Abstract
Grafts can be rejected even when matched for MHC because of differences in the minor histocompatibility Ags (mH-Ags). H4- and H60-derived epitopes are known as immunodominant mH-Ags in H2b-compatible BALB.B to C57BL/6 transplantation settings. Although multiple explanations have been provided to explain immunodominance of Ags, the role of vascularization of the graft is yet to be determined. In this study, we used heart (vascularized) and skin (nonvascularized) transplantations to determine the role of primary vascularization of the graft. A higher IFN-γ response toward H60 peptide occurs in heart recipients. In contrast, a higher IFN-γ response was generated against H4 peptide in skin transplant recipients. Peptide-loaded tetramer staining revealed a distinct antigenic hierarchy between heart and skin transplantation: H60-specific CD8+ T cells were the most abundant after heart transplantation, whereas H4-specific CD8+ T cells were more abundant after skin graft. Neither the tissue-specific distribution of mH-Ags nor the draining lymph node-derived dendritic cells correlated with the observed immunodominance. Interestingly, non-primarily vascularized cardiac allografts mimicked skin grafts in the observed immunodominance, and H60 immunodominance was observed in primarily vascularized skin grafts. However, T cell depletion from the BALB.B donor prior to cardiac allograft induces H4 immunodominance in vascularized cardiac allograft. Collectively, our data suggest that immediate transmigration of donor T cells via primary vascularization is responsible for the immunodominance of H60 mH-Ag in organ and tissue transplantation.
Minor histocompatibility Ags (mH-Ags) are naturally processed polymorphic peptides presented by MHC molecules (1, 2). T cell reactivity to mH-Ags can induce expansion of CTLs and rejection of MHC-matched allografts. In MHC-matched, multiple mH-Ag–mismatched transplants, only a limited number of antigenic epitopes are exposed, resulting in oligoclonal expansion of CTLs (3-5). This is in contrast to MHC-mismatched transplants, which induce polyclonal expansion of CTLs (6, 7). By studying skin transplants and CTL responses in mice, more than 61 mH-Ag loci were identified, and a similar number is highly plausible in humans. Although more than 26 mismatched mH-Ags have been defined in the MHC-matched BALB.B to C57BL/6 strain combination, CD8+ T cell responses are predominantly directed to a few of these (8). Immune response-inducing mH-Ags (dominant mH-Ags) are limited because of the phenomenon of immunodominance (5, 9-14). Similar to the hierarchy among the epitope specificity of MHC class I-restricted responses to microbial pathogens in different experimental systems, there is a hierarchy of immunodominance among these mH-Ags in the transplant setting (15-19). Immunodominant epitopes generate vigorous responses, whereas those Ags eliciting lesser responses are considered subdominant (20). Likewise, when multiple mH-Ags coexist in an allograft, some mH-Ags dominate over others in the host immune response. The hierarchy of mH-Ag immunodominance in C57BL/6 mice was evaluated by tracking mH-Ag–specific CD8+ T cells after immunization with BALB.B spleen cells.
Among the mH-Ags, H60 is of hematopoietic origin and was found to dominate the B6 anti-BALB.B immune response during both primary and secondary challenges (21, 22). H60 immunodominance in the C57BL/6 mice after BALB.B splenocytes transfusion was explained by the unusually high precursor frequency of H60-specific CD8+ T cells (22). C57BL/6 mice do not transcribe the H60 RNA, so that T cell clones recognizing H60 mH-Ag peptide were not negatively selected in C57BL/6 mice (21). H4 mH-Ag is also of hematopoietic origin, yet is widely expressed in epithelial cells and other cell types (23) and was found to be a dominant mH-Ag in skin transplantation (14). Differential T cell expansion in these two immunizations could also represent the differential allospecific T cell expansion in solid organ transplantation achieved by primary vascularization and skin transplantation without primary vascularization but with revascularization process. Unlike cell immunization, vascularization includes many aspects that increase the complexity of the immune response because it provides the conduit structure for passenger leukocytes, the Ag presentation via endothelium, and alteration of graft injury. Although multiple explanations have been provided to explain immunodominance of these mH-Ags, the role of vascularization of the graft has not been evaluated.
In this article, we demonstrate differential expansion hierarchy of mH-Ag–specific CD8+ T cells in response to different types of transplants, and we have investigated the possible confounding factors that could affect mH-Ag immunodominance and its corresponding mH-Ag–specific T cell expansion in organ transplantation. Unexpectedly, neither the tissue distribution nor the conventional APC (CD11c+ dendritic cells) from draining lymph nodes of the recipients showed direct correlation to the host T cell response. Instead, we found that vascularization of the transplant is the deciding factor determining clonal T cell expansion after transplantation. These data emphasize the critical role of primary vascularization in mH-Ag immunodominance after organ transplantation.
Materials and Methods
Animals
Male BALB/c (H-2d), BALB.B (H-2b), C57BL/6 (H-2b), and B6.CB17-Prkdcscid/SzJ (H-2b), 6–8 wk of age, were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in plastic cages with controlled light–dark cycles and provided with food and water ad libitum at the University of Wisconsin-Madison animal resources facility (Madison, WI). All mouse experiments were performed in accordance with the guidelines and in compliance with the University of Wisconsin-Madison Animal Research Ethics Committee.
Skin transplantation
Skin was recovered and placed in Eurocollins solution for a maximum of 30 min until used for transplantation. Full-thickness abdominal skin (~1 cm diameter) derived from BALB/c (H-2d) or BALB.B (H-2b) donor mice was transplanted on the right/left dorsal area of C57BL/6J (H-2b) or B6.CB17-Prkdcscid/SzJ (H-2b) recipients. Recipient mice were anesthetized with isoflurane for the entire procedure. The skin graft was secured with a plastic adhesive bandage for 7 d. Graft survival was evaluated by daily visual inspection. Necrosis of ≥50% of the transplanted skin surface was defined as rejection.
Heart transplantation
Primarily vascularized heart transplantation was performed using a modification of the methods described by Corry et al. (24). Briefly, the C57BL/6 recipient mouse was anesthetized with isoflurane. A segment of descending aorta and vena cava below the renal vessels was dissected. The heart was immediately removed from the donor and placed in chilled Eurocollins solution on ice. The BALB.B donor heart was then placed in the abdominal cavity of the recipient, and the donor aorta and pulmonary artery were anastomosed in an end-to-side manner to the recipient abdominal aorta and vena cava using 10-0 nylon suture. For donor T cell-depleted donor heart transplantation, BALB.B donor mice were given i.p. administrations of 100 mg anti-CD8 mAb (clone 53.6.72; Bio X cell, West Lebanon, NH) and 200 mg anti-CD4 mAb (GK1.5; Bio X cell) at 24 h prior to the primarily vascularized heart transplantation. The grafts were monitored by daily palpation and graded from 4+ (strongest beat) to 0 (no beat).
Ear-pinna cardiac allograft
Nonvascularized ear-pinna cardiac allografts were performed as previously described (25). BALB.B newborn mice (<24 h old) were sacrificed, and the hearts were excised and bisected longitudinally. Half of the heart was placed in ice-cold Eurocollins solution and immediately implanted in the C57BL/6 mouse ear pinna. Grafts were inspected visually under the microscope for cardiac contractions. Cessation of cardiac contractions was defined as rejection.
Histology
Histopathologic analysis was performed on paraffin-embedded sections of heart allografts removed at necropsy. Sections were stained with either H&E or trichrome and were evaluated blindly according to the established clinical criteria for diagnosing heart transplant rejection (26). Grafts were also prepared and snap-frozen to −180°C in embedding medium (Tissue-Tek OCT compound; Miles, Elkhart, IN) in a bath of 2-methylbutane (Sigma-Aldrich) and liquid nitrogen. Frozen sections (5 μm) were cut from tissue blocks onto coated 3-in. glass slides (Fisher Scientific, Pittsburgh, PA) at −19°C on a Leica CM1800 cryostat, fixed in acetone (Fisher Scientific), and immediately stained with H&E (Fisher Scientific) or stored at −80°C until used for immunohistochemical staining.
