Abstract
BACKGROUND
The current understanding of hormonal regulation of matrix metalloproteinase-26 (MMP-26) in the primate endometrium is incomplete. The goal of this work was to clarify estrogen and progesterone regulation of MMP-26 in the endometrium of ovariectomized, hormone-treated rhesus macaques.
METHODS
Ovariectomized rhesus macaques (n= 66) were treated with estradiol (E2), E2 plus progesterone, E2 followed by progesterone alone or no hormone. Endometrium was collected from the hormone-treated animals during the early, mid- and late proliferative and secretory phases of the artificial menstrual cycle. MMP-26 expression was quantified by real-time PCR, and MMP-26 transcript and protein were localized by in situ hybridization and immunohistochemistry and correlated with estrogen receptor 1 and progesterone receptor (PGR).
RESULTS
MMP-26 was localized to glandular epithelium and was undetectable in the endometrial stroma and vasculature. MMP-26 transcript levels were minimal in the hormone-deprived macaques and treatment with E2 alone did not affect MMP-26 levels. Treatment with progesterone both in the presence and absence of E2 stimulated MMP-26 expression in the early and mid-secretory phases (P < 0.001). MMP-26 expression preceded decidualization of endometrial stroma. MMP-26 levels then declined to baseline in the late secretory phase (P < 0.01) despite continued E2 plus progesterone treatment. Loss of detectable MMP-26 expression in the late secretory phase was correlated with late secretory phase loss of glandular epithelial PGR.
CONCLUSIONS
Endometrial MMP-26 expression is dependent on the presence of progesterone in the early secretory phase and then gradually becomes refractory to progesterone stimulation in the late secretory phase. In the macaque, MMP-26 is a marker of the pre-decidual, secretory endometrium. During the second half of the late secretory phase, and during decidualization, MMP-26 loses its response to progesterone concurrent with the loss of epithelial PGR. The decline in MMP-26 levels between the mid- and late secretory phases may play a role in the receptive window for embryo implantation.
Keywords: matrix metalloproteinase-26, estradiol, progesterone, rhesus macaque, endometrium
Introduction
The primate endometrium undergoes extensive steroid-dependent remodeling during the menstrual cycle (Slayden and Keator, 2007). In the follicular phase of the cycle, estradiol (E2) stimulates endometrial cell proliferation and growth. Following ovulation in the luteal phase of the cycle, E2 and progesterone stimulate endometrial secretory differentiation and the endometrium becomes receptive to embryo implantation. If pregnancy does not occur, the corpus luteum regresses and circulating levels of E2 and progesterone decline, which triggers degradation and shedding of the endometrial functionalis zone during menses. Tissue sloughing during menses is mediated by cycle-dependent expression of tissue degrading matrix metalloproteinase (MMP) enzymes (Goffin et al., 2003). The MMPs are a family of 23 endopeptidases that degrade extracellular matrix (ECM) substrates and play roles in ECM homeostasis (Sternlicht and Werb, 2001). Endometrial expression of MMP-1, -3, -7, -8, -9, -10, -11 and -12 is strikingly up-regulated following progesterone withdrawal in the menstrual phase of the cycle (Goffin et al., 2003).
Matrix metalloproteinase-26 (MMP-26), also referred to as matrilysin-2 or endometase, displays a unique expression pattern compared with other endometrial MMPs. In women, MMP-26 is up-regulated during the early secretory phase of the cycle immediately preceding the period of embryo implantation (Li et al., 2002; Pilka et al., 2003; Pilka et al., 2004a,b). Several reports indicate that estrogen, acting through estrogen receptor 1 (ESR-1), regulates MMP-26 expression and that MMP-26 levels are elevated in estrogen-dependent endometrial hyperplasia (Pilka et al., 2004a,b, 2006). These authors suggest a complex web of regulation of the MMP-26 gene via indirect estrogen regulation of pathways including Ras and Jun families of proteins and/or by β–catenin via the Wnt signal transduction pathway. However, careful review of published data does not support a role for estrogens in stimulating MMP-26 expression because there appears to be no positive correlation between levels of MMP-26 and circulating levels of E2 or the expression of ESR1.
The regulation of MMP-26 is complicated in that both estrogen and progesterone are secretory products of the corpus luteum during the secretory phase. However, progesterone action typically opposes many of the effects of estrogen on the endometrium. A clear understanding of MMP-26 regulation is further confounded by reports that MMP-26 was elevated in subjectively staged samples of endometrial tissue collected from women during the late proliferative phase (Pilka et al., 2003). However, the same report indicated that MMP-26 was undetectable in endometrial explants collected from the proliferative stage and then subsequently treated with high levels of E2 in vitro (Pilka et al., 2003). In that same study, MMP-26 was detectable when similar explants were cultured in the presence of E2 plus progesterone (Pilka et al., 2003) indicating that progesterone, not estrogen, regulates the expression of MMP-26. Moreover, in a recent study, levels of MMP-26 were examined in the secretory endometrium collected from women during the natural cycle or from women given supplemental E2 and progesterone, with the authors concluding that exogenous estrogen therapy stimulated MMP-26 despite elevated levels of progesterone (Pilka et al., 2006). All of these studies reaffirm a possible role of progesterone in stimulating MMP-26 expression. Therefore, hormonal regulation of MMP-26 expression in the secretory phase is unclear, and the broad perception that MMP-26 is regulated by estrogen alone is a gross misinterpretation.
The regulation of MMP-26 is most likely a complex system and assessing hormonal regulation of the endometrium in women is complicated in and of itself. The staging of human endometrium following the Noyes criteria (Noyes et al., 1950) is a subjective system that has been scrutinized in recent years because of its inaccuracies in properly dating the endometrial phase of the menstrual cycle (Murray et al., 2004; Fadare and Zheng, 2005). In addition, clinical studies on MMP-26 are limited by practical considerations, including unwanted effects resulting from manipulating the hormonal milieu in women.
In this study we assessed MMP-26 expression and localization in the endometrium from ovariectomized hormone-treated rhesus macaques, a model in which steroid hormone levels can be tightly regulated. We focused our experiments on the action of E2, E2 plus progesterone and progesterone alone following E2 priming. This study achieves what is not possible in women and lays an important foundation for future functional studies. We report that MMP-26 is minimal in the endometrium from hormone-deprived animals and maximal in macaques during the first 3–7 days of progesterone treatment, with or without E2 present, in the artificial secretory phase of the cycle. Thereafter, MMP-26 expression was refractory to progesterone stimulation. Reduced expression of MMP-26 in the secretory phase was closely associated with the loss of endometrial ESR1 and PGR in the glandular epithelium during the late secretory phase of the menstrual cycle.
