantation of endometrial tissue into the peritoneal lining results in active wounding of the mesothelial surface and likely triggers apoptotic clearance of ectopic endometrial debri (reviewed in Taniguchi et al., 2011) As this clearance does not occur in patients with endometriosis, we speculate that ectopic endometrial fragments evade the innate immune system using similar methods to tumor cells. The primary method to acquire immune tolerance in tumor cells is through recruitment and active differentiation of Tregs in the tumor microenvironment (reviewed in Wang et al., 2012). Analysis of Foxp3 transcript levels and Foxp3-positive cells in ectopic endometrial lesions that were excised at the15-month terminal procedure indicates that animals that had sham operational procedures had higher levels of Foxp3 transcript and an increased abundance of Foxp3-positive cells, indicative of immune tolerance. Animals that had received excisional surgeries at either 1 or 6 months had lower Foxp3 transcript levels and reduced abundance of Foxp3-positive cells indicating reduced immune tolerance. This was supported by our results indicating that animals receiving excisional surgeries at 6 months had increased lesion regression over the time course of disease. The role of Tregs at ectopic sites may be to enhance immune tolerance at the invasive edge of the lesion, as indicated by our localization of Tregs to the mesenchymal/endometrial border. These data are similar to those reported in tumor cell lines (Bergmann et al., 2007; Strauss et al., 2007) and multiple tumor types (Katz et al., 2010; Kim et al., 2011; Righi et al., 2011).
Here we demonstrate that the immune environment of animals was altered upon the induction of endometriosis. Alteration of Tregs was observed in the peripheral circulation and eutopic or ectopic endometrial tissues. Peripheral cells and the eutopic endometrium had decreased Tregs, most likely contributing to endometriosis-associated infertility, while ectopic endometrial tissues had enhanced Tregs, thus attaining immune tolerance from the innate immune system. Ongoing studies will elucidate the regulation and function of Tregs at ectopic sites to develop therapeutic targets for the regression of ectopic lesions. This study supports the utilization of surgical intervention as a method for potentially increasing fecundity rates in patients with endometriosis. Additional studies are warranted to determine the precise physiological changes induced by surgical intervention, with a long-term aim of designing more effective surgical treatment options for patients with endometriosis.
Authors' roles
A.B. was involved in experimental design, execution, analysis and manuscript preparation. K.J., J.H. were involved in experimental design and execution. J.K. was involved in execution. R.N. was involved in funding and manuscript preparation. A.F. was involved in funding, experimental design, execution and manuscript preparation.
Funding
These studies were supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD 40093 as part of the Specialized Cooperative Centers Program in Reproductive Research to ATF.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank the generous efforts by the following people: Mark Olson, May Miguel, Kavita Sapru, Denisse Yanez and Rachel Rey. We would also like to thank the biological research laboratory staff (UIC) and also Drs. Shane Lipskin and Magdy Milad for assistance with surgeries.
References
- Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18:1922–1927. doi: 10.1093/humrep/deg275. doi:10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150:3863–3870. doi: 10.1210/en.2009-0008. doi:10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan ZM, Denis D, Kagan D, Grund EM, Palmer SS, Nataraja SG. A long-acting tumor necrosis factor alpha-binding protein demonstrates activity in both in vitro and in vivo models of endometriosis. J Pharmacol Exp Ther. 2010;334:460–466. doi: 10.1124/jpet.110.166488. doi:10.1124/jpet.110.166488. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. doi:10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18:756–759. doi: 10.1093/humrep/deg136. doi:10.1093/humrep/deg136. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. doi: 10.1210/jc.2008-1180. doi:10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. doi:10.1210/jc.85.8.2897. [DOI] [PubMed] [Google Scholar]
- Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–161. doi: 10.1146/annurev.immunol.19.1.131. doi:10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril. 2008;90:972–987. doi: 10.1016/j.fertnstert.2007.07.1358. doi:10.1016/j.fertnstert.2007.07.1358. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. doi:10.1016/S0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- Basta P, Majka M, Jozwicki W, Lukaszewska E, Knafel A, Grabiec M, Stasienko E, Wicherek L. The frequency of CD25+CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol. 2011;8:116. doi: 10.1186/1477-7827-8-116. doi:10.1186/1477-7827-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149–155. doi: 10.1016/j.jri.2010.11.004. doi:10.1016/j.jri.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Berbic M, Hey-Cunningham AJ, Ng C, Tokushige N, Ganewatta S, Markham R, Russell P, Fraser IS. The role of Foxp3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod. 2010;25:900–907. doi: 10.1093/humrep/deq020. doi:10.1093/humrep/deq020. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother. 2007;56:1429–1442. doi: 10.1007/s00262-007-0280-9. doi:10.1007/s00262-007-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. doi:10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT, Nowak RA. Extracellular matrix metalloproteinase inducer (EMMPRIN) expression in the baboon endometrium: menstrual cycle and endometriosis. Reproduction. 2010;140:911–920. doi: 10.1530/REP-09-0481. doi:10.1530/REP-09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. doi:10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. doi:10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Immune-endocrine interactions in endometriosis. Front Biosci (Elite Ed) 2009;1:429–443. doi: 10.2741/E39. [DOI] [PubMed] [Google Scholar]
- Chavele K-M, Ehrenstein MR. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett. 2011;585:3603–3610. doi: 10.1016/j.febslet.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance. Eur J Contracept Reprod Health Care. 2007;12:194–202. doi: 10.1080/13625180701387266. doi:10.1080/13625180701387266. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril. 1997;68:613–625. doi: 10.1016/s0015-0282(97)00277-x. doi:10.1016/S0015-0282(97)00277-X. [DOI] [PubMed] [Google Scholar]
- Elsheikh E, Sylven C, Ericzon BG, Palmblad J, Mints M. Cyclic variability of stromal cell-derived factor-1 and endothelial progenitor cells during the menstrual cycle. Int J Mol Med. 2011;27:221–226. doi: 10.3892/ijmm.2010.570. doi:10.3892/ijmm.2010.570. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. doi:10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci. 2002;955:308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. discussion 340–302, 396–406 doi:10.1111/j.1749-6632.2002.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Folse D, Stout L. Endometriosis in the baboon. Lab Anim Sci. 1978;28:217–219. [PubMed] [Google Scholar]
- Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics. 2004;4:299–312. doi: 10.2165/00129785-200404050-00003. doi:10.2165/00129785-200404050-00003. [DOI] [PubMed] [Google Scholar]
- Hey-Cunningham AJ, Fazleabas AT, Braundmeier AG, Markham R, Fraser IS, Berbic M. Endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. Reprod Sci. 2011;18:747–754. doi: 10.1177/1933719110397210. doi:10.1177/1933719110397210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. doi:10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Sun L, Gao L, Liao L, Mao Y, Liu J. Cytokine array analysis of peritoneal fluid between women with endometriosis of different stages and those without endometriosis. Biomarkers. 2009;14:604–618. doi: 10.3109/13547500903183970. doi:10.3109/13547500903183970. [DOI] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. doi:10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- Inagaki J, Hao L, Nakatsuka M, Yasuda T, Hiramatsu Y, Shoenfeld Y, Matsuura E. A possible mechanism of autoimmune-mediated infertility in women with endometriosis. Am J Reprod Immunol. 2011;66:90–99. doi: 10.1111/j.1600-0897.2010.00956.x. doi:10.1111/j.1600-0897.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14:137–150. doi: 10.1177/1933719106298409. doi:10.1177/1933719106298409. [DOI] [PubMed] [Google Scholar]
- Jackson K, Hastings J, Mavrogianis P, Bagchi I, Fazleabas A. Alterations in the calcitonin and calcitonin modulated proteins, E-cadherin and the enzyme tissue transglutaminase II during the window of implantation in a baboon model of endometriosis. J Endometriosis. 2009;1:57–67. [Google Scholar]