Peptides
H4b (SGIVYIHL), H28 (FILENFPRL), H60 (LTFNYRNL), H7b, and H13b were synthesized and purified (>90%) by Proimmune. As a source of donor Ag for studies of indirect allorecognition, the BALB.B splenocytes were suspended at concentrations of 40 × 106/ml in HBSS, sonicated with ten 1-s pulses on ice, frozen in a dry ice/ethanol bath, and then thawed at room temperature. Any residual intact cells or cell membranes were removed by centrifugation at 1400 rpm for 20 min at room temperature. The concentration of allogenic peptide was estimated using a microbicinchoninic acid assay. 100 μg/ml of the resultant supernatant was added to pulse the dendritic cells (DCs).
Flow cytometry and cell sorting
Cells from spleen, lymph node, blood, and graft were stained with biotin-, PE-, FITC-, PerCP-, or allophycocyanin-conjugated Abs directed at mouse CD4 (H129.19), CD8 (53-6.7), CD44 (IM7), CD11a (2D7), CD62L (MEL-14), and isotype controls (BD Pharmingen, San Diego, CA). PE-conjugated MHC class I tetramer (Beckman Coulter) or pentamer (Proimmune) was costained. Cytometric analysis was performed using a FACSCaliber cytometer (BD Biosciences, San Jose, CA) and analyzed using Cell Quest (BD Biosciences) and FlowJo (Tree Star, San Carlos, CA) software. CD11c+ DCs were enriched from draining lymph nodes (dLNs) of skin or heart-transplanted recipients [postoperation day (POD) 10]. Briefly, dLNs were mechanically dissociated, and cell suspension was subjected to hypotonic lysis of RBCs. CD11c+ cells were sorted by FACSVantage (BD Biosciences).
Measuring antidonor response using MLR
The IFN-γ expression kinetics assay was performed using a modification of the methods described (27). For the direct MLR, recipient (C57BL/6) splenocytes (5 × 105) were cocultured in culture medium with equal numbers of irradiated (2000 rad) donor cells (BALB.B). For the indirect MLR, artificial H60 mH-Ag peptide or donor cell sonicates were applied to recipient splenocytes (5 × 105/200 μl) in a 96-well plate (15 wells per recipient) for 5 d at 37°C in a 5% CO2 incubator. Culture supernatant was collected daily for 5 d. The concentration of IFN-γ in the culture supernatant was measured with a mouse IFN-γ ELISA kit (R&D Systems, Minneapolis, MN). IFN-γ–producing cell numbers were quantified with ELISPOT assay using splenocytes from C57BL/6 recipient mice. C57BL/6 mouse spleen cells (4 × 105) were added to each well of the plate in triplicate. K89 cells (5 × 103) pulsed with either H60 or H4 mH-Ag peptide were added. Cells were incubated for 48 h at 37°C. Spots were visualized with the BCIP/NBT chromogen (R&D Systems). Each spot represented an IFN-γ–secreting cell, and the spots were enumerated using an ImmunoSpot analyzer (AID, Strassberg, Germany).
Peptide extractions and HPLC analysis
Total acid-soluble peptide pool from three to five mice for each organ was extracted as previously described (28-30). Briefly, homogenized tissue was lysed in 2 ml 10% formic acid in water and homogenized by ultrasonication. The homogenate was spun at 12,000 × g for 30 min. The supernatant was passed through a 10-kDa Ultra Free-MC filter (Millipore). The filtrate was dried in a vacuum centrifuge, resuspended in up to 500 μl of 10% formic acid, and fractionated by HPLC. For naturally occurring peptides, the filtered sample was separated by reverse-phase HPLC using the elution gradient indicated in the figures accompanying this article: buffer A, 0.1% trifluoroacetic acid in H2O; buffer B, acetonitrile with 0.1% TFA. Flow rate 0.35 ml/min, fraction size 0.5 ml. Individual fractions of all HPLC separations were dried in a Speed-Vac concentrator (Savant).
T cell activation assays
EL4-B7 (H-2Db) and K89B7 (H-2Kb) were used as APCs. The lacZ-inducible T cell hybridomas BCZ103 (anti-H60; LYL8), BCZ1755 (anti-H7; KDL9), and BCZ1644 (anti-H4; SEL8) were used for T cell activation assays as described (31, 32). T cell hybrids (3 × 104 to 10 × 104) were cocultured overnight (18 h) with APCs (2 × 104 to 5 × 104) either expressing the Ag endogenously or with exogenous peptides in 96-well plates. The peptide–MHC–induced T cell response was assayed as lacZ activity using the substrate chlorophenol red β-galactoside (CPRG). The conversion of CPRG to chlorophenol red was measured at 595 nm and 655 nm as a reference wavelength with a 96-well microplate reader (Bio-Rad, Richmond, CA). Data show the mean absorbance of replicate cultures and are representative of at least three independent experiments.
Statistical analysis
Standard statistical methods were used to calculate mean and SD. Log-rank test was used for graft survival. Otherwise, a Student t test was used. A p value <0.05 was considered to be statistically significant.
Results
Differential graft survival in multiple mH-Ag–mismatched heart and skin transplantation
To establish the role of mH-Ag in rejecting vascularized or non-vascularized grafts, we performed heterotopic heart transplants and skin transplants in C57BL/6 (H-2b) mice using either fully allogeneic (major histocompatibility Ag mismatched/minor histocompatibility Ag mismatched) BALB/c (H-2d) or less allogeneic (only mH-Ag mismatched) BALB.B (H-2b) mice as a donor. In the fully MHC-mismatched combination (BALB/c into C57BL/6), heart grafts were rejected promptly within 10 d [mean survival time (MST) = 9 d], whereas the multiple mH-Ag–mismatched combination (BALB.B into C57BL/6) showed either delayed acute rejection (MST = 14 d) or spontaneous long-term allograft survival with chronic allograft vasculopathy in ~50% of animals. However, spontaneous graft prolongation was not identified in multiple mH-Ag–mismatched skin transplantation (Fig. 1A). Histological examination revealed comparable lymphocytic infiltration in the mH-Ag–only and the fully mismatched skin grafts. In contrast, mH-Ag–mismatched heart grafts showed lesser degrees of infiltration than fully mismatched grafts at the same time points (Fig. 1B). Based on these data, we conclude different graft survival trends are induced in skin and heart transplantation even with same strain combination. mH-Ag–mediated rejection occurs at a slower tempo than rejection mediated across a complete MHC mismatch in heart transplantation, whereas mH-Ag skin grafts reject as fast as complete MHC mismatch.
FIGURE 1.
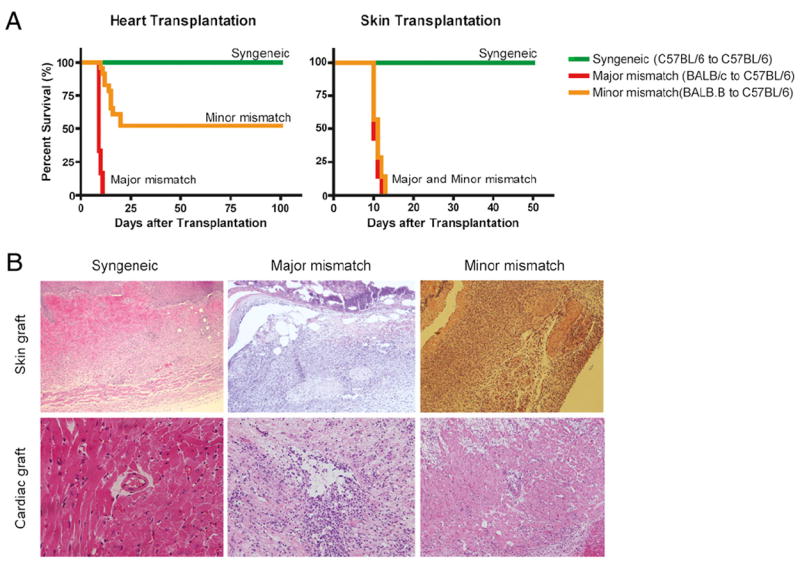
Differential graft survival of cardiac versus skin allograft in BALB.B (H-2Kb) donor to C57BL/6 (H-2Kb) recipient with MHC-matched/multiple minor Ag-mismatched strain combination. A, In BALB/c (H-2d) to C57BL/6 (H-2b), fully MHC-mismatched combination, both cardiac and skin grafts were rejected acutely (red line; MST = 10 versus 12 d, respectively). The MHC-matched, BALB.B (H-2b) to C57BL/6 (H-2b), minor histocompatibility-mismatched skin grafts were also rejected around 10 d (yellow line; n = 7, MST = 11 d), whereas 50% of cardiac allograft was spontaneously accepted (yellow line; n = 24; MST = 54.6 d). Syngeneic (C57BL/6 to C57BL/6) combination showed no sign of rejection for both types of transplantation (green line; n = 7, MST > 100 d). B, Representative histology of mouse cardiac and skin grafts at POD 10. A syngeneic graft in a mouse was accepted without lymphocytic infiltration. Allogeneic graft (both major and minor mismatch) without any treatment showed numerous infiltrating cells in the graft regardless of tissue type. Formalin-fixed, paraffin-embedded sections were prepared and stained with H&E. All mice were sacrificed at POD 10. Original magnification ×100.
