Abstract
The visual recognition of letters dissociates from the recognition of numbers at both the behavioral and neural level. In this article, using fMRI, we investigate whether the visual recognition of numbers dissociates from letters, thereby establishing a double dissociation. In Experiment 1, participants viewed strings of consonants and Arabic numerals. We found that letters activated the left midfusiform and inferior temporal gyri more than numbers, replicating previous studies, whereas numbers activated a right lateral occipital area more than letters at the group level. Because the distinction between letters and numbers is culturally defined and relatively arbitrary, this double dissociation provides some of the strongest evidence to date that a neural dissociation can emerge as a result of experience. We then investigated a potential source of the observed neural dissociation. Specifically, we tested the hypothesis that lateralization of visual number recognition depends on lateralization of higher-order numerical processing. In Experiment 2, the same participants performed addition, subtraction, and counting on arrays of nonsymbolic stimuli varying in numerosity, which produced neural activity in and around the intraparietal sulcus, a region associated with higher-order numerical processing. We found that individual differences in the lateralization of number activity in visual cortex could be explained by individual differences in the lateralization of numerical processing in parietal cortex, suggesting a functional relationship between the two regions. Together, these results demonstrate a neural double dissociation between letter and number recognition and suggest that higher-level numerical processing in parietal cortex may influence the neural organization of number processing in visual cortex.
INTRODUCTION
The visual recognition of letters has been found to be dissociable from the visual recognition of numbers in behavioral studies (Hamilton, Mirkin, & Polk, 2006; Polk & Farah, 1995; Jonides & Gleitman, 1972), neuropsychological studies (Ingles & Eskes, 2008; Starrfelt, 2007; Anderson, Damasio, & Damasio, 1990), electrophysiological studies (Wong, Gauthier, Woroch, DeBuse, & Curran, 2005; Allison, McCarthy, Nobre, Puce, & Belger, 1994), and neuroimaging studies (Joseph, Cerullo, Farley, Steinmetz, & Mier, 2006; James, James, Jobard, Wong, & Gauthier, 2005; Pernet, Celsis, & Demonet, 2005; Flowers et al., 2004; Polk et al., 2002; Polk & Farah, 1998; Puce, Allison, Asgari, Gore, & McCarthy, 1996). This finding is significant because letters and numbers are arbitrary symbols, and the distinction between them is simply a cultural convention. If they are processed differently at both behavioral and neural levels, it suggests that early schooling experiences can lead to qualitative changes in neurocognitive architecture.
Nevertheless, inferring that two tasks depend on separate underlying processes on the basis of single or one-way dissociations is problematic (Shallice, 1988). For example, if Task A is harder than Task B, then brain damage might selectively impair Task A even if the two tasks depend on the same neural substrates. Similarly, if Task A produces greater breadth or more areas of activation than Task B in a neuroimaging experiment, it might merely reflect the fact that Task A is more demanding, not that the tasks depend on dissociable neural systems.
Ideally then, one would like to demonstrate that number recognition is also dissociable from letter recognition, thereby establishing a double dissociation and undermining alternative hypotheses. Some electrophysiological studies have found number-specific responses from specific inferotemporal electrodes (Roux, Lubrano, Lauwers-Cances, Giussani, & Demonet, 2008; Allison et al., 1994) consistent with the hypothesis that number recognition is dissociable from letter recognition, but evidence from patient studies and neuroimaging is scarce. Although patients with numerical processing deficits (acalculia) are commonly reported, we know of no reports of patients with a specific deficit in the visual recognition of numbers relative to letters and words (but see the following reports for descriptions of patients with deficits in the verbal production of number names: Marangolo, Nasti, & Zorzi, 2004; Cipolotti, Warrington, & Butterworth, 1995). And very few neuroimaging studies have directly contrasted the visual recognition of numbers with the recognition of letters. Polk et al. (2002) attempted to do so but only reported significant activation for numbers in three subjects, and no significant activation was reported at the group level. In Experiment 1, we use new procedures and study a larger group of subjects to examine directly the neural dissociation of number recognition from letter recognition.