Materials and Methods
Animals
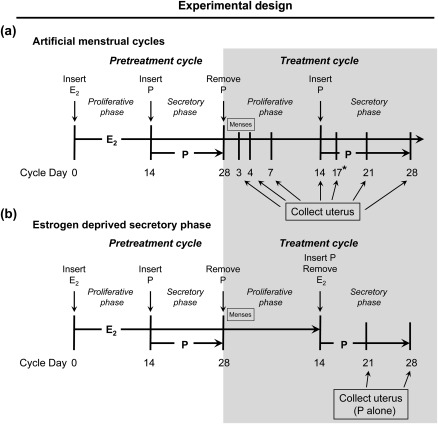
Archived endometrial tissues from a total of 66 adult rhesus macaques (Macaca mulatta) at the Oregon National Primate Research Center (ONPRC) were used in this study. Animal care was provided by the veterinary staff at ONPRC and animal use was reviewed and approved by the ONPRC Institutional Animal Care and Use Committee. The animals were ovariectomized and treated with defined regimens of E2 and progesterone to stimulate artificial menstrual cycles (Rudolph-Owen et al., 1998; Slayden et al., 2001; Slayden and Keator, 2007). These cycles were created by s.c. insertion of a 5-cm E2-releasing Silastic capsule (0.34 cm i.d.; 0.46 cm o.d.; Dow Corning, Midland, MI, USA) to stimulate an artificial proliferative phase. After 14 days of E2 priming, a 6-cm progesterone-releasing capsule was implanted s.c. for 14 days to stimulate an artificial secretory phase. Removal of the progesterone implant on Day 28 completed the cycle and induced menstruation on Days 2–4 of the next cycle (Fig. 1). Endometrial tissues were analyzed from artificial cycle day 3 (menstruation; n = 8), day 7 (mid-proliferative phase; n = 4), day 14 (late proliferative phase; n = 5), day 17 (early secretory phase; n = 3), day 21 (mid-secretory phase; n = 4) and day 28 (late secretory phase; n = 14; Fig. 1a).
Figure 1.
Diagram depicting rhesus macaque treatments. The typical and well-established artificial menstrual cycle, which shows time points for tissue collection, is depicted in (a). The newly developed model to assess the effects of progesterone-only treatment, and the time points for tissue collection, are shown in (b). D, day of cycle.
Six artificially cycled animals had E2 withdrawn at the onset of the secretory phase (Fig. 1b) and samples were then collected after 7 and 14 days of treatment with progesterone alone (n = 3 each). Control tissues were obtained from ovariectomized animals that were not treated with exogenous hormones for at least 2 months (no hormone; n = 8); animals treated with 28 days of E2 alone (n = 10) and animals treated for 14 days with progesterone without estrogen priming (n = 4).
Tissue processing
Reproductive tracts were prepared by dissecting the uterus from the oviducts and cervix. The uterine body was cut into equal quarters along the longitudinal axis. The endometrium from two uterine quarters was cut into full-thickness cross-sectional slices spanning from the lumen to the myometrium. Representative slices were fixed overnight (∼18h) in 4% paraformaldehyde, dehydrated, cleared with xylene and embedded in paraffin. Similar slices were treated with microwave irradiation for 7 s (Slayden et al., 1995), embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA, USA) and frozen in liquid propane. The remaining endometrium was separated from the myometrium and frozen in liquid nitrogen. All frozen samples were stored at −80°C.
Real-time PCR
Frozen endometrium was thawed in TRIzol (Invitrogen, Carlsbad, CA, USA) and homogenized with a Polytron (Brinkman Instruments, Westbury, NY, USA). Total RNA was extracted with TRIzol; precipitated with ethanol and further purified via DNase digestion on Qiagen Quick columns (Qiagen, Valencia, CA, USA). RNA concentration was determined on a Nanodrop Spectrophotometer (Model ND-1000; Thermo Scientific, Wilmington, DE, USA) and RNA integrity was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The RNA exhibited high 260/280 ratios (1.91 ± 0.03) and RNA integrity number values (8.72 ± 0.17) indicating sufficient quality for analysis by real-time PCR (Schroeder et al., 2006).
Total RNA (1 µg) was reverse transcribed into cDNA, in the presence of random hexamer primers (Promega Corporation, Madison, WI, USA) using an Omniscript RT kit (Qiagen). Real-time PCRs were on a 7500 Fast Real-time PCR System (ABI; Applied Biosystems, Carlsbad, CA, USA) as described previously (Keator et al., 2011). Real-time PCRs were performed in duplicate on 2 µl of diluted (1:20) cDNA and compared against a five-point standard curve of pooled endometrial cDNA. Each reaction contained 250-nM TaqMan® probe specific for rhesus macaque MMP-26 (FAM-labeled) and probe (VIC-labeled) specific for the ribosomal protein gene RPL32 or mitochondrial ribosomal protein S10, described previously (Keator et al., 2011). The reactions contained 900-nM MMP-26 or 300-nM housekeeping gene sense and antisense primers and 5 μl of 2× Fast Universal PCR Master Mix (ABI). MMP-26 transcript was normalized to the expression of each housekeeping gene and the values were then averaged to yield a single expression level of MMP-26 for each sample, according to the methods reviewed by Nolan et al. (2006).
In situ hybridization
Macaque-specific riboprobes for MMP-26 were prepared as follows: gene-specific PCR products were amplified from macaque cDNA using gene-specific PCR primers (Table I) designed with Oligoperfect™ software (Invitrogen). The identity of the PCR products was confirmed by sequence analysis on a 3130XL Genetic analyzer (ABI) and subsequent BLAST comparison on the National Center for Biotechnology Information website. PCR primers containing the original sequences and a T7 recognition sequence (TAATACGACTCACTATAGGG) were purchased (Invitrogen) and used to synthesize two DNA templates, one with the T7 recognition sites in 5′ sense and another with the T7 site in the 5′ antisense orientation. Antisense and sense single-stranded riboprobes were synthesized using the MaxiScript Kit (Ambion, Foster City, CA, USA) with the gene-specific DNA templates in the presence of 35S-UTP (1250 Ci/mmol; Perkin Elmer, Waltham, MA, USA) and T7 RNA polymerase.
Table I.
Primer and TaqMan probe sequences (all 5′ to 3′) used to detect matrix MMP-26 in rhesus macaque tissues.
| Application | Sense | Antisense | TaqMan probe | Region |
|---|---|---|---|---|
| qPCR | caacctgtcctttcagggaatt | ccaaggctgctgtagtgcttt | ttgaggaccaaaaac | 829–891 |
| ISH (set 1) | ccccctgctacagtccataa | tttcactgtggatggcttca | 82–384 | |
| ISH (set 2) | caacctgtcctttcagggaatt | aacctgactgggttgcattc | 829–935 |
Regions listed coincide with the human mRNA sequence (accession no. NM_021801). qPCR, quantitative real-time PCR; ISH, in situ hybridization.