Differential expansion hierarchy of mH-Ag–specific CD8+ T cells after multiple mH-Ag–mismatched heart and skin transplantation
To evaluate the potency of alloimmune responses from both tissue transplantation model systems, we performed MLRs and measured IFN-γ daily for 5 d. Naive splenocytes showed typical IFN-γ expression patterns (27) and kinetics (primary response) against MHC-mismatching stimulators in MLR. However, there was no detectable IFN-γ against either BALB.B donor splenocytes or H60 mH-Ag peptide in MHC-matched, multiple mH-Ag–mismatched MLR (Fig. 2A). These data suggested that the multiple mH-Ag–mismatch combination has limited allogeneic T cell numbers compared with the MHC-mismatched combination. Splenocytes from both skin and heart transplantation recipients showed rapid increases of IFN-γ production against donor splenocytes (Fig. 2B). IFN-γ expression kinetics induced by H60 mH-Ag (LTFNYRNL; LYL8) peptide-pulsed autologous DC shows a higher contribution of H60-specific CD8+ T cells in heart transplantation but not in skin transplantation (Fig. 2A). IFN-γ expression was not detected with nonpulsed autologous DC (data not shown). Notably, IFN-γ production was higher against H4 mH-Ag (SGIVYIHL; SIL8) peptide-pulsed DC in skin graft recipients (Fig. 2B). These data suggest that different mH-Ag–specific T cells tracked according to graft types.
FIGURE 2.
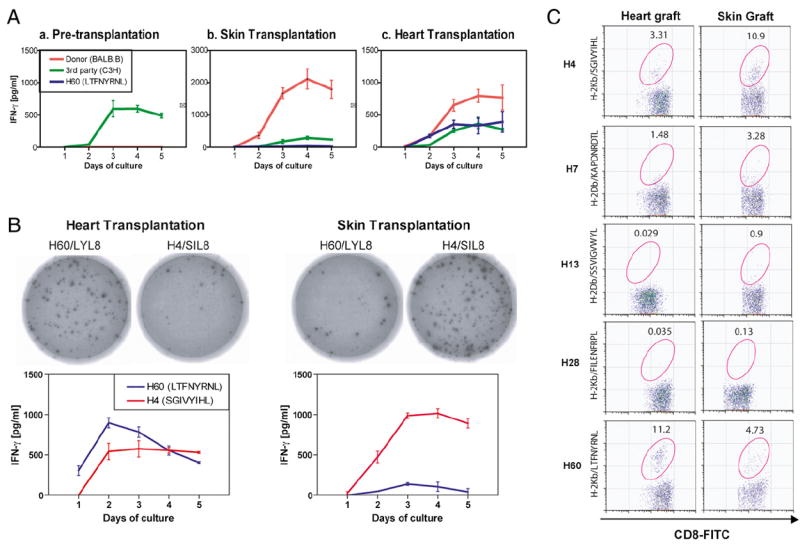
Effect of transplanted tissues on alloimmune response with mH-Ag mismatch. A, Spleen cells derived from naive, skin transplantation, and cardiac allograft recipients were challenged by irradiated donor (BALB.B; H-2b; red) cell, third-party (C3H; H-2k; green) cell, or H60 molecule-derived peptide (LTFNYRNL; blue). Naive C57BL/6 splenocytes showed primary IFN-γ production kinetics but cannot induce IFN-γ production against irradiated BALB.B splenocytes or H60 peptide (a). Production of IFN-γ is dramatically increased from skin graft C57BL/6 splenocytes against BALB.B splenocytes compared with pretransplant naive mice, whereas in skin-grafted mice, IFN-γ production against immunodominant H60 mH-Ag was detected at a low level (b). Anti-H60 mH-Ag response was relatively higher in heart transplantation compared with skin graft recipients (c). The experiment was repeated three times with the same results. B, ELISPOT detection of cardiac and skin transplanted C57BL/6 recipient reactivity to allogeneic mH-Ag peptides. Representative IFN-γ ELISPOT wells using 4 × 106 responder splenocytes per well plus H4 or H60 mH-Ag peptide (100 nM as final concentration). IFN-γ kinetic assay showed differential reactivity against H4 and H60 mH-Ag peptides from skin and heart graft recipients. The experiment was repeated three times with the same results. C, Differential hierarchy of mH-Ag–specific CD8 T cell immunodominance in heart and skin transplantation. Splenocytes were recovered on posttransplant day 10 from heart or skin graft recipients and stained with CD3 mAb, CD8 mAb and PE-conjugated H4/H-2Kb, H7/H-2Db, H13/H-2Db, H28/H-2Kb, or H60/H-2Kb multimer. Gates in the figures represent the percentages of multimer-positive cells within CD3+CD8+ T cells. In repeat experiments, similar data were obtained.
Next, we assessed the number of Ag-specific T cells directed against a particular mH-Ag epitope using multimers that bind to the Ag-specific TCR on effectors and memory T cells. To measure H4 and H60 mH-Ag–specific CD8+ T cells at the time of maximal allogeneic response, splenocytes from heart and skin transplant recipients at POD 10 were stained with anti-CD8, anti-CD3, and mH-Ag–specific multimers. Naive, pre-transplanted C57BL/6 mice were used as negative controls for mH-Ag–specific CD8+ T cells. Notably, splenocytes from heart transplant recipients showed higher frequency of H60-specific CD8+ T cells than H4-specific CD8+ T cells (2 versus 0.3% of total T cell). On the contrary, H4-specific CD8+ T cells were more abundant in skin transplant recipients than H60-specific CD8 T cells (3 versus 0.7% of total T cell). These data suggested that H60 mH-Ag is more immunodominant than H4 in heart transplantation, whereas H4 is more immunodominant in skin transplantation. We evaluated the complete panel of mismatched mH-Ag–specific (H4, H7, H13, H28, and H60 mH-Ag) CD8+ T cells by multimers and defined the hierarchy of mH-Ag–specific CD8+ T cells. The relative amount and hierarchy of mH-Ag–specific CD8+ T cells was H60 ≫ H4 > H7 > H13, H28 in heart transplantation. However, in skin transplantation, the hierarchy of immunodominance was H4 ≫ H60 > H7 > H13, H28 (Fig. 2C). Thus, clearly, differential hierarchy of immunodominant CD8 T cells is observed in heart and skin transplantations.
Tissue-specific expression of mH-Ags does not account for their immunodominance
Multiple mH-Ag peptides are generated due to genetic polymorphism that results in amino acid variations in normal house-keeping genes (33). As expected, the expression of many of these genes is tissue specific (29). This uneven distribution of mH-Ags in different tissues could contribute to tissue-specific T cell responses. Because H60 mH-Ag is known as the most immunodominant Ag among the mH-Ag disparities in BALB.B to C57BL/6 strain combination (21, 22), we hypothesized that high expression of H4 mH-Ag in skin overwhelmed H60 immunodominance after skin transplantation. Therefore, we performed transplantation with larger skin grafts. More skin provides more of both Ags, and increasing the quantity of these two mH-Ags could have favored H60 response if it is the only immunodominant mH-Ag. An additional skin patch was transplanted to achieve this. Interestingly, the increased amount of skin does not affect survival of the grafts (Fig. 3A). Furthermore, an increased amount of skin tissue did not induce H60 immunodominance but it did increase the number/proportion of both H4 and H60 mH-Ag–specific CD8 T cells. It could be interpreted that both mH-Ags are coimmunodominant (Fig. 3B). It is known that donor splenocyte transfusion (DST) induces H60 immunodominance similar to heart transplantation (i.e., more H60- than H4-specific CD8+ T cells) (21). H60 and H4 immunodominance was evaluated from the C57BL/6 mice manipulated with a) skin graft and b) skin graft with DST. Frequency of H60-specific CD8+ T cells was 0.6 and 1.8% of total T cells whereas the frequency of H4-specific CD8+ T cells was 4 and 2% of total T cells for skin graft and skin graft with DST, respectively (Fig. 3C). These data suggest that in vivo allogeneic T cell responses could vary by route of immunization. DST skews the response toward H60 and away from H4 when combined with a skin graft.