If letter and number recognition rely (at least in part) on different neural systems, a natural question is why. Dehaene and colleagues’ neuronal recycling hypothesis proposes that acquired functions, like letter and number recognition, exploit and reorganize evolutionarily older neural circuits originally performing similar functions (Dehaene & Cohen, 2007). According to this view, one of the reasons that the so-called visual word form area (VWFA) tends to develop in the left occipito-temporal cortex is because this area has relatively direct connections to and from anterior language processing sites in the left hemisphere (McCandliss, Cohen, & Dehaene, 2003).
A recent EEG study has provided indirect support for this hypothesis (Cai, Lavidor, Brysbaert, Paulignan, & Nazir, 2008). Although it has been known that VWFA lies in the left inferior temporal region in most right-handed subjects (Cohen et al., 2002), Cai et al. (2008) tested its lateralization in left-handed subjects in whom anterior language processing regions are commonly right-lateralized. Four of the nine left-handed subjects showed right-lateralized frontal activity in a verb generation task, and these four subjects also exhibited stronger right-sided negativity in inferior temporal sites. On the basis of these results, they argued that the localization of word recognition in ventral visual cortex depends on the localization of spoken language processes in frontal cortex.
Following this reasoning, we hypothesized that the location of cortical areas involved in visual number recognition might depend on the location of cortical areas involved in higher-order numerical processing in parietal cortex. Specifically, participants whose higher-order numerical processing is more right-lateralized in parietal cortex would also tend to exhibit more right-lateralized number recognition in ventral visual cortex and vice versa. We tested these predictions in Experiment 2 by measuring neural activity in response to higher-order numerical processing tasks (addition, subtraction, and counting) in the same participants. We then investigated whether the lateralization of this higher-order numerical processing activity correlated with the lateralization of lower-order number recognition activity in ventral visual cortex.
EXPERIMENT 1
Methods
Participants
Twenty healthy young adults (ages 18–29 years with mean of 23.4 years; nine men) participated in the study. All participants were screened through self-report questionnaires to ensure they were right-handed, native English speakers, psychologically and physically healthy, not taking medications with psychotropic or vascular effects, and free of any other MRI safety contraindications. All study procedures were reviewed and approved by the institutional review boards at the University of Texas at Dallas, the University of Texas Southwestern Medical School, and the University of Michigan. All participants provided detailed written consent before their involvement in the study.
Stimulus Materials
Five types of stimuli were created for use in the study. All five types consisted of two strings, 4.2° above and below the central cross (examples are shown in Figure 1). Participants made “same/different” judgment on each pair of strings. The primary stimuli of interest were letter consonant strings and number strings. All strings were composed of four letters/numbers (monospaced type-face with 2° visual angle in height), and only capital letters were used. Three other types of stimuli—words, pseudowords, and false fonts (adapted from Vinckier et al., 2007)—were included, although the focus of this study was on consonant and number strings. Pseudowords (pronounceable nonwords) were created with constrained trigram-based strings (Medler & Binder, 2005), and real words with mean word frequency of 323.6 per million (ranging from 205.4 to 497.3) were included.
Figure 1.
Examples of stimuli used in Experiment 1. Participants performed a visual matching task and judged whether the two items were the same or different.
Procedure
The task consisted of five 5-min runs with eighteen 16-sec blocks, pseudorandomly ordered (three for each of the five categories in addition to three blocks of fixation). Each block consisted of eight trials (1.5 sec of presentation and 0.5 sec of intertrial interval) in which participants made a same/different judgment on each pair of strings. The correct answer was “same” in half of all the trials. All visual stimuli were presented via E-prime (Psychology Software Tools, Pittsburgh, PA) and displayed by a back-projection system. Participants made responses using buttons under the right index and middle fingers (Lumina response pad; Cedrus, San Pedro, CA).