OCT-embedded samples of macaque endometrium were cryosectioned at 7 µm, mounted on Super Frost Plus slides (Fisher Scientific, Pittsburgh, PA, USA) and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at 4°C. The tissue sections were rinsed in 2× standard saline citrate (SSC), acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) for 10 min and then air dried. One slide per tissue group was treated with RNAse A [20 mg/ml, 0.5 M NaCl, 0.01 M Tris and 1 mM EDTA (pH 8.0)] as a negative control. All the slides were prehybridized for 1 h at 42°C in 10 mM dithiothreitol, 0.3 M NaCl, 20 mN Tris (pH 8.0), 5 mM EDTA, 1× Denhardt solution, 10% dextran sulfate and 50% formamide. The sections were then subjected to in situ hybridization (ISH) with 5 × 106 cpm/ml [35S]-labeled riboprobes to MMP-26 at 55°C overnight in the same solution containing the appropriate concentration of the antisense probe. Representative slides were also incubated with sense probe as another negative control. The slides were treated with RNase A at 37°C for 30 min to inactivate non-hybridized probe and then subjected to high stringency rinses in a descending series of SSC as recently described (Keator et al., 2011). Sections were dehydrated in an ascending series of alcohol dilutions, vacuum dried, coated with NTB autoradiographic emulsion (Eastman Kodak, Rochester, NY, USA), stored at 4°C for 9–10 days, developed in aqueous D-19 (Eastman Kodak), lightly counterstained with hematoxylin, dehydrated in an ascending series of alcohol dilutions, cleared with xylene and cover slipped with Permount (Fisher Scientific).
Immunohistochemistry
Qualitative immunohistochemistry (IHC) for MMP-26 was conducted on 5-µm sections of paraformaldehyde-fixed, paraffin-embedded endometrium mounted on poly-L-lysine-coated slides (Fisher Scientific). The slides were deparaffinized in xylene, rehydrated stepwise in ethanol and rinsed briefly in deionized H2O. Sections were heated in citrate buffer (BioGenex, San Ramon, CA, USA) for 10 min in a household-type pressure cooker to facilitate antigen retrieval. The antigen-retrieved slides were allowed to cool to room temperature, rinsed three times (3×) in PBS and then used immediately. The slides were incubated in 3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidases, quickly rinsed in deionized H2O and then rinsed (3×) in PBS. Slides were incubated (∼20 min) with a blocking solution (normal goat serum) in a moisture chamber at room temperature and then incubated overnight at 4°C with a polyclonal (goat) anti-MMP-26 immunoglobulin (Ig)G (Abcam, Cambridge, MA, USA). The standard working concentration of MMP-26 antibody (5 µg/ml) was determined by performing a series of serial antibody dilutions. Representative sections were incubated with normal serum, or PBS and 1% bovine serum albumin (omission of the first antibody), to assess non-specific staining.
Following overnight incubation, the slides were rinsed (3×) with 0.075% polyoxyethylene 23 laurylether (BRIJ) in PBS (4°C). Slides were incubated with a blocking solution (goat serum) and then incubated with a biotinylated anti-goat secondary antibody for 30 min at room temperature. The slides were rinsed (3×) with 0.075% BRIJ in PBS and the biotinylated antibody complexes were then reacted with an avidin–biotin peroxidase kit (Vector Laboratories, Burlingame, CA, USA) for 60 min at room temperature. The slides were again rinsed (3×) with 0.075% BRIJ in PBS and then treated for 15–20 min with 0.025% 3,3′-diaminobenzidine/4HCl (Dojindos DAB; Wako Chemicals, Richmond, VA, USA) in Tris buffer and 0.03% hydrogen peroxide (Fisher Scientific). Slides were rinsed repeatedly in water, treated with 0.05% OsO4 for 1 min, lightly counterstained with Mayer's hematoxylin, dehydrated, cleared with xylene and mounted with Permount (Fisher Scientific).
IHC for ESR1 and PGR was conducted on OCT-embedded cryosections as previously described (Slayden and Brenner, 2004). Briefly, cryosections (7 μm) were mounted on SuperFrost Plus slides and fixed in 2% paraformaldehyde in PBS (pH 7.3; Sigma) for 10 min at room temperature (∼23°C). Slides were rinsed and then incubated in a buffered solution containing glucose oxidase (1 U/ml), sodium azide (1 mM/l) and glucose (10 mM/l) for 45 min to inhibit endogenous peroxidase activity. The sections were treated with normal horse serum (20 min) and then with an endogenous biotin blocking solution (Vector Labs) for 30 min. The sections were incubated overnight at 4°C with mouse monoclonal antibodies directed against ESR1 (4 μg/ml; Clone 1D5; Thermo Fisher Scientific, Rockford, IL, USA) or PGR (1 μg/ml; Ab-8; Thermo). Non-specific mouse IgG antibodies directed against timothy pollen, and the omission of primary antibody were used as negative controls. Following incubation with primary antibody, the slides were rinsed in 0.075% BRIJ 35 (30% Stock; Sigma Chemicals, St. Louis, MO, USA) in PBS. The sections were incubated with blocking serum for 20 min and then with biotinylated anti-mouse IgG for 45 min at room temperature. The slides were rinsed again with PBS and reacted with an avidin–biotin peroxidase kit (Vector Labs) for 60 min (at room temperature), then rinsed in Tris buffer (pH 7.6). The slides were incubated for 15 min in 0.025% DAB to visualize antibody–antigen complexes, which were further stabilized by incubating the slides in 0.026% OsO4 for 1 min (room temperature). Slides were lightly counterstained with hematoxylin, dehydrated in ethanol, cleared with xylene and mounted with Permount.
Photomicrography
IHC and ISH stained slides were viewed through a Zeiss AxioImager A.1 microscope (Carl Zeiss, Inc., Oberkochen, Germany) fitted with planapochromatic lenses. Digital microphotographs were captured with a Leica DFC 480 camera (Leica, Wetzlar, Germany). Darkfield images were illuminated with a DarkliteTM stage illuminator (Micro Video Instruments, Inc., Avon, MA, USA).
Statistical analysis
Real-time PCR data were examined for statistical outliers using Grubbs' test (Sokal and Rohlf, 1987) and evaluated for homoscedasticity by Levene's test. Values were subsequently transformed (log 10 + 1) to correct for hetereogeneity of variance. Main effects on mean transcript expression related to hormonal status were tested by a one-way analysis of variance using the mixed procedure subroutine within SAS® software (SAS Institute, Inc., 2008). Significant differences between means (P≤ 0.05) were separated by a (protected; Petersen, 1985) least-square means analysis. All data are presented as the untransformed mean ± SE.