FIGURE 3.
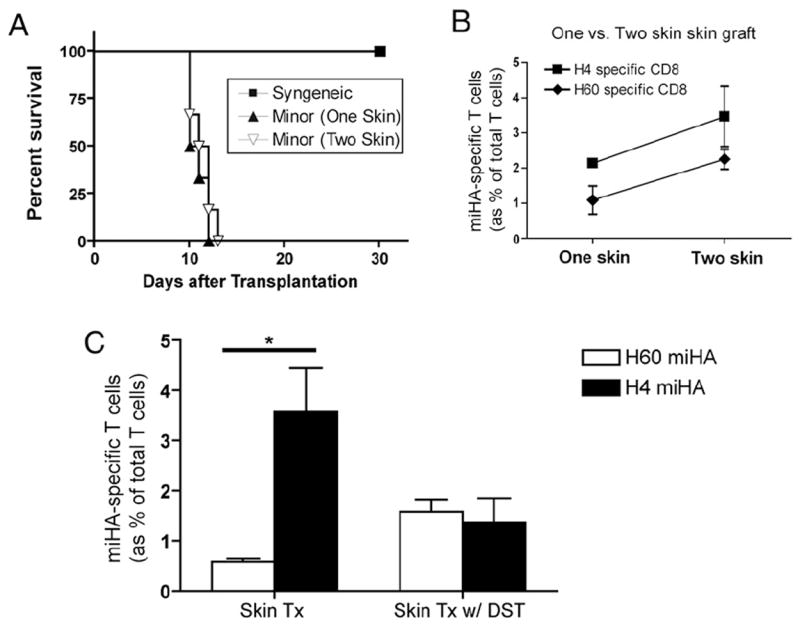
Competition between H4 and H60 mH-Ag on immunodominance in combination of skin transplantation and DST. A, Graft survival of one- (n = 6) or two-patch (n = 6) skin transplantation was not significantly different. Increased amount of skin showed neither prevention nor acceleration of graft rejection (p > 0.05). B, Both H4- and H60-specific CD8 T cell number was increased in accordance with amount of skin tissue. Splenocytes from one- or two-patch skin graft recipient were used for FACS analysis. C, Interference/competition of mH-Ags on immunodominance after DST and skin transplantation. BALB.B splenocytes (20 × 106) were administered with or without BALB.B skin transplantation. C57BL/6 recipients were sacrificed, and splenocytes were stained for H4 or H60 mH-Ag–specific CD8 T cells at 10 d after either skin transplantation (n = 6) or skin transplantation with DST (n = 6). The frequency of H60 and H4 mH-Ag–specific CD8+ T cells was significantly different in the skin transplantation-alone group (p < 0.05) but not in the skin transplantation with DST group. *p < 0.05.
To correlate tissue mH-Ag quantity and immunodominance after corresponding tissue transplantation, we extracted each mH-Ag peptide from spleen. Naturally processed H60/LYL8 and H4/SIL8 peptides in BALB.B tissue were evaluated with BCZ103 and BCZ1755 lacZ transfected T cell hybridomas, respectively. Attempts to extract the antigenic peptide from the skin and heart of BALB.B were unsuccessful, suggesting that both the H4 and H60 mH-Ag peptides were expressed in the tissue at a low level. However, a greater abundance of the SIL8 peptide than the LYL8 peptide in BALB.B spleen (Fig. 4A, 4B) was paradoxical to the hierarchy of immunodominance after BALB.B splenocytes transfusion (Fig. 4C). Therefore, we conclude that tissue concentrations of these mH-Ags are not responsible for differential T cell expansion after tissue transplantation.
FIGURE 4.
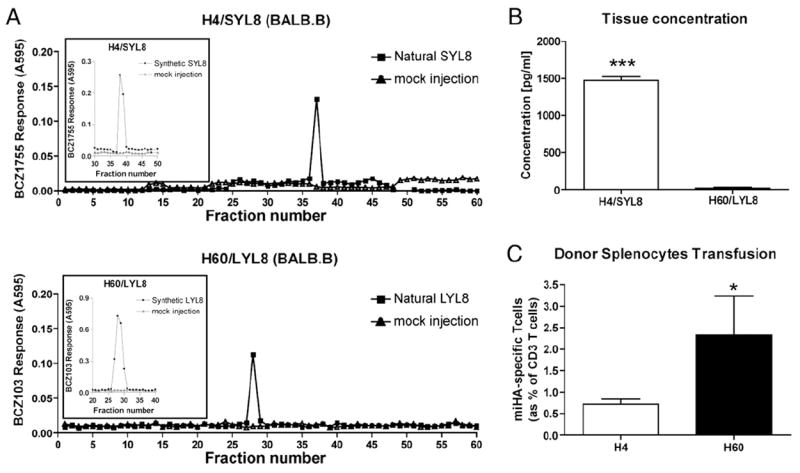
Identification of tissue concentration of H4 and H60 mH-Ag peptide. A, Spleen, heart, and skin (whole) extract pooled from four BALB.B mice were fractionated by HPLC, and fractions were assayed for the presence of H4 and H60 mH-Ag peptide using Kb-K89 cells as APCs. Natural peptide or synthetic peptide (inset) was eluted in 96-well plates. K89 cells (5 × 104 per well) plus 1 × 105 T cell hybridomas (lacZ transfected BCZ103 or BCZ1755 T cell hybridomas) were added per well. The T cell response was measured as the lacZ activity by conversion of the substrate CPRG at 595 nm and 655 nm as reference. “Mock” indicates fractions collected in identical run with sample buffer alone prior to extract sample. Inset shows synthetic mH-Ag peptide elution with HPLC. B, Concentration of H4 and H60 mH-Ag peptide was calculated by standard curve from T cell activation against corresponding synthetic peptides. H4 mH-Ag peptide showed higher concentration in the spleen compared with H60 mH-Ag peptide (***p < 0.001). C, H4- and H60-specific CD8 T cells were measured from the recipients at 10 d after DST. More H60 mH-Ag–specific CD8 T cell after DST was observed (*p <0.05).
Role of tissue-specific APCs on immunodominance of mH-Ags
It has long been suggested that graft-derived APCs can efficiently activate host T cells (34). While the heart contains interstitial DCs, skin has dermal DCs and epidermal DCs called Langerhans cells (35, 36). It could be argued that skin-specific DCs (Langerhans cells) present more H4 mH-Ag peptide than H60 mH-Ag, whereas common DC populations (dermal DC, cardiac DC, and spleen DC) in the tissue preferentially present H60 mH-Ag. Therefore, to determine whether APCs from different tissue can modulate immunodominance after transplantation, we evaluated donor-derived APC populations in dLNs. We used H4 and H60 mH-Ag peptides recognizing BCZ103 and BCZ1755 T cell hybridomas. Splenocytes from BALB.B mouse stimulate both T cell lines whereas cells from C57BL/6 did not (data not shown). We recovered dLNs (both axillary and inguinal lymph nodes) from heart and skin recipients 10 d after transplantation and cocultured total dLN-derived cells with BCZ103 or BCZ1755 T cell lines. The T cell activation results correlated with immunodominance of each tissue transplantation in that more H60 response was measured from heart graft dLN cells, whereas more H4 response was elicited from skin graft dLN cells (Fig. 5A). Composition of dLN DC showed different populations reconstituted in dLN after skin and heart transplantation. There were more interstitial DCs (CD11c+CD8−CD205−) in heart transplant recipients, but more lymphoid DCs (CD11c+CD8+CD205−) were found in skin transplant recipients. Interestingly, the proportion of Langerhans cells (CD11c+CD8−CD205+) in the dLN was not different in the two types of transplants (Fig. 5B). Because T cell activation would be determined by peptide presented by DC, we sorted the CD11c+ population and used these as stimulator cells (APC) for T cell hybridomas. However, differential T cell activation between skin versus heart grafts disappeared when sorted dLN-derived CD11c+ cells were used (Fig. 5C). This suggests that the CD11c+ DC population is not responsible for the difference seen in alloimmunity in this model.
FIGURE 5.