- Jones CJ, Inuwa IM, Nardo LG, Litta P, Fazleabas AT. Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls—a pilot observational study. Reprod Sci. 2009;16:559–572. doi: 10.1177/1933719109332825. doi:10.1177/1933719109332825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. doi:10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gonen M, Espat NJ, Klimstra DS, D'Angelica MI, Allen PJ, Jarnagin W, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxford) 2010;12:674–683. doi: 10.1111/j.1477-2574.2010.00231.x. doi:10.1111/j.1477-2574.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, Park CG, Kim CB. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol. 2011;104:728–733. doi: 10.1002/jso.22038. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89:2470–2476. doi: 10.1210/jc.2003-031293. doi:10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- Krikun G, Hu Z, Osteen K, Bruner-Tran KL, Schatz F, Taylor HS, Toti P, Arcuri F, Konigsberg W, Garen A, et al. The immunoconjugate "icon" targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am J Pathol. 2010;176:1050–1056. doi: 10.2353/ajpath.2010.090757. doi:10.2353/ajpath.2010.090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Kitade M, Kikuchi I, Kumakiri J, Matsuoka S, Kuroda M, Takeda S. Peritoneal vascular density assessment using narrow-band imaging and vascular analysis software, and cytokine analysis in women with and without endometriosis. J Minim Invasive Gynecol. 2010;17:21–25. doi: 10.1016/j.jmig.2009.09.003. doi:10.1016/j.jmig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, Peeraer K, Tomassetti C, Meuleman C, D'Hooghe TM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed) 2009;1:444–454. doi: 10.2741/e40. [DOI] [PubMed] [Google Scholar]
- Laird SM, Widdowson R, El-Sheikhi M, Hall AJ, Li TC. Expression of CXCL12 and CXCR4 in human endometrium; effects of CXCL12 on MMP production by human endometrial cells. Hum Reprod. 2011;26:1144–1152. doi: 10.1093/humrep/der043. doi:10.1093/humrep/der043. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011;187:1778–1787. doi: 10.4049/jimmunol.1003919. doi:10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002;955:265–280. doi: 10.1111/j.1749-6632.2002.tb02787.x. ; discussion 293–265, 396–406 doi:10.1111/j.1749-6632.2002.tb02787.x. [DOI] [PubMed] [Google Scholar]
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84:1574–1578. doi: 10.1016/j.fertnstert.2005.02.059. doi:10.1016/j.fertnstert.2005.02.059. [DOI] [PubMed] [Google Scholar]
- Luo Q, Chen XJ, Ding GL, Dong MY, Huang HF. Downregulative effects of nitric oxide on oocyte fertilization and embryo development: possible roles of nitric oxide in the pathogenesis of endometriosis-associated infertility. Cell Physiol Biochem. 2011;26:1023–1028. doi: 10.1159/000323977. doi:10.1159/000323977. [DOI] [PubMed] [Google Scholar]
- Lv D, Song H, Shi G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD008088.pub2. CD008088. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011;335:42–51. doi: 10.1016/j.mce.2010.08.006. doi:10.1016/j.mce.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Medicine ASfR. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. doi:10.1016/S0015-0282(97)81391-X. [DOI] [PubMed] [Google Scholar]
- Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand. 2009;88:968–975. doi: 10.1080/00016340903176826. doi:10.1080/00016340903176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. Spontaneous endometriosis in the Kenya baboon. Am J Obstet Gynecol. 1968;101:569–570. doi: 10.1016/0002-9378(68)90572-3. [DOI] [PubMed] [Google Scholar]
- Meyer R. uber den Stand der Frage der Ademomyositis and Ademomyome serosepithelialis und Adenomyometritis sarcomatosa. Zentrakbl Gynako. 1919;43:745–750. [Google Scholar]
- Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2009;82:698–705. doi: 10.1095/biolreprod.109.081208. doi:10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- Monckedieck V, Sannecke C, Husen B, Kumbartski M, Kimmig R, Totsch M, Winterhager E, Grummer R. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol Hum Reprod. 2009;15:633–643. doi: 10.1093/molehr/gap063. doi:10.1093/molehr/gap063. [DOI] [PubMed] [Google Scholar]
- Nielsen NM, Jorgensen KT, Pedersen BV, Rostgaard K, Frisch M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum Reprod. 2011;26:1555–1559. doi: 10.1093/humrep/der105. doi:10.1093/humrep/der105. [DOI] [PubMed] [Google Scholar]