Results
Endometrial morphology
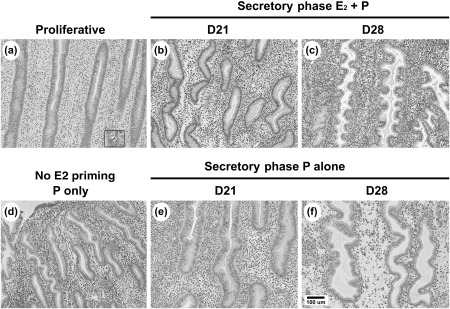
Sequential treatment of ovariectomized animals with E2 followed by E2 plus progesterone resulted in endometrial morphology and thickness that was similar to the natural menstrual cycle in macaques (Brenner and Slayden, 1994). Compared with the controls with no hormone treatment, treatment with E2 in the first 14 days of the artificial cycle stimulated endometrial growth and resulted in a ‘proliferative’ endometrium, similar to the follicular phase of the menstrual cycle, indicated by tubular glands and abundant mitotic cells (Fig. 2a; inset). Further treatment of E2-primed animals with E2 plus progesterone resulted in a ‘secretory’ differentiation of the endometrium, with minimal mitotic activity and torturous sacculated glands identical to the glands of the endometrium in the mid- [Day 21 (D21); Fig. 2b] and late (D28; Fig. 2c) luteal phases of the natural cycle (Brenner and Slayden, 1994). No decidual or predecidual cells were observed in the endometrium on D21. Predecidual cells (histochemically positive for tissue inhibitor of metalloproteinases-3; not shown) were observed cuffing the spiral arterioles on D28 of the artificial cycle. Interestingly, treatment with progesterone alone after 2 weeks of E2 priming also resulted in a secretory morphology (Fig. 2e and f) identical to that observed after E2 plus progesterone. Treatment with progesterone alone in the absence of E2 priming (Fig. 2d) resulted in underdeveloped secretory differentiation with notably smaller endometrial glands and a strikingly thin endometrium compared with E2 plus progesterone.
Figure 2.
Microphotographs showing the general endometrial histology (haematoxylin and eosin stain) in paraformaldehyde-fixed paraffin-embedded sections of macaque endometrium.
Cyclic regulation of MMP-26 expression
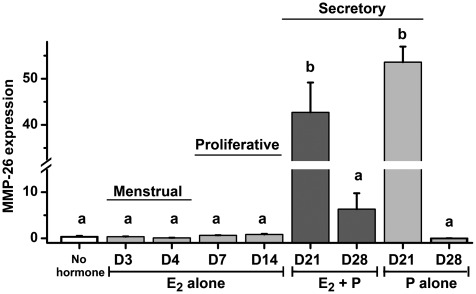
Figure 3 shows changes in the expression of MMP-26 transcript determined by real-time PCR. Levels of MMP-26 mRNA were minimal (at the low limit of detection) in control samples from ovariectomized macaques treated with no hormone. MMP-26 levels were also virtually undetectable under E2 treatment, during the menstrual phase of the cycle (D3 and D4) and during the artificial proliferative phase (D7 and D14) and after prolonged 28-day E2 treatment (not shown). Treatment with E2 plus progesterone resulted in a >50-fold significant increase (P< 0.001) by cycle day 21, prior to the onset of morphological decidualization of the endometrium. MMP-26 mRNA levels then declined significantly (P< 0.001) after 14 days of E2 plus progesterone treatment (cycle day 28). Treatment with progesterone alone for 7 days following E2 priming (D21 P alone) also resulted in a significant increase in MMP-26 expression (P< 0.001). MMP-26 levels declined after extended treatment with progesterone alone for 14 days following E2 priming (D28 P alone), which paralleled the changes between animals treated with 7 and 14 days of E2 plus progesterone. MMP-26 was almost undetectable in animals treated with P alone for 14 days without E2 priming (data not shown).
Figure 3.
Expression of MMP-26 in macaque detected by real-time PCR measures of mRNA transcript. Values represent the mean (±SE) level of transcript after being normalized to two housekeeping genes (ribosomal RPL32 and S10). Treatment with estradiol (E2) plus progesterone and progesterone alone for 7 days (D21) resulted in a significant increase in MMP-26 transcript.
Localization of MMP-26 transcript
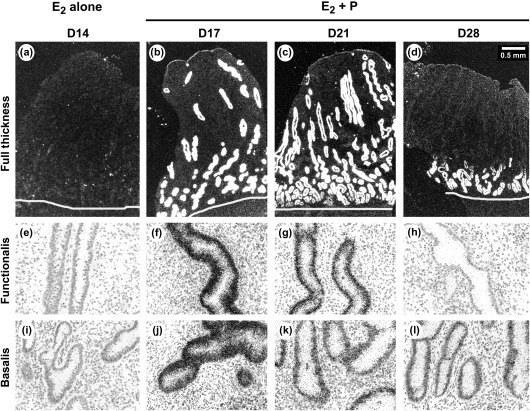
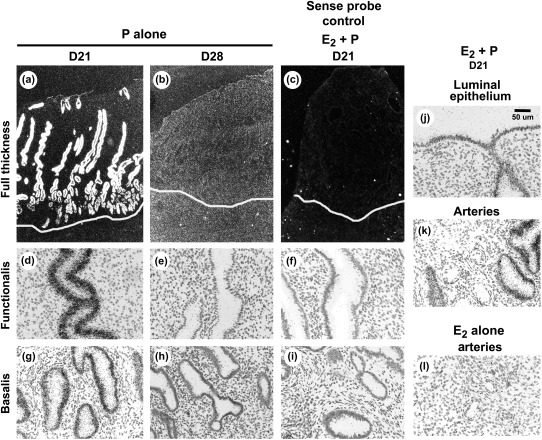
Figures 4 and 5 show images of ISH for endometrial sections hybridized with 35S-labeled riboprobes to MMP-26. Dark-field images (Figs 4a–d and 5a–c) show full-thickness endometrium. Strong hybridization for MMP-26 transcript was localized to the endometrial glands and weak hybridizations were seen over the luminal epithelia (Fig. 5) and no specific signal was detected in endometrial arteries (Fig. 5k and l), stroma or myometrium (Figs 4a–d and 5a–c). No specific hybridization for MMP-26 was detected during menses (not shown) or after E2 treatment alone in the proliferative phase (Fig. 4a). Epithelial expression of MMP-26, especially in the endometrial glands, was strikingly increased in the secretory phase and was maximal after 3 and 7 days of E2 plus progesterone treatment (D17 and D21; Fig 4b and c, respectively). Hybridization for the MMP-26 transcript declined in the functionalis zone during the late secretory phase (D28) but was still detected in the basalis zone (Fig. 4d).
Figure 4.
Micrographs showing MMP-26 ISH with 35S-labeled riboprobes during the artificial menstrual cycle in macaques. (a–d) Show low-power, full thickness darkfield images. (e–l) Show high-power, brightfield images highlighting the endometrial glands in the functionalis and basalis zones.
Figure 5.
MMP-26 ISH from macaques after treatment with progesterone alone following E2 priming. (a–c) Show low-power, full thickness darkfield images. (d–l) show high-power, brightfield images highlighting the endometrial glands in the functionalis and basalis zones. Sense controls are shown in c, f and i. Note long exposure for (b) in an attempt to identify the low-level signal.
MMP-26 transcript was also strongly expressed in the glands throughout the endometrium of animals treated with progesterone alone for 7 days following E2 priming (Fig. 5a). However, prolonged treatment with progesterone only resulted in minimal hybridization for MMP-26 transcript in both the functionalis and basalis zones by D28 of progesterone alone (Fig. 5b). No specific hybridizations were detected in sense-probe controls (Fig. 5c,f,i).