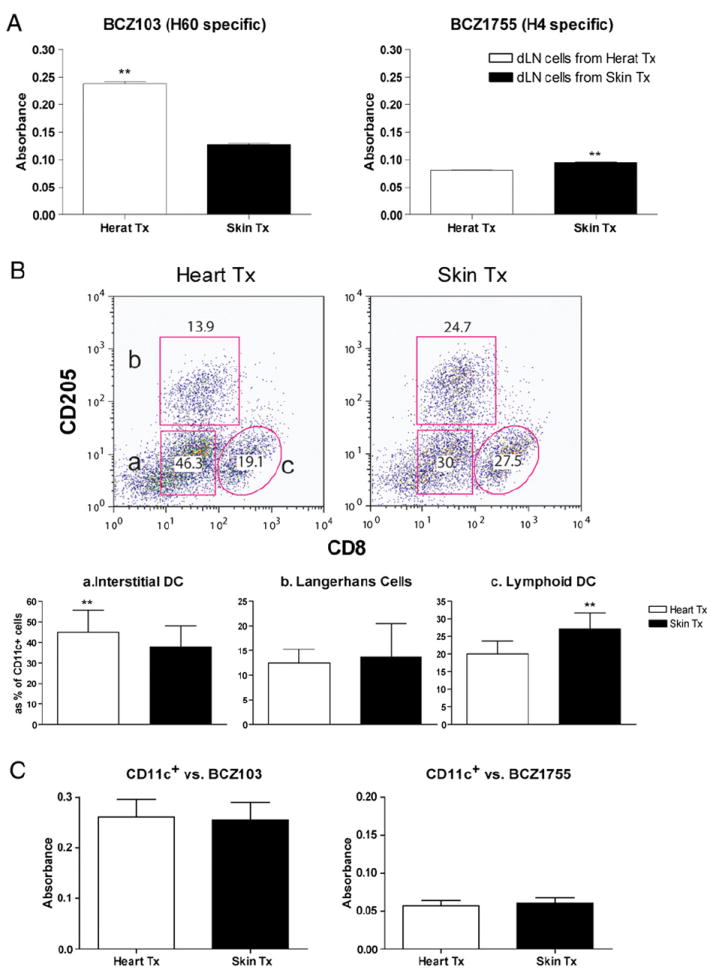
Total dLN cells, not CD11c+ DC, represent differential T cell activation in skin and heart transplantation. A, Axillary and inguinal lymph nodes were collected from skin transplantation recipients (n = 6) and heart transplantation recipients (n = 6). Cell suspension was cocultured with BCZ103 or BCZ1755 lacZ transfected T cell hybridoma. BCZ103 activation was significantly higher with dLN cells from cardiac allograft recipients, whereas BCZ1755 activation was higher with dLN cells from skin graft recipients (**p < 0.01). B, Differential dLN DC composition in between skin and heart transplantation. Cell suspension from dLN was stained with CD11c, CD205, and CD8. Dot plot represents CD11c gated cells. Gating strategy represents (a) CD205− CD8−CD11c+ (interstitial DC), (b) CD205+CD8−CD11c+ (Langerhans cells), and (c) CD205−CD8+CD11c+ (lymphoid DC). Data shown are representative of six independent experiments (**p < 0.01). C, CD11c+ cell was enriched by FACS sorting. CD11c+ cells (5 × 105) were cocultured with BCZ103 or BCZ1755 T cell hybridoma. Neither BCZ103 nor BCZ1755 showed different level of activation against heart or skin transplanted dLN-driven CD11c+ cells.
Effect of vascularization of the graft on immunodominance
Technically, skin transplantation does not involve immediate revascularization but requires ingrowth of new blood supply over days. In contrast, heart transplantation involves vascular anastomoses and immediate blood supply. Vascularization itself could induce several different outcomes on donor cell migration, intensity of ischemic injury, accessibility of recipient cells, etc. To determine whether vascularization itself affects differential T cell expansion in these transplant model systems, we performed non-primarily vascularized cardiac allografts with neonatal hearts. H4- and H60-specific CD8 T cells were measured 10 d after transplantation. Unlike vascularized heart transplantation, nonvascularized ear-pinna cardiac allografts were all rejected and, surprisingly, showed H4 immunodominance (Fig. 6A). It could be argued that the location (route) rather than vasculature is the reason for this. We mimicked the situation of vascularized skin transplantation by adoptive transfer of wild-type C57BL/6 splenocytes to BALB.B skin graft-bearing B6.SCID recipients. H4 immunodominance that was seen in nonvascularized skin transplantation was reduced in vascularized skin transplantation, while H60 immunodominance was somewhat restored when the B6.SCID recipient was reconstituted with B6 splenocytes 10 d after transplantation. Notably-whereas H60 immunodominance fully recovered when BALB.B skin graft-bearing B6.SCID recipients were reconstituted 25 d after transplantation (Fig. 6B). Thus, we concluded that vascularization paralleled H60 immunodominance after transplantation.
FIGURE 6.
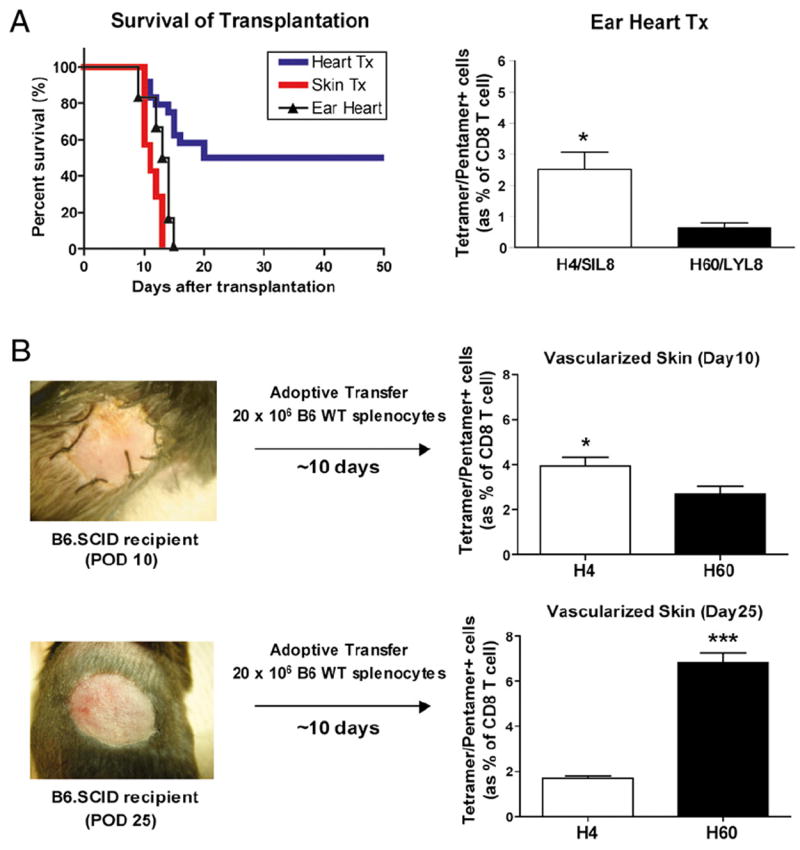
Vascularization-dependent H60 immunodominance. A, Minor mismatched ear-pinna cardiac allografts (n = 6, MST = 12 d) developed a similar rate of acute rejection and lymphocytic infiltration compared with skin transplantation at POD 10. Splenocytes were collected 10 d after transplantation and stained for H4- and H60-specific CD8 T cells. H4-specific CD8 T cells were significantly higher than H60-specific CD8 T cells (*p < 0.05). B, Vascularized skin transplantation was set up using B6.SCID mice as a recipient. BALB.B skin was transplanted to B6.SCID mice, and recipients were reconstituted with 2 × 106 wild-type C57BL/6 splenocytes 10 or 25 d after transplantation. H4-specific CD8 T cells were higher when B6.SCID recipients were reconstituted on day 10 (*p < 0.05), whereas H60-specific CD8 T cells prevailed when B6.SCID recipients were reconstituted on day 25 (***p < 0.001).