Data Acquisition
Brain images were acquired with a Philips Achieva 3T whole-body scanner at University of Texas Southwestern Medical School using the Philips SENSE parallel acquisition technique. Functional scans were acquired as axial slices, with a voxel size of 3.4 mm × 3.4 mm × 3.5 mm. At each of 148 BOLD acquisitions per run, 43 axial slices were acquired (covering the whole brain; repetition time [TR] = 2.0 sec, echo time [TE] = 25 msec). A high-resolution axial T1 MPRAGE was acquired primarily to facilitate group registration (voxel size 1 mm isotropic; TR = 8.27 msec, TE = 3.82 msec).
Activation Analysis
Data were preprocessed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk). Functional images underwent slice-timing correction and realignment to the mean volume. Each participant’s T1 anatomical image was coregistered with the functional images and then segmented into gray matter, white matter, and cerebral spinal fluid. The gray matter was normalized into the default gray matter probability template in standard Montreal Neurological Institute space, and the acquired normalization parameters were used to normalize the functional images for each individual with a spatial resolution of 3 mm × 3 mm × 3 mm.
Activations in response to each stimulus type (numbers, letters, words, pseudowords, and false fonts) in contrast to fixation were estimated using the standard general linear model with a high-pass filter at 128 Hz and correcting for temporal autocorrelation with an AR(1) model. The model included separate regressors for each of the experimental conditions convolved with a canonical hemodynamic response function as well as six nuisance covariates modeling head translation and rotation. In each subject, “letter-preferred activation” was defined by contrasting consonant strings to number strings, and “number-preferred activation” was defined by contrasting number strings to consonant strings. These contrast maps from each subject were then entered into a second-level random effects group analysis to identify letter- and number-preferred activity at the group level (see Figure 3).
Figure 3.
Letter- and number-preferred activation maps. Letters in contrast to numbers activated the left midfusiform/inferior temporal area (p = .005, cluster-level correction for multiple comparisons), whereas numbers in contrast to letters activated the right lateral occipital area (p = .010). Complete coordinates of clusters are reported in Table 2. For visualization, these group-level functional maps (p < .005, cluster extent > 20 voxels) were overlaid on to a 3-D inflated surface as in Figure 2.
Laterality Analysis
To assess laterality for letter-preferred activity, we first created an ROI based on the letter-preferred (letter vs. number) group activation map within a bilateral anatomical mask including the fusiform, inferior and middle temporal, and inferior occipital gyri (defined using Pick-Atlas AAL toolbox, www.fmri.wfubmc.edu/cms/software). The activation map was thresholded at a relatively lenient threshold (p < .05, extent > 20 voxels) within the anatomical mask to accommodate individual differences in the location of letter-preferred activation in individual participants. This approach yielded 216 voxels, all of which were in left ventral visual cortex. Finally, we added all the corresponding 216 voxels in the right hemisphere (flipping the sign of the x coordinates) to make this ROI symmetric. This bilateral ROI constructed from the group-level letter-preferred activation map is referred to as the visual letter ROI.
We applied the same procedure to create a visual number ROI. The number-preferred (number vs. letter) group activation yielded 343 voxels within the same anatomical mask, all in the right ventral visual cortex. We then added the 343 corresponding voxels from the left hemisphere to create the visual number ROI.
We then computed laterality indices (LIs) for each individual participant’s letter-preferred activity in the visual letter ROI and number-preferred activity in the visual number ROI using a threshold-independent LI that was computed following Suarez et al. (2009). This approach was validated to be more robust and unambiguous compared with threshold-dependent methods in a study determining language dominance validated against the intracarotid amytal test (Suarez et al., 2009). In this method, histograms of the number of voxels with positive t values were generated separately in the left and the right hemisphere of each bilateral ROI. These histograms were multiplied by a weighting function defined as t2 (as suggested by Suarez et al., 2009), and areas under each of the weighted histograms were computed. Then, the LI was computed using the conventional ratio, LI = (QL − QR)/(QL + QR), wherein QL denotes the area under the weighted histograms from the left hemisphere and QR denotes the same area from the right hemisphere. An LI of 1 indicates complete left hemisphere dominance, and an LI of −1 indicates complete right hemisphere dominance.