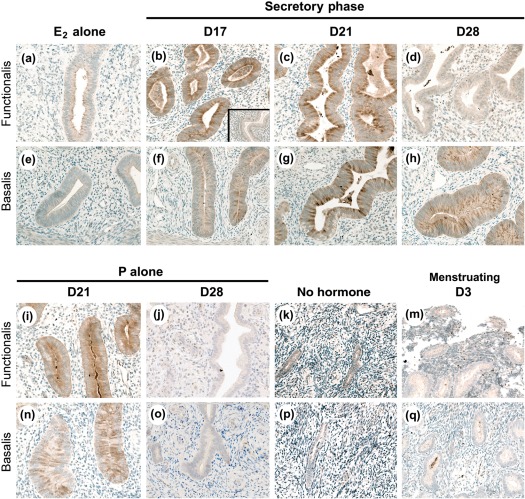
Immunohistochemistry
Figure 6 shows immunostaining for MMP-26 protein, which was localized exclusively to the endometrial glands, paralleling the localization of MMP-26 transcript. MMP-26 staining was not detected in the vascular or stromal cells, or the stromal ECM. MMP-26 was detected in scattered individual cells (likely the cells of the immune system) during menses (D3). MMP-26 was undetectable during the early and late proliferative phases (not shown), and the extended proliferative phase (E2 alone).
Figure 6.
Micrographs showing MMP-26 IHC conducted on paraformaldehyde-fixed paraffin-embedded macaque tissue sections. Staining was absent and/or minimal in animals with E2 alone or following progesterone withdrawal (menstruating D3). Sections from the typical artificial cycle (E2 plus progesterone) show strong staining after 3 (D17) and 7 days (D21) of treatment, with marked reductions following 14 days of treatment (D28). Treatment with progesterone alone following E2 priming for 7 (D21) and 14 (D28) days showed similar patterns. Inset shows negative control; images are ∼640× magnification.
MMP-26 staining was readily detectable in the glands of the functionalis and basalis zones after 3 days (D17) and 7 days (D21) of treatment with E2 plus progesterone. Strong staining was observed in the apical tips of the glandular epithelial cells and in cellular debris within the glandular lumen. MMP-26 staining was noticeably weaker in the late secretory phase (D28) in the upper functionalis zone, whereas moderate staining was retained in the basalis zone. Strong staining for MMP-26 was observed in the mid-secretory phase (e.g. D21) of animals treated with progesterone alone following E2 priming. Staining was then strikingly reduced in both the functionalis and basalis regions by D28 of progesterone alone following E2 priming. Staining for MMP-26 was not observed in the endometrium of animals treated with progesterone alone without E2 priming (P alone; data not shown).
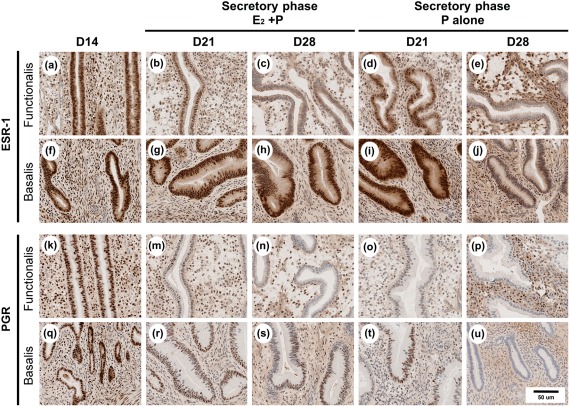
We compared the localization of MMP-26 with localization of ESR1 and PGR. Strong staining for ESR1 and PGR was observed in the stroma and epithelia in the proliferative phase of the cycle (Fig. 7). Strong-to-moderate staining for ESR1 and PGR was also observed throughout the endometrium on D21 of the artificial secretory phase. Staining intensity declined in the glandular epithelia in the upper functionalis zones by D28. As previously observed (Slayden and Brenner, 2004), ESR1 and PGR were retained in the basalis zone of animals treated with E2 plus progesterone. In the glands of the functionalis and basalis zones of animals treated with 14 days of progesterone alone following E2 priming (D28), staining for ESR1 was markedly reduced and staining for PGR was absent. In animals treated with progesterone alone without E2 priming (P alone), staining for ESR1 and PGR1 was markedly reduced or absent, further supporting the long-standing observation that estrogens are required to up-regulate PGR (data not shown).
Figure 7.
Micrographs showing IHC for estrogen receptor alpha (ESR1) and PGR on fresh frozen tissue sections of macaque endometrium. The localization patterns of ESR1 and PGR in animals treated with E2 alone (D14) and E2 plus progesterone (D21 and D28) were consistent with previous reports. Note the absence of PGR and the marked reduction in ESR1 in the basalis glands of animals treated with progesterone alone for 14 days following E2 priming (P alone, D28).
Discussion
The non-human primate and human endometrium are rich sources of MMPs capable of degrading ECM and remodeling the endometrium during the menstrual cycle. The expression of the majority of the endometrial MMPs (e.g. MMP-1, -2, -3, -7, -10, -11, -14 and -20) is increased during the menstrual phase of the cycle and these MMPs are often undetectable during the late follicular and secretory phase in macaques (Rudolph-Owen et al., 1998; Carroll et al., 2007) and women (Goffin et al., 2003). The majority of these MMPs are localized to the ECM and/or fibroblasts of the endometrial stroma (Salamonsen and Woolley, 1996; Chakraborti et al., 2003). Two notable exceptions to this pattern are MMP-7 and MMP-26, which are localized to the epithelial cells of the primate endometrium. MMP-26 has been reported to be up-regulated by estrogen and implicated in endometrial cancer and hyperplasia (Pilka et al., 2004a,b). Other reports suggest that MMP-26 may increase during the secretory phase and be important for the epithelial remodeling that occurs during early pregnancy in macaques (Li et al., 2002).
During the menstrual cycle, E2 and progesterone exert their effects through specific nuclear receptors (Clark and Peck, 1979). E2 induces the expression of ESR-1 as well as PGR, and progesterone acts to suppress both ESR-1 and PGR in glandular epithelial cells (Slayden and Brenner, 2004). ESR1 and PGR are retained in the basalis zone during the secretory phase (Slayden and Brenner, 2004). We hypothesized that steroid receptor expression was requisite for hormone stimulation of MMP-26, and our goal was to provide a detailed picture of MMP-26 co-localization and expression with ESR-1 and PGR. Our conclusions regarding MMP-26 in the macaque endometrium are as follows.