- Opøien HK, Fedorcsak P, Ãbyholm T, Tanbo T. Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod BioMed Online. 2011;23:389–395. doi: 10.1016/j.rbmo.2011.06.002. doi:10.1016/j.rbmo.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Koga K, Hirota Y, Hirata T, Yoshino O, Taketani Y. Lymphocytes in endometriosis. Am J Reprod Immunol. 2011;65:1–10. doi: 10.1111/j.1600-0897.2010.00887.x. doi:10.1111/j.1600-0897.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- Podgaec S, Dias Junior JA, Chapron C, Oliveira RM, Baracat EC, Abrao MS. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev Assoc Med Bras. 2011;56:92–98. doi: 10.1590/s0104-42302010000100022. doi:10.1590/S0104-42302010000100022. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. doi: 10.1002/jnr.20881. doi:10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. doi:10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi E, Kashiwagi S, Yuan J, Santosuosso M, Leblanc P, Ingraham R, Forbes B, Edelblute B, Collette B, Xing D, et al. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res. 2011;71:5522–5534. doi: 10.1158/0008-5472.CAN-10-3143. doi:10.1158/0008-5472.CAN-10-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Sasaki Y, Sakai M. CD4(+)CD25high regulatory T cells in human pregnancy. J Reprod Immunol. 2005;65:111–120. doi: 10.1016/j.jri.2005.01.004. doi:10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. doi:10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Sampson J. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. doi:10.1001/archsurg.1925.01120100007001. [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. doi:10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Sherwin JR, Sharkey AM, Mihalyi A, Simsa P, Catalano RD, D'Hooghe TM. Global gene analysis of late secretory phase, eutopic endometrium does not provide the basis for a minimally invasive test of endometriosis. Hum Reprod. 2008;23:1063–1068. doi: 10.1093/humrep/den078. doi:10.1093/humrep/den078. [DOI] [PubMed] [Google Scholar]
- Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr Med Chem. 2011;18:200–208. doi: 10.2174/092986711794088416. doi:10.2174/092986711794088416. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–2724. doi: 10.1093/humrep/17.10.2715. doi:10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. doi:10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- Sundqvist J, Falconer H, Seddighzadeh M, Vodolazkaia A, Fassbender A, Kyama C, Bokor A, Stephansson O, Padyukov L, Gemzell-Danielsson K, et al. Endometriosis and autoimmune disease: association of susceptibility to moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil Steril. 2011;95:437–440. doi: 10.1016/j.fertnstert.2010.07.1060. doi:10.1016/j.fertnstert.2010.07.1060. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. doi:10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi F, Kaponis A, Izawa M, Kiyama T, Deura I, Ito M, Iwabe T, Adonakis G, Terakawa N, Harada T. Apoptosis and endometriosis. Front Biosci (Elite Ed) 2011;3:648–662. doi: 10.2741/e277. doi:10.2741/e277. [DOI] [PubMed] [Google Scholar]
- Tomassetti C, Meuleman C, Pexsters A, Mihalyi A, Kyama C, Simsa P, D'Hooghe TM. Endometriosis, recurrent miscarriage and implantation failure: is there an immunological link? Reprod Biomed Online. 2006;13:58–64. doi: 10.1016/s1472-6483(10)62016-0. doi:10.1016/S1472-6483(10)62016-0. [DOI] [PubMed] [Google Scholar]
- Tran LV, Tokushige N, Berbic M, Markham R, Fraser IS. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod. 2009;24:835–841. doi: 10.1093/humrep/den483. doi:10.1093/humrep/den483. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. doi:10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. doi:10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa M, Tanaka N, Tainaka H, Takeda K, Ihara T, Sugamata M. Microarray analysis provides insight into the early steps of pathophysiology of mouse endometriosis model induced by autotransplantation of endometrium. Life Sci. 2009;84:832–837. doi: 10.1016/j.lfs.2009.03.015. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. doi:10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol. 2009;16:713–719. doi: 10.1016/j.jmig.2009.07.012. doi:10.1016/j.jmig.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, Li DJ. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010a;45:291–299. doi: 10.1677/JME-09-0177. doi:10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu J, Luo X, Wang X, Li M, Wang L, Li D. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol. 2010b;7:51–60. doi: 10.1038/cmi.2009.102. doi:10.1038/cmi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D, Shen X. Regulatory T cell: a protection for tumor cells. J Cell Mol Med. 2012;16:425–436. doi: 10.1111/j.1582-4934.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. doi:10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidual and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. doi:10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Zhou WH, Wang L, He YY, Li DJ. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. doi:10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. doi:10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]