The effect of donor-derived T cells on H60 immunodominance
We hypothesized that donor-derived T cells are a major source of anti-H60 response after vascularized skin and heart transplantation. To test this hypothesis, first we measured H60 surface expression on BALB.B T cells. As expected, C57BL/6-derived T cells did not express the H60a molecule. However, neither did resting BALB.B-derived T cells (Fig. 7A). H60 mH-Ag peptide seemed constitutively presented by BALB.B cells, but H60 molecule was not detectable in naive mice. Interestingly, in vitro-activated (Con A, PMA/ionomycin, and MLR) BALB.B T cells upregulated H60 surface expression (Fig. 7A). This finding suggested that activated T cells would have increased H60 peptide presentation. In the dLN, the alloimmune response is not unidirectional but bidirectional in that donor-derived T cells will also proliferate after exposure to recipient APCs. Expanded donor T cell numbers provide increased numbers of H60 peptide during their activation. We measured BALB.B skin or cardiac graft-derived T cell numbers in B6.SCID recipients. Spleen, dLN, and peripheral blood were recovered 10 d after transplantation, and T cell number/proportion was determined by flow cytometric analysis. We found that cardiac grafts release more donor-derived T cells (p < 0.05) compared with that of skin grafts (Fig. 7B). Finally, to confirm the role of BALB.B T cells on H60 immunodominance, we measured H60-specific CD8 T cells after adoptive transfer of enriched CD3+ T cells from BALB.B donors. Without any professional donor APC, sorted CD3 T cells induced host immune response against H60 mH-Ag (Fig. 7C). To test the actual role of graft-derived T cells in influencing immunodominance, we depleted donor T cells from the graft using anti-CD4 (GK1.5) and anti-CD8 (53.6.72) mAbs prior to the transplantation. Profound T cell depletion was confirmed from the spleen at 24 h (data not shown). T cell-depleted BALB.B donor hearts were transplanted to C57BL/6 recipients (n = 6), and two immunodominant mH-Ag–specific T cells were evaluated during T cell expansion (at day 10). Surprisingly, more H4-specific CD8 T cells were found from the spleen compared with H60-specific CD8 T cells even with the primarily vascularized cardiac allograft (Fig. 8).
FIGURE 7.
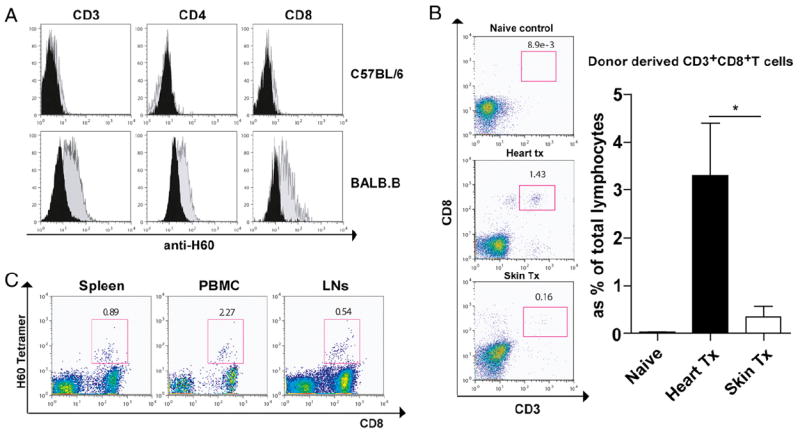
Allogeneic/homeostatic proliferation of donor-derived T cells and their upregulation of surface H60 molecule. A, Upregulation of surface H60 in T cells after activation. Surface H60 expression level on T cells was measured with antimouse H60 mAb. Splenocytes from C57BL/6 or BALB.B were cultured with or without Con A. After 3 d, cells were stained for H60 molecule with CD3, CD4, or CD8 mAbs. H60 molecule was observed only after Con A stimulation in BALB.B splenocytes but not without Con A stimulation or from C57BL/6 splenocytes. B, Donor-derived T cell in the B6.SCID recipient after skin and heart transplantation. B6.SCID recipient mice showed reconstitution of T cells in the spleen by graft 10 d after transplantation. Naive, skin transplanted, and heart transplanted SCID mice were sacrificed 10 d after transplantation. Splenocytes were stained for donor-derived CD3+ CD8+ T cells. Significantly increased donor-derived T cell was found after heart transplantation compared with skin transplantation (*p < 0.05). C, Adoptively transferred BALB.B CD3+ T cells showed induction of H60-specific CD8 T cells in dLNs, blood, and spleen.
FIGURE 8.
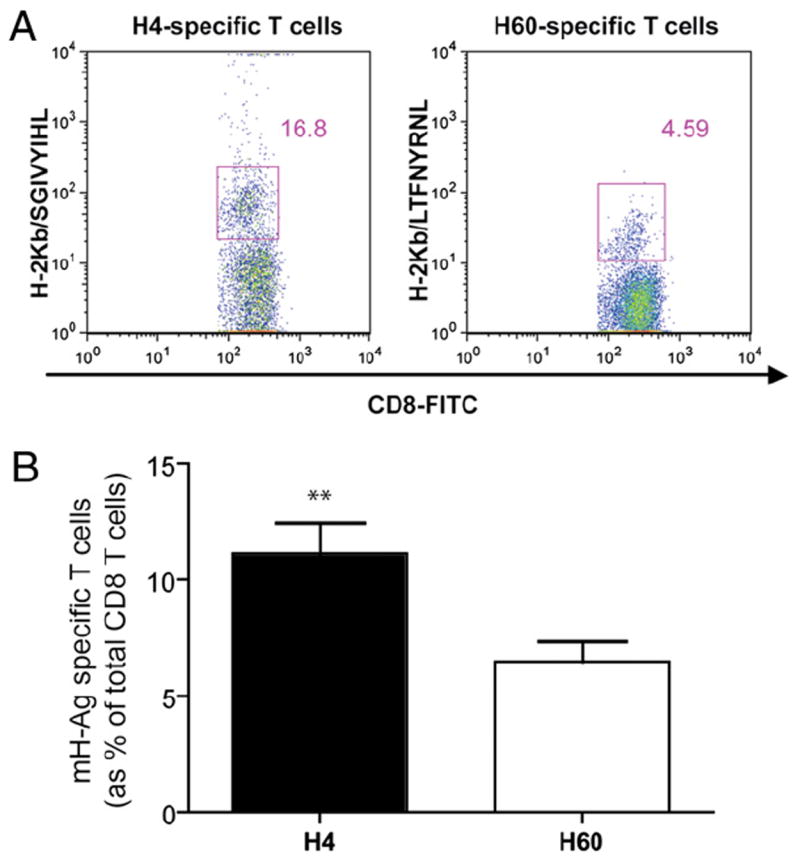
Effect of donor T cell depletion on H60 immunodominance after cardiac allograft. BALB.B donors were treated with anti-CD4 mAb (100 μg) and anti-CD8 mAb (200 μg) 24 h prior to heart transplantation. The donor T cell-depleted BALB.B hearts were transplanted to wild-type B6 mice (n = 6). Splenocytes were recovered posttransplant day 10 from the recipients and stained with CD3 mAb, CD8 mAb and PE-conjugated H4/H-2Kb or H60/H-2Kb multimer. A, Representative dot plots of H4- and H60-specific CD8 T cells in spleen of recipients receiving T cell-depleted heart. Gates in the figure represent the percentages of multimer-positive cells within CD3+CD8+ T cells. B, Percentage of H4- and H60-specific T cells of six recipients. Bar graph represents the average frequency of H4- and H60-specific CD8+ T cells in the spleen at POD 10 (n = 6). H4-specific CD8+ T cells were significantly higher than H60-specific CD8+ T cells. Values represent mean ± SEM of six animals. **p < 0.01 (paired Student t test).
Taken together, these results imply that immediate transmigration of donor T cells via vascularization provides an increased quantity of H60 mH-Ag peptide to the recipient immune system.
Discussion
Minor Ag mismatch organ transplantation is a useful model to study immunodominance. In particular, H60 and H4 mH-Ags are known to be the most immunodominant peptides in this mouse model, although not without discrepancy of reports. Wettstein and Colombo (14) reported H4b mH-Ag showed the highest binding affinity (Kb) to MHC molecule. Furthermore, the CTL assay revealed H4-specific T cells to be most efficient after skin transplantation. In contrast, Choi et al. (21, 22) showed the immunodominance of H60 in BALB.B splenocyte transfusion by their exceptionally high clonal frequency. It was also documented that H60 mH-Ag–specific CD8+ T cell numbers were decreased after skin transplantation compared with DST, and the authors attributed this to broad antigenic diversity and route of immunization. However, each mH-Ag peptide showed a similar binding affinity to H-2Kb, and similar binding avidity of peptide-conjugated MHC molecules to their cognate TCRs was observed (23, 37). These in vitro data showed that H4 mH-Ag is immunodominant as much as H60 mH-Ag. We also reported H60 immunodominance in BALB.B to C57BL/6 heart transplantation (38). Unfortunately, H4 and H60 mH-Ag responses were never directly compared using two different immunization methods.