Progesterone action is required for expression of MMP-26 in the endometrium
MMP-26 was strikingly up-regulated during the early to mid-secretory phase of the artificial menstrual cycle in estrogen-primed macaque endometrial tissues. MMP-26 was co-localized only to the glandular epithelial cells that were ESR1 and PGR positive, and MMP-26 was up-regulated in the glands but not in the stroma of the endometrium during the first half of the secretory phase. These observations support the results of Pilka et al. (2003) who reported that the up-regulation of MMP-26 in the human endometrium is strongly correlated with the onset of progesterone action in the early secretory phase of the cycle. Maximal expression of MMP-26 in the macaque endometrium requires progesterone stimulation of estrogen-primed endometrial tissue, which is in accordance with studies conducted with glandular epithelial cells from human endometrial explants (Pilka et al., 2003). Further, MMP-26 is not detectable in macaque or human tissues when treated with estrogens alone, and MMP-26 was not detected in the endometrium of macaques treated only with progesterone (no estrogen priming).
Interestingly, MMP-26 expression declined in the late secretory phase to baseline levels, and this striking down-regulation of MMP-26 occurred in the presence or absence of E2. Kao et al. (2002) speculate that because ESRs are down-regulated in endometrial epithelial cells during the secretory phase, E2-regulated genes would decrease owing to the loss of ESR-1. In support of this hypothesis, animals treated with E2 plus progesterone for 14 days displayed no staining for MMP-26, ESR1 or PGR in the glandular epithelium of the functionalis zone, whereas MMP-26, ESR1 and PGR were retained in the endometrial glands of the deep basalis, where ESR-1 and PGR are retained. On the other hand, animals treated with progesterone alone for 14 days (following E2 priming) exhibited no immunoreactivity for PGR or MMP-26, and markedly reduced staining for ESR1, in the glandular epithelia of the basalis region. These results suggest that the expression and translation of MMP-26 in the basalis glands during the late secretory phase is dependent on the expression of PGR. However, using publicly available software (www.gene-regulation.com) we could not identify PGR response elements on the upstream promoter region of the MMP-26 gene, therefore progesterone likely acts via an indirect mechanism to regulate MMP-26 transcription. This is in agreement with Pilka et al. (2003), who hypothesize that the regulation of MMP-26 may involve enhancer elements further upstream or MMP-26 may be regulated indirectly via induction of unknown intermediary proteins or cytokines, such as transforming growth factor beta (TGFβ) or interleukin-1α. Because progesterone acted to both stimulate and suppress MMP-26, i.e. a short-term progesterone treatment for 3–7 days rapidly stimulated MMP-26, whereas an extended exposure (e.g. 14 days or longer) to progesterone reduced MMP-26, it is reasonable to presume that progesterone indirectly regulates MMP-26 expression.
We propose that progesterone is likely acting via progestomedins, such as the TGFβ superfamily, to regulate the expression of MMP-26 in the macaque endometrium. To support this idea, TGFβ1 and TGFβ2 appear to cause the progesterone-induced down-regulation of matrilysin-1 (MMP-7) in the human endometrium (Bruner et al., 1995), and TGFβ response elements [upstream stimulatory factor (USF)-binding motifs] are present in the promoter region of MMP-7 (Shibata et al., 2009). We identified more than 10 USF motifs on the 10-kB putative upstream promoter region of the macaque MMP-26 gene. More importantly, the expression pattern of TGFβ2 increases markedly during the early to mid-secretory phase and then plummets by the late secretory premenstrual period in women (Chegini et al., 1994; Gaide Chevronnay et al., 2008). This pattern of TGFβ2 expression directly parallels the expression of MMP-26.
During the late secretory phase of the natural menstrual cycle in macaques and women, levels of E2 rise and it is possible that the fall in MMP-26 may be estrogen related. Although plasma E2 was not detectable in the macaques treated with progesterone alone, it is possible (although unlikely) that progesterone could have been converted to E2 locally within the tissues of the reproductive tract, and that these low levels of E2 inhibited MMP-26 expression after treatment with progesterone alone for 14 days. Nevertheless, these results collectively suggest that progesterone, in the presence or absence of estrogens, stimulates MMP-26. Because levels of MMP-26 are very low or undetectable in the presence of estrogens during the artificial proliferative phases, these results suggest that E2 inhibits MMP-26 production. Estradiol may directly inhibit gene transcription through the estrogen response element by acting as a co-repressor but this inhibitory effect by E2 on MMP-26 may be an indirect action of an E2-dependent signaling paracrine pathway through ESR1.
MMP-26 is correlated with sacculation of the functionalis glands
As previously published, MMP-26 transcript is expressed exclusively in the epithelial cells, glandular as well as luminal, and is not detected in the stroma of the human endometrium (Pilka et al., 2003). In accordance with that study, we were also unable to detect MMP-26 transcript and protein in the ECM or fibroblasts of the macaque endometrial stroma. Altogether, it seems unlikely that MMP-26 facilitates remodeling of the stromal compartment during the secretory phase of the cycle. However, previous studies suggested MMP-26 may play an important role in the ECM remodeling associated with rapid epithelial growth (Uria and Lopez-Otin, 2000; Marchenko et al., 2001). Therefore, MMP-26 may be acting to degrade the basement membrane (i.e. likely collagen I–IV) of the epithelial cells to facilitate the progesterone-driven sacculation of the functionalis glands.
Specific enzymes and pathways involved in the sacculation of glands are unknown. Sacculation of the glands is a complex process that requires complete but localized cellular remodeling, whereby the outlying stromal cells and ECM must yield to the migrating and expanding glandular epithelium. Several lines of evidence exist to support a role for MMP-26 in facilitating epithelial remodeling. First, MMP-26 is over-expressed in aggressive cancers that exhibit hyperplasia of the epithelium (Marchenko et al., 2001; Marchenko et al., 2002), most notably epithelial cells lining endometrial cancers (Pilka et al., 2004a,b), intestinal and colon cancers (Bister et al., 2004) and benign skin disorders, such as dermatofibromas (Skoog et al., 2006). Second, MMP-26 promotes invasion of human glioma U251 cells in matrigel substrates in vitro, suggesting MMP-26 stimulates proteolysis and cell invasion (Deng et al., 2010). Third, MMP-26 can directly cleave the inactive pro-MMP-9 protein into the mature active-MMP-9 gelatinase isoform (Uria and Lopez-Otin, 2000; Zhang et al., 2002; Galewska et al., 2010). MMP-9 is localized to the stroma and abundant in the secretory endometrium, where it has been proposed to play a role during trophoblast invasion during early pregnancy. Thus, MMP-26 could be acting directly, in a proteolytic manner, to degrade the basement membrane permitting invasion/migration of the epithelial cells, and by activating MMP-9 in the adjacent stromal cells. Collectively, MMP-26 could directly facilitate the aggressive and rapid remodeling of the functionalis glands during the early secretory phase. Further studies are warranted to investigate this proposed role for MMP-26 in the primate secretory endometrium.