Results
Behavioral Results
Accuracy on the visual matching task was above 95% for all conditions (Table 1). RT for the letter (consonant strings) condition was slightly faster than for the number condition (t(19) = 2.007, p < .059, paired t test). The RTs for the three orthographic conditions (real words, pseudowords, and consonant strings) did not show any significant difference (p > .220 for the three paired t tests). RT to the false fonts was the slowest of all the conditions (t(19) = 11.602, p < .001 for the contrast of false fonts vs. all other conditions).
Table 1.
Behavioral Results of the Visual-matching Task for Each Experimental Condition Performed in the Scanner (n = 20)
| Word | Pseudoword | Consonant Strings | Number Strings | False Fonts | |
|---|---|---|---|---|---|
| Accuracy (%) | 98.9 (1.20) | 97.9 (1.85) | 98.1 (1.70) | 99.0 (1.29) | 96.2 (2.91) |
| Reaction time (msec) | 679.30 (91.21) | 679.65 (95.16) | 691.00 (111.49) | 712.60 (98.68) | 801.75 (111.02) |
Mean accuracy and median RT for the correct trials were measured for each subject, and the average (SD) of these scores across subjects are reported.
Letter- and Number-preferred Activation
Both the letter and number conditions showed greater BOLD response compared with the fixation condition in various regions including the bilateral ventral visual cortex and the left sensorimotor cortex (Figure 2). Moreover, when the letter condition and the number condition were contrasted directly, letters activated an area in left ventral visual cortex more than numbers whereas numbers activated an area in right ventral visual cortex more than letters at the group level (Figure 3). The left midfusiform and inferior temporal area and the left angular gyrus were the only two regions that passed the cluster-level threshold in the letter-preferred (letter vs. number) activation map, and the right lateral occipital area was the only region that passed the threshold in the number-preferred (number vs. letter) activation map (Table 2).
Figure 2.
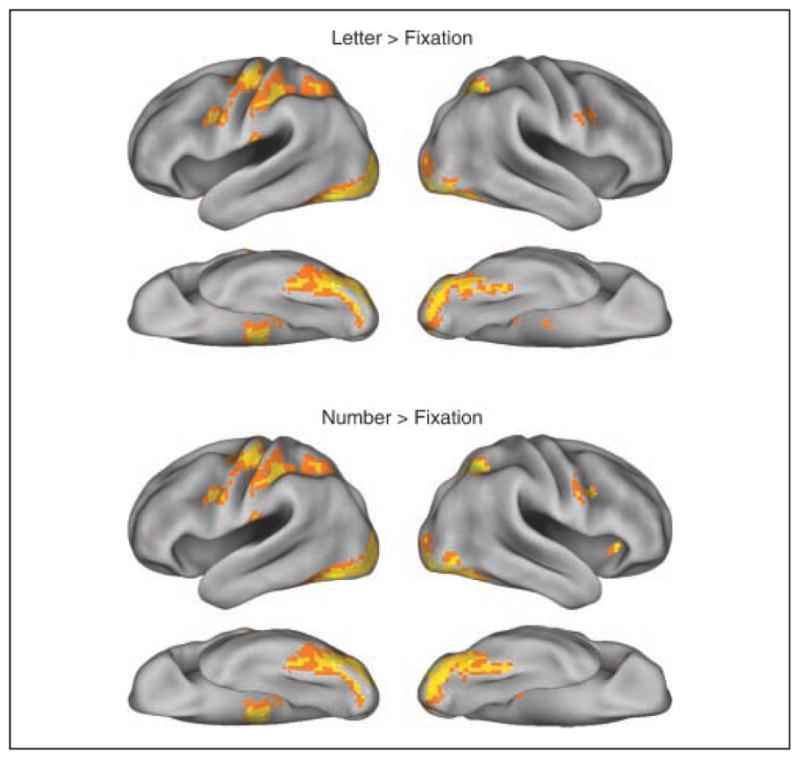
Group-level (n = 20) activation maps of letters and numbers relative to fixation, thresholded at p < .001 (uncorrected) with extent of greater than 20 voxels, overlaid on a 3-D inflated surface using Caret PALS atlas (brainvis.wustl.edu/wiki/index.php/Caret:About) for visualization.