We had two interesting observations by comparing BALB.B with C57BL/6 heart transplantation and skin transplantation. These two transplant models showed not only different graft survival trends but also differential expansion hierarchy of mH-Ag–specific CD8+ T cells after each transplantation (Figs. 1, 2). Briefly, H60-specific CD8+ T cells were the most abundant after heart transplantation, whereas H4-specific CD8+ T cells were more abundant after skin graft. To elucidate the possible mechanisms for this differential T cell expansion or hierarchy of immunodominance, we evaluated several hypotheses that 1) uneven distribution of mH-Ags, 2) differential APC distributions, and 3) different vascularization status in these two different tissues could affect T cell expansion.
Augmenting tissue Ags by increasing size of the donor skin was not successful for restoring H60 immunodominance after skin transplantation (Fig. 3). Our HPLC data from the spleen confirmed that concentration of minor Ag peptide in the tissue is not the only factor influencing T cell immunodominance (Fig. 4). However, under certain conditions such as skin transplantation, Ag density may be important. We also evaluated dLN-derived cells for their ability to differentially activate T cells as the dLN is the anatomic location where host T cells are activated by donor-derived or donor Ag-bearing host APCs. We found that these dLN cells can differentially activate mH-Ag–specific CD8+ T cells. It is natural to consider that dermal DC is equivalent to cardiac DC whereas Langerhans cells are unique to the skin. Skin-derived Langerhans cells are greater in number and may be more immunogenic than heart-derived DCs (39). In addition, because skin grafts are not initially vascularized but undergo gradual revascularization over several days, ischemia–reperfusion injury is likely to be more prolonged and severe than in vascularized heart transplantation, possibly leading to increased activation and immunogenicity of donor DCs migrating to the recipient secondary lymphoid organs. Potential consequences of these differences might be higher frequencies of anti–mH-Ag effector T cells and/or induction of more potent (pathogenic) T cell effector functions in recipients of skin versus heart transplants. However, sorted CD11c+ cells, which include interstitial DC, Langerhans cell, and lymphoid DC, did not induce differential T cell activation (Fig. 5). It is still notable that the T cell activation induced by total dLN cells correlated with immunodominance of each tissue transplantation, which represents confined effect of each transplantation in the dLNs.
Finally, we evaluated whether vascularization of the graft affects T cell immunodominance. By comparing nonvascularized heart transplantation with skin transplantation or vascularized skin transplantation with heart transplantation, we could evaluate the role of tissue vasculature after transplantation. Surprisingly, CD8 T cell immunodominance depended on whether tissues were vascularized rather than what tissues were transplanted (Fig. 6). One interpretation of our finding is that donor passenger leukocytes that we think of as the trigger for host T cells to mobilize in the lymph nodes vary in their immunogenicity. H60 is not immunodominant for a skin graft (whereas H4 is) but only for a primarily vascularized organ like the heart. It is possible that smaller amounts of H60-expressing cells (hematopoietic origin) transfer into the recipient in skin compared with heart transplantation. Combining T cell activation and dLN data, we hypothesized that passenger leukocytes other than APC contribute to differential T cell activation.
Induction of H60 as an NKG2D ligand has been reported with several different models including transplantation (40, 41). Interestingly, BALB.B donor T cells upregulated surface H60 expression after activation both in vitro and in vivo. BALB.B donor heart transplantation into B6.SCID mice revealed allogeneic/homeostatic proliferation of donor-derived T cells in the recipients (Fig. 7). We found definite quantitative differences of transmigrated donor-derived T cells in vascularized versus nonvascularized transplants. H60 immunodominance after adoptive transfer of sorted CD3 T cells showed that donor-derived alloreactive T cells could be the source of mH-Ag immunodominance after organ transplantation. Furthermore, initial quantity of donor-derived T cells in the recipient contributed to H60 immunodominance in vascularized skin transplantation (Fig. 6). Finally, we depleted donor T cells using anti-CD4 and anti-CD8 mAbs from the BALB.B donors and transplanted T cell-depleted donor hearts to wild-type C57BL/6 recipients. Removing donor T cells changes H60 immunodominance over H4 mH-Ags after primarily vascularized cardiac allograft (Fig. 8). It is also notable that donor T cell depletion does not abolish the anti-H60 response completely. This could be due to 1) incomplete donor T cell depletion in the graft, 2) other source of H60 molecule in the passenger leukocytes, or 3) H60 expression in the graft tissue. Nevertheless, it shows that donor T cells are essential for H60 immunodominance after vascularized heart transplantation. Unexpectedly, the graft survival of donor T cell-depleted heart transplantation was shortened compared with the untreated group, as they were all rejected at POD 12 (n = 6). It shows that H60 immunodominance is actually beneficial to graft survival possibly due to suppressing (or dominating) tissue-specific (H4 mH-Ag) T cell, which should be more efficient on rejecting BALB.B graft. These data indicate that donor-derived T cells from the graft could profoundly affect a host’s antidonor response. In accordance with this, Russel et al. (42) recently showed that severe chronic rejection is developed in single H4 mH-Ag–mismatched cardiac allograft, whereas chronic rejection is less developed with single H60 mH-Ag disparity. Because the CD3 T cell is not the professional APC, its contribution may be through augmenting the quantity of H60 mH-Ag peptides via cross-presentation. Here, the direct effect of H60 molecule as an NKG2D receptor has not been evaluated. However, a graft-versus-host response itself can be the source of a graft-prolonging effect in that reduced graft acceptance after donor T cell depletion could be due to removing the tolerogenic effect (i.e., donor regulatory T cells) of the donor T cells (43).
The donor-derived lymphocytes are one of the most overlooked cells in organ transplantation, although they are considered a major effector cell in bone marrow transplantation, in particular for inducing graft-versus-host disease. The T cells from a graft should experience a similar activation process as recipient T cells (a two-way MLR rather than one-way in dLNs). Donor T cells would activate and expand like host T cells, even though they would eventually be abolished by overwhelming numbers of recipient T cells. This is why they are generally neglected in organ transplantation. According to our data, donor-derived T cells are not a drop in the ocean. By upregulating shared donor mH-Ag in the graft, they may contribute to expanding recipient effector T cells. In contrast, this could be extended to specific immunomodulation if the Ag presented on donor-derived lymphocytes is not shared with those on the graft. Currently, the role of H60-specific CD8+ T cells in BALB.B cardiac allograft is under investigation.
Nevertheless, these data establish a direct causal link between vascularization and immune responses to graft Ag and association of the migrating donor T cells via the vasculature. These findings may affect future protocols for tolerance induction in transplantation and indicate a mechanism of immunodominance induced by rapid transit from graft to host dLN of donor passenger T cells.
Acknowledgments
We thank Dr. Maria Alegre (Department of Pathology, University of Chicago) for insightful suggestions on the study. We also thank Drs. Allan Kirk and William Kitchens (Department of Surgery, Emory University) for helpful comments on the manuscript and Kathleen Schell and the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Facility (Madison, WI) for assistance with cell sorting.