MMP-26 may facilitate remodeling associated with trophoblast invasion
The significance of MMP-26 in the glandular epithelium is not presently clear; therefore, it is reasonable to propose a role for MMP-26 during implantation. The up-regulation and apical cellular localization of MMP-26 in the glandular epithelium imply that it may be secreted into the endometrial lumen during the early to mid-secretory phase, analogous with the window of implantation, and suggests a possible role during early pregnancy. Substances secreted by the endometrial glands are essential for uterine receptivity and embryo implantation (Gray et al., 2001). Mammalian implantation is a complex process involving invasion of the maternal endometrium by the trophoblast in a spatio-temporally limited manner (Paria et al., 2000). Three families of proteases, the cysteine-, serine- and metallo-proteinases (Grevin et al., 1993; Alexander et al., 1996; Afonso et al., 1999; Salamonsen, 1999; Fata et al., 2000; Zhao et al., 2002), have all been demonstrated to participate in the matrix degradation that is required for implantation. Although the exact roles of all these proteases are not known, several MMPs (e.g. MMP-9) have been shown to play direct roles in embryonic implantation (Alexander et al., 1996; Menino et al., 1997; Hurst and Palmay, 1999; Bischof and Campana, 2000; Paria et al., 2000).
MMP-26 is likely secreted into the uterine lumen, and may be up-regulated during the early secretory phase to inactivate α1-antitrypsin (AAT), which is a serine proteinase inhibitor that is also secreted into the endometrial lumen (Parmar et al., 2009). The role of AAT is currently not known but the AAT protein has been shown to increase during the mid-secretory phase of the cycle and then fall during the late secretory phase (Parmar et al., 2009), a pattern similar to that of MMP-26. Thus, a delicate balance of active and inactive proteolytic enzymes in the uterine lumen is likely essential for proper blastocyst invasion, even the partial suppression of AAT by MMP-26 would help maintain consistent levels of proteolytic enzymes required during early pregnancy.
MMP-26 may also be up-regulated and secreted in order to cleave pro-MMP-9 into the active gelatinase. MMP-9 is a key enzyme that is activated during implantation to cleave fibronectin, degrade collagen components within the basement membranes of the stromal cells and possibly facilitate invasion of the placental villi into the endometrial stroma [reviewed in (Cohen and Bischof, 2007) and (Singh and Aplin, 2009)]. MMP-9 has recently been localized to the endometrial vessels in rhesus macaques during the window of implantation (Ghosh et al., 2011), and MMP-26 was localized to the spiral arterioles of the basalis region of the macaque endometrium at Days 18 and 26 of gestation (Li et al., 2002). Collectively, MMP-26 may be necessary to activate MMP-9 to facilitate stromal remodeling and, more importantly, vascular remodeling of the endometrial blood vessels during early pregnancy. In vivo studies using specific MMP-26 antagonists and/or inhibitors will be required to further define these putative roles.
Conclusions
Progesterone action is required for MMP-26 expression, which is maximal during the early to mid-secretory phase. MMP-26 levels plummeted to baseline levels during the late secretory phase of the artificial menstrual cycle. We propose that the primary regulator of MMP-26 is the level of glandular PGR, which likely regulates the transcription of TGFβ2 which in turn results in the striking changes in the MMP-26 transcript and protein observed during the secretory phase in the macaque endometrium. Functionally, the rapid decline in MMP-26 levels between the mid- and late secretory phases may be responsible for closing the window of embryo implantation. MMP-26 is a unique protease, co-regulated by progesterone and PGR, that is secreted into the uterine lumen during the window of embryo implantation which, together with MMP-9, likely co-ordinates blastocyst invasion and the stromal and vascular remodeling necessary for successful pregnancy.
Authors' roles
O.D.S. and C.A.F. contributed equally to the study design. K.M, L.H., C.H. and C.S.K performed laboratory and statistical analyses. All authors critically revised the article and approved the final version.
Funding
Funds were provided by NIH grants HD18185 and RR000163 awarded to the ONPRC, and the NIH Fogarty International Center grant TW/HD-00668 (P. Michael Conn P.I.).
Conflict of interest
None declared.
References
- Afonso S, Romagnano L, Babiarz B. Expression of cathepsin proteinases by mouse trophoblast in vivo and in vitro. Dev Dyn. 1999;216:374–384. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<374::AID-DVDY6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- Bischof P, Campana A. Molecular mediators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:801–814. doi: 10.1053/beog.2000.0120. [DOI] [PubMed] [Google Scholar]
- Bister VO, Salmela MT, Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P, Lopez-Otin C, Saarialho-Kere U. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci. 2004;49:653–661. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. Cyclic changes in the primate oviduct and endometrium. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 541–569. [Google Scholar]
- Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, Osteen KG. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci USA. 1995;92:7362–7366. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RL, Mah K, Fanton JW, Maginnis GN, Brenner RM, Slayden OD. Assessment of menstruation in the Vervet (Cercopithecus aethiops) Am J Primatol. 2007;69:901–916. doi: 10.1002/ajp.20396. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135:439–449. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- Clark JH, Peck EJ., Jr . Female sex steroids: receptors and function. In: Gross F, Grumbach MM, Labhart A, Lipsett MB, Mann T, Samuels LT, Zander J, editors. ‘Monographs on Endocrinology’. NY: Springer-Verlag; 1979. [PubMed] [Google Scholar]
- Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64:126–130. doi: 10.1159/000101734. [DOI] [PubMed] [Google Scholar]
- Deng Y, Li W, Li Y, Yang H, Xu H, Liang S, Zhang L, Li Y. Expression of matrix metalloproteinase-26 promotes human glioma U251 cell invasion in vitro and in vivo. Oncol Rep. 2010;23:69–78. [PubMed] [Google Scholar]
- Fadare O, Zheng W. Histologic dating of the endometrium: accuracy, reproducibility, and practical value. Adv Anat Pathol. 2005;12:39–46. doi: 10.1097/01.pap.0000155051.91366.bf. [DOI] [PubMed] [Google Scholar]
- Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci. 2000;57:77–95. doi: 10.1007/s000180050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide Chevronnay HP, Cornet PB, Delvaux D, Lemoine P, Courtoy PJ, Henriet P, Marbaix E. Opposite regulation of transforming growth factors-beta2 and -beta3 expression in the human endometrium. Endocrinology. 2008;149:1015–1025. doi: 10.1210/en.2007-0849. [DOI] [PubMed] [Google Scholar]