Table 2.
Statistics on the Clusters of Letter-preferred Activation (Letter vs. Number) and Number-preferred Activation (Number vs. Letter) Surviving the Threshold of p < .005 and Extent of >20
| Z Score (t Score) | Coordinates (x y z in mm) | p (Cluster-level Corrected) | p (Voxelwise Uncorrected) | |
|---|---|---|---|---|
| Letter > Number | 4.60 (6.36) | −57 −28 28 | <0.001 | 2.11 × 10−6 |
| 4.12 (5.33) | −36 −37 −23 | 0.005 | 1.91 × 10−5 | |
| 3.81 (4.75) | 24 −43 58 | 0.328 | 0.005 | |
| 3.47 (4.16) | 51 −25 34 | 0.118 | 0.002 | |
| 3.32 (3.92) | 3 20 34 | 0.721 | 0.016 | |
| Number > Letter | 3.65 (4.47) | 48 −73 −2 | 0.010 | 7.12 × 10−5 |
The peak z scores and coordinates (in Montreal Neurological Institute space) are reported with the voxelwise uncorrected p value and cluster-level corrected p value.
Examination of the letter- and number-preferred activation at the individual subject level confirmed this overall pattern identified in the group-level results. With a relatively strict threshold for multiple comparisons correction (False Discovery Rate q < 0.05), 14 subjects exhibited a significant dissociation (either single or double) between letter and number recognition within the pre-specified anatomical ventral visual mask. Of the 14, seven showed significant activation for letter compared with number recognition, and all seven had more voxels activated in the left hemisphere than in the right hemisphere. Ten of the 14 subjects showed significant activation for number compared with letter recognition, and 9 of the 10 had more voxels activated in the right hemisphere. With a more lenient threshold (p < .001, uncorrected), all 20 subjects showed a significant dissociation (either single or double) between letter and number recognition within the anatomical mask. Seventeen subjects exhibited letter-preferred activation, and 14 of the 17 had more voxels activated in the left hemisphere than in the right hemisphere. Fifteen subjects exhibited number-preferred activation, and 12 of these subjects showed more voxels activated in the right hemisphere than in the left hemisphere. These results collectively suggest that the dissociation is observable on an individual level and that individuals typically show more activation in the left visual cortex for letters and more activation in the right visual cortex for numbers. More quantitative measures of laterality are reported below in the Laterality Analysis section.
Additionally, we examined the pattern of activation in response to other types of orthographic stimuli (i.e., pseudowords and real words). A 5-mm spherical ROI was constructed around the peak in the visual letter ROI [−36 −37 −23] and the peak in the visual number ROI [48 −73 2] and the average of the mean beta values across subjects within these spheres was compared across conditions (Figure 4). In the visual letter sphere, there was a linear increase in the beta values from consonant strings to pseudowords to real words (F(1, 19) = 16.006, p = .001, repeated measures ANOVA with linear contrast). This pattern of activation in the left ventral visual area is consistent with previous findings demonstrating hierarchical organization in the VWFA (Vinckier et al., 2007). In the visual number sphere, no such pattern was observed; this area showed greater BOLD response to numbers than to both pseudowords (t(19) = 5.858, p < .001, paired t test) and real words (t(19) = 7.431, p < .001, paired t test).1
Figure 4.
Average of the mean beta values within the 5-mm spherical ROI around the peaks in the visual letter area and the visual number area. The error bar denotes SEM across 20 subjects (WD = word, PW = pseudoword, L = letter, N = number).