Abbreviations used in this article
- CPRG
chlorophenol red β-galactoside
- DC
dendritic cell
- dLN
draining lymph node
- DST
donor splenocyte transfusion
- mH-Ag
minor histocompatibility Ag
- MST
mean survival time
- POD
postoperation day
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wallny HJ, Rammensee HG. Identification of classical minor histocompatibility antigen as cell-derived peptide. Nature. 1990;343:275–278. doi: 10.1038/343275a0. [DOI] [PubMed] [Google Scholar]
- 2.Wettstein PJ, Bailey DW. Immunodominance in the immune response to “multiple” histocompatibility antigens. Immunogenetics. 1982;16:47–58. doi: 10.1007/BF00364441. [DOI] [PubMed] [Google Scholar]
- 3.Teh HS, Bennink J, Von Boehmer H. Selection of the T cell repertoire during ontogeny: limiting dilution analysis. Eur J Immunol. 1982;12:887–892. doi: 10.1002/eji.1830121016. [DOI] [PubMed] [Google Scholar]
- 4.Theobald M, Bunjes D. Pretransplant detection of human minor histocompatibility antigen-specific naive and memory interleukin-2-secreting T cells within class I major histocompatibility complex (MHC)-restricted CD8+ and class II MHC-restricted CD4+ T-cell subsets. Blood. 1993;82:298–306. [PubMed] [Google Scholar]
- 5.Perreault C, Roy DC, Fortin C. Immunodominant minor histocompatibility antigens: the major ones. Immunol Today. 1998;19:69–74. doi: 10.1016/s0167-5699(97)01185-7. [DOI] [PubMed] [Google Scholar]
- 6.Martin S, Dyer PA. The case for matching MHC genes in human organ transplantation. Nat Genet. 1993;5:210–213. doi: 10.1038/ng1193-210a. [DOI] [PubMed] [Google Scholar]
- 7.Pimsler M, Forman J. Estimates of the precursor frequency of cytotoxic T lymhocytes against antigens controlled by defined regions of the H-2 gene complex: comparison of the effect of H-2 differences due to intra-H-2 recombination vs mutation. J Immunol. 1978;121:1302–1305. [PubMed] [Google Scholar]
- 8.Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat Med. 2001;7:80–87. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 9.Adorini L, Appella E, Doria G, Nagy ZA. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988;168:2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perreault C, Jutras J, Roy DC, Filep JG, Brochu S. Identification of an immunodominant mouse minor histocompatibility antigen (MiHA). T cell response to a single dominant MiHA causes graft-versus-host disease. J Clin Invest. 1996;98:622–628. doi: 10.1172/JCI118832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pion S, Fontaine P, Desaulniers M, Jutras J, Filep JG, Perreault C. On the mechanisms of immunodominance in cytotoxic T lymphocyte responses to minor histocompatibility antigens. Eur J Immunol. 1997;27:421–430. doi: 10.1002/eji.1830270212. [DOI] [PubMed] [Google Scholar]
- 12.Wolpert EZ, Grufman P, Sandberg JK, Tegnesjö A, Kärre K. Immunodominance in the CTL response against minor histocompatibility antigens: interference between responding T cells, rather than with presentation of epitopes. J Immunol. 1998;161:4499–4505. [PubMed] [Google Scholar]
- 13.Mori S, El-Baki H, Mullen CA. Analysis of immunodominance among minor histocompatibility antigens in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:865–875. doi: 10.1038/sj.bmt.1704021. [DOI] [PubMed] [Google Scholar]
- 14.Wettstein PJ, Colombo MP. Immunodominance in the T cell response to multiple non-H-2 histocompatibility antigens. IV. Partial tissue distribution and mapping of immunodominant antigens. J Immunol. 1987;139:2166–2171. [PubMed] [Google Scholar]
- 15.Andreansky SS, Stambas J, Thomas PG, Xie W, Webby RJ, Doherty PC. Consequences of immunodominant epitope deletion for minor influenza virus-specific CD8+-T-cell responses. J Virol. 2005;79:4329–4339. doi: 10.1128/JVI.79.7.4329-4339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, Chen W, Turner SJ, Doherty PC. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc Natl Acad Sci USA. 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sijts AJ, Neisig A, Neefjes J, Pamer EG. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J Immunol. 1996;156:683–692. [PubMed] [Google Scholar]
- 18.van der Most RG, Murali-Krishna K, Whitton JL, Oseroff C, Alexander J, Southwood S, Sidney J, Chesnut RW, Sette A, Ahmed R. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology. 1998;240:158–167. doi: 10.1006/viro.1997.8934. [DOI] [PubMed] [Google Scholar]
- 19.Vijh S, Pamer EG. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J Immunol. 1997;158:3366–3371. [PubMed] [Google Scholar]
- 20.Busch DH, Pamer EG. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 21.Choi EY, Yoshimura Y, Christianson GJ, Sproule TJ, Malarkannan S, Shastri N, Joyce S, Roopenian DC. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: the H60 histocompatibility antigen dominates over all others. J Immunol. 2001;166:4370–4379. doi: 10.4049/jimmunol.166.7.4370. [DOI] [PubMed] [Google Scholar]
- 22.Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung N, Joyce S, Roopenian DC. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002;17:593–603. doi: 10.1016/s1074-7613(02)00428-4. [DOI] [PubMed] [Google Scholar]
- 23.Yadav R, Yoshimura Y, Boesteanu A, Christianson GJ, Ajayi WU, Shashidharamurthy R, Stanic AK, Roopenian DC, Joyce S. The H4b minor histocompatibility antigen is caused by a combination of genetically determined and posttranslational modifications. J Immunol. 2003;170:5133–5142. doi: 10.4049/jimmunol.170.10.5133. [DOI] [PubMed] [Google Scholar]
- 24.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Fey TA, Krause RA, Hsieh GC, Andrews JM, Bretheim PT, Morgan SJ, Luly JR, Mollison KW. Improved methods for transplanting split-heart neonatal cardiac grafts into the ear pinna of mice and rats. J Pharmacol Toxicol Methods. 1998;39:9–17. doi: 10.1016/s1056-8719(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A The International Society for Heart Transplantation. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 27.Kwun J, Knechtle SJ, Hu H. Determination of the functional status of alloreactive T cells by interferon-gamma kinetics. Transplantation. 2006;81:590–598. doi: 10.1097/01.tp.0000196353.04494.14. [DOI] [PubMed] [Google Scholar]
- 28.Rötzschke O, Falk K, Wallny HJ, Faath S, Rammensee HG. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990;249:283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- 29.Griem P, Wallny HJ, Falk K, Rötzschke O, Arnold B, Schönrich G, Hämmerling G, Rammensee HG. Uneven tissue distribution of minor histocompatibility proteins versus peptides is caused by MHC expression. Cell. 1991;65:633–640. doi: 10.1016/0092-8674(91)90095-g. [DOI] [PubMed] [Google Scholar]
- 30.Malarkannan S, Goth S, Buchholz DR, Shastri N. The role of MHC class I molecules in the generation of endogenous peptide/MHC complexes. J Immunol. 1995;154:585–598. [PubMed] [Google Scholar]
- 31.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 32.Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998;161:3501–3509. [PubMed] [Google Scholar]
- 33.Simpson E, Roopenian D, Goulmy E. Much ado about minor histocompatibility antigens. Immunol Today. 1998;19:108–112. doi: 10.1016/s0167-5699(97)01213-9. [DOI] [PubMed] [Google Scholar]
- 34.Elkins WL, Guttmann RD. Pathogenesis of a local graft versus host reaction: immunogenicity of circulating host leukocytes. Science. 1968;159:1250–1251. doi: 10.1126/science.159.3820.1250. [DOI] [PubMed] [Google Scholar]
- 35.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura Y, Yadav R, Christianson GJ, Ajayi WU, Roopenian DC, Joyce S. Duration of alloantigen presentation and avidity of T cell antigen recognition correlate with immunodominance of CTL response to minor histocompatibility antigens. J Immunol. 2004;172:6666–6674. doi: 10.4049/jimmunol.172.11.6666. [DOI] [PubMed] [Google Scholar]
- 38.Kwun J, Hu H, Schadde E, Roenneburg D, Sullivan KA, DeMartino J, Burlingham WJ, Knechtle SJ. Altered distribution of H60 minor H antigen-specific CD8 T cells and attenuated chronic vasculopathy in minor histocompatibility antigen mismatched heart transplantation in Cxcr3-/- mouse recipients. J Immunol. 2007;179:8016–8025. doi: 10.4049/jimmunol.179.12.8016. [DOI] [PubMed] [Google Scholar]
- 39.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galazka G, Jurewicz A, Orlowski W, Stasiolek M, Brosnan CF, Raine CS, Selmaj K. EAE tolerance induction with Hsp70-peptide complexes depends on H60 and NKG2D activity. J Immunol. 2007;179:4503–4512. doi: 10.4049/jimmunol.179.7.4503. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Chang CK, Hayden T, Liu FC, Benjamin J, Hamerman JA, Lanier LL, Kang SM. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–6420. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 42.Russell PS, Chase CM, Madsen JC, Hirohashi T, Cornell LD, Sproule TJ, Colvin RB, Roopenian DC. Coronary artery disease from isolated non-H2-determined incompatibilities in transplanted mouse hearts. Transplantation. 2011;91:847–852. doi: 10.1097/TP.0b013e3182122f82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011;11:1296–1301. doi: 10.1111/j.1600-6143.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