- Galewska Z, Romanowicz L, Jaworski S, Bankowski E. Matrix metalloproteinases, MMP-7 and MMP-26, in plasma and serum of control and preeclamptic umbilical cord blood. Eur J Obstet Gynecol Reprod Biol. 2010;150:152–156. doi: 10.1016/j.ejogrb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Najwa AR, Khan MA, Sengupta J. IGF2, IGF binding protein 1, and matrix metalloproteinases 2 and 9 in implantation-stage endometrium following immunoneutralization of vascular endothelial growth factor in the rhesus monkey. Reproduction. 2011;141:501–509. doi: 10.1530/REP-10-0475. [DOI] [PubMed] [Google Scholar]
- Goffin F, Munaut C, Frankenne F, Perrier DS, Beliard A, Fridman V, Nervo P, Colige A, Foidart JM. Expression pattern of metalloproteinases and tissue inhibitors of matrix-metalloproteinases in cycling human endometrium. Biol Reprod. 2003;69:976–984. doi: 10.1095/biolreprod.103.015933. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001;65:1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- Grevin D, Chen JH, Raes MB, Stehelin D, Vandenbunder B, Desbiens X. Involvement of the proto-oncogene c-ets 1 and the urokinase plasminogen activator during mouse implantation and placentation. Int J Dev Biol. 1993;37:519–529. [PubMed] [Google Scholar]
- Hurst PR, Palmay RD. Matrix metalloproteinases and their endogenous inhibitors during the implantation period in the rat uterus. Reprod Fertil Dev. 1999;11:395–402. doi: 10.1071/rd99021. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Keator CS, Mah K, Ohm L, Slayden OD. Estrogen and progesterone regulate expression of the endothelins in the rhesus macaque endometrium. Hum Reprod. 2011;26:1715–1728. doi: 10.1093/humrep/der115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang H, Zhao Y, Lin H, Sang QA, Zhu C. Identification and specific expression of matrix metalloproteinase-26 in rhesus monkey endometrium during early pregnancy. Mol Hum Reprod. 2002;8:934–940. doi: 10.1093/molehr/8.10.934. [DOI] [PubMed] [Google Scholar]
- Marchenko GN, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY. Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J. 2001;356:705–718. doi: 10.1042/0264-6021:3560705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko GN, Marchenko ND, Leng J, Strongin AY. Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J. 2002;363:253–262. doi: 10.1042/0264-6021:3630253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menino AR, Jr, Hogan A, Schultz GA, Novak S, Dixon W, Foxcroft GH. Expression of proteinases and proteinase inhibitors during embryo-uterine contact in the pig. Dev Genet. 1997;21:68–74. doi: 10.1002/(SICI)1520-6408(1997)21:1<68::AID-DVG8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–1343. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Paria BC, Lim H, Das SK, Reese J, Dey SK. Molecular signaling in uterine receptivity for implantation. Semin Cell Dev Biol. 2000;11:67–76. doi: 10.1006/scdb.2000.0153. [DOI] [PubMed] [Google Scholar]
- Parmar T, Gadkar-Sable S, Savardekar L, Katkam R, Dharma S, Meherji P, Puri CP, Sachdeva G. Protein profiling of human endometrial tissues in the midsecretory and proliferative phases of the menstrual cycle. Fertil Steril. 2009;92:1091–1103. doi: 10.1016/j.fertnstert.2008.07.1734. [DOI] [PubMed] [Google Scholar]
- Petersen RG. Design and Analysis of Experiments. 66th edn. New York: Marcel Dekker, Inc.; 1985. [Google Scholar]
- Pilka R, Whatling C, Domanski H, Hansson S, Eriksson P, Casslen B. Epithelial expression of matrix metalloproteinase-26 is elevated at mid-cycle in the human endometrium. Mol Hum Reprod. 2003;9:271–277. doi: 10.1093/molehr/gag039. [DOI] [PubMed] [Google Scholar]
- Pilka R, Domanski H, Hansson S, Eriksson P, Casslen B. Endometrial TIMP-4 mRNA is high at midcycle and in hyperplasia, but down-regulated in malignant tumours. Coordinated expression with MMP-26. Mol Hum Reprod. 2004a;10:641–650. doi: 10.1093/molehr/gah092. [DOI] [PubMed] [Google Scholar]
- Pilka R, Norata GD, Domanski H, Andersson C, Hansson S, Eriksson P, Casslen B. Matrix metalloproteinase-26 (matrilysin-2) expression is high in endometrial hyperplasia and decreases with loss of histological differentiation in endometrial cancer. Gynecol Oncol. 2004b;94:661–670. doi: 10.1016/j.ygyno.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Pilka R, Oborna I, Lichnovsky V, Havelka P, Fingerova H, Eriksson P, Hansson S, Casslen B. Endometrial expression of the estrogen-sensitive genes MMP-26 and TIMP-4 is altered by a substitution protocol without down-regulation in IVF patients. Hum Reprod. 2006;21:3146–3156. doi: 10.1093/humrep/del180. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen LA, Slayden OD, Matrisian LM, Brenner RM. Matrix metalloproteinase expression in Macaca mulatta endometrium: evidence for zone-specific regulatory tissue gradients. Biol Reprod. 1998;59:1349–1359. doi: 10.1095/biolreprod59.6.1349. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA. Role of proteases in implantation. Rev Reprod. 1999;4:11–22. doi: 10.1530/ror.0.0040011. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Woolley DE. Matrix metalloproteinases and their tissue inhibitors in endometrial remodelling and menstruation. Reprod Med Rev. 1996;5:185–203. [Google Scholar]
- SAS Institute, Inc. SAS/INSIGHT 9.2 User's Guide. 2nd edn. Vol. 1. Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- doi: 10.1186/1471-2199-7-3. Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 2006;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Marushima H, Asakura T, Matsuura T, Eda H, Aoki K, Matsudaira H, Ueda K, Ohkawa K. Three-dimensional culture using a radial flow bioreactor induces matrix metalloprotease 7-mediated EMT-like process in tumor cells via TGFbeta1/Smad pathway. Int J Oncol. 2009;34:1433–1448. [PubMed] [Google Scholar]
- Singh H, Aplin JD. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215:3–13. doi: 10.1111/j.1469-7580.2008.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog T, Ahokas K, Orsmark C, Jeskanen L, Isaka K, Saarialho-Kere U. MMP-21 is expressed by macrophages and fibroblasts in vivo and in culture. Exp Dermatol. 2006;15:775–783. doi: 10.1111/j.1600-0625.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67:393–409. doi: 10.1679/aohc.67.393. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Keator CS. Role of progesterone in nonhuman primate implantation. Semin Reprod Med. 2007;25:418–430. doi: 10.1055/s-2007-991039. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Koji T, Brenner RM. Microwave stabilization enhances immunocytochemical detection of estrogen receptor in frozen sections of macaque oviduct. Endocrinology. 1995;136:4012–4021. doi: 10.1210/endo.136.9.7649110. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Chwalisz K, Brenner RM. Reversible suppression of menstruation with progesterone antagonists in rhesus macaques. Hum Reprod. 2001;16:1562–1574. doi: 10.1093/humrep/16.8.1562. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Introduction to Biostatistics. 2nd edn. New York: W.H. Freeman and Company; 1987. [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745–4751. [PubMed] [Google Scholar]
- Zhang J, Li S, Tian Y, Zhao Y, Qing-Xiang AS, Duan E. Effect of matrix metalloproteinase-26 (MMP-26) during embryo implantation in the mouse. Chin Sci Bull. 2002;47:1884–1888. [Google Scholar]
- Zhao YG, Xiao AZ, Cao XM, Zhu C. Expression of matrix metalloproteinase -2, -9 and tissue inhibitors of metalloproteinase -1, -2, -3 mRNAs in rat uterus during early pregnancy. Mol Reprod Dev. 2002;62:149–158. doi: 10.1002/mrd.10123. [DOI] [PubMed] [Google Scholar]