Laterality Analysis
As can be seen in Figure 3, letter-preferred activity was left-lateralized whereas number-preferred activity was right lateralized at the group level. This pattern was quantitatively confirmed at the individual level by the laterality analysis in which letter-preferred activity was left-lateralized across subjects on average (LI = 0.715 ± 0.329, mean and standard deviation) and number-preferred activity was right-lateralized across subjects on average (LI = −0.544 ± 0.305). Positive LI represents left lateralization, and negative LI represents right lateralization. However, as indicated by the standard deviations, these LIs varied across subjects. The letter-preferred activity LI ranged from 0.451 to 0.987 (excluding one outlier who had a LI = −0.486; see Figure 7 subpanel), and the number-preferred activity LI ranged from −0.984 to 0.118. The letter activity LI and the number activity LI were not correlated (r = −0.124, p = .614), suggesting that laterality of letter and number recognition is likely to be independent.
Figure 7.
Co-lateralization of the visual number area LI and the parietal numerical area LI. The main panel shows the scatter plot between the visual number area LI and the parietal numerical area LI (r = 0.782, p < .001). The smaller subpanel shows how the visual letter area LI is related to the parietal numerical area LI (r = −0.315, p = .189). Positive LI represents left lateralization, and negative LI represents right lateralization. In the subpanel, one outlying subject was excluded from the calculation of the correlation coefficients.
Discussion
In Experiment 1, we confirmed a left-lateralized ventral visual area that responded significantly more to letters than numbers, replicating previous studies (Reinke, Fernandes, Schwindt, O’Craven, & Grady, 2008; Baker et al., 2007; James et al., 2005; Polk et al., 2002; Polk & Farah, 1998). More importantly, we also identified a right-lateralized ventral visual area that responded significantly more to numbers compared with letters. Together, these results constitute a double dissociation between letter and number recognition and provide perhaps the strongest evidence to date that letter and number recognition, at least in part, rely on different neural systems.
Although number-preferred activation was strongly right-lateralized in the group analysis, there were substantial individual differences in the laterality of individual participants. What factors might influence the lateralization of number recognition in the brain? One hypothesis is that lateralization depends on patterns of neural connectivity. For example, Cai et al. (2008) found that the laterality of ventral visual activity in response to visual word recognition correlated with the laterality of frontal activity for spoken language (which varied in left- and right-handers). They argued that this finding was because of the important functional connections between written and spoken language.
Following this reasoning, in Experiment 2, we investigated whether a similar mechanism might be at work in the lateralization of number recognition. Specifically, we tested the hypothesis that the lateralization of number recognition might be significantly correlated with the lateralization of higher-order numerical processing (addition, subtraction, and counting), which is known to depend on parietal cortex.
EXPERIMENT 2
Methods
Participants
The same 20 healthy young adults (ages 18–29 years) who participated in Experiment 1 also participated in Experiment 2 within the same fMRI session.
Stimulus Materials and Procedure
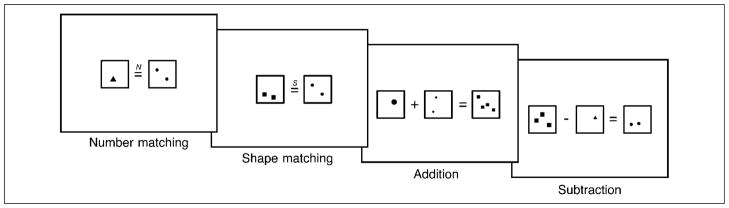
There were four types of stimuli used to perform different simple mathematical judgments (examples are shown in Figure 5). The number-matching stimuli were composed of two arrays—one on the left and the other on the right of an equal sign. The items within the two arrays varied in numerosity (from one to four) and shape (triangles, squares, or circles). Participants viewed each trial and were asked to make a numerosity judgment, determining whether the number of items in the left array matched the number on the right (regardless of shape). The same stimuli were used for the shape-matching blocks, and participants judged whether the left and right arrays contained the same shapes (regardless of numerosity). The only difference between shape and numerosity stimuli was that an “S” appeared above the equal sign for shape-matching trials and an “N” appeared above the equal sign for number-matching trial.
Figure 5.
Examples of stimuli used in Experiment 2. Participants performed simple numerical tasks on sets of dot arrays differing in numerosity and in shape. They judged whether the numerical operation was correct or not.
Similar stimuli elicited addition and subtraction operations from participants. In addition blocks, participants viewed stimuli consisting of three arrays. On the left were two arrays with a plus sign in the middle, followed by an equal sign and a third array on the right. Participants judged whether the numerosity of stimuli on the right of the equal sign was the same as the sum of the numerosities on the left. The subtraction blocks were similar, except that the plus sign was replaced with a minus sign and participants judged whether the numerosity of stimuli on the right was the same as the difference of the numerosities on the left.
This task consisted of five 5-min runs with twenty 16-sec blocks, pseudorandomly ordered (four for each of the four categories in addition to four fixation blocks). Each block was preceded by a 2-sec instruction screen in which the name of the task was displayed on the screen so that the participants could prepare for the upcoming block. The task was self-paced within each block, and the correct answer was “yes” in half of all the trials.
Data Acquisition
Functional scans were acquired as axial slices, with a faster acquisition (TR = 1.5 sec, TE = 25 msec) compared with Experiment 1. This resulted in 246 BOLD repetitions per run with 33 axial slices. Because of the narrowed slice range, cerebellums of some participants were not fully imaged. Other data acquisition parameters were identical to that of Experiment 1.
Activation Analysis
As in Experiment 1, functional images underwent slice-timing correction, realignment, coregistration, segmentation, and normalization as part of preprocessing. The functional images were then modeled using a general linear model as in Experiment 1, which included separate regressors for each of the experimental conditions convolved with a canonical hemodynamic response function as well as six nuisance covariates modeling head translation and rotation. An additional covariate was included to model the 2-sec instructions at the beginning of each experimental block. The contrast maps for numerical processing (addition, subtraction, and number matching vs. shape matching) from each subject were entered into a second-level random effects group analysis to estimate neural activity for numerical processing at the group level (see Figure 6).
Figure 6.
Neural activity for numerical processing in the whole brain. Group-level (n = 20) activation map of the addition + subtraction + number matching > shape matching contrast is displayed on a 3-D inflated surface for visualization as in Figure 2 (p < .001, cluster extent > 20 voxels).
Co-lateralization Analysis
To test the hypothesis of co-lateralization of the visual number activity with the parietal activity for numerical processing, we first defined a parietal ROI associated with numerical operations. Similar to the approach used in Experiment 1, we contrasted an aggregate of the three numerical operation conditions (addition, subtraction, and number matching) with the shape-matching condition at the group level (p < .05, extent > 20 voxels) within a bilateral mask of the superior and inferior parietal cortex. The area isolated from the contrast consisted of 617 voxels in left parietal cortex and 451 voxels in right parietal cortex. The ROI was then defined bilaterally to include the voxels in the opposite hemisphere by flip-ping the x coordinates. This bilateral ROI is referred to as the parietal numerical ROI.
In each individual subject, the t values associated with numerical processing (addition, subtraction, number matching vs. the shape-matching condition) in this parietal numerical ROI were used to compute the LI for that subject (see Methods in Experiment 1 for precise methods). The LI for this parietal numerical activity was then correlated with both the LI for the visual letter activity and the visual number activity.
Laterality Analysis in Individualized ROIs
To ensure that co-lateralization results are not an artifact of ROIs defined from the group map, co-lateralization analysis was also performed with neural activities within the ROIs defined at the level of individual subjects. In each subject, the visual letter ROI, visual number ROI, and the parietal numerical ROI were defined by each subject’s own letter-preferred, number-preferred, and numerical processing activation maps (using a lenient threshold of p < .05), respectively, within prespecified anatomical regions (identical to the anatomical masks used in the group-level ROI approach). Then, all the suprathreshold voxels and their opposite hemisphere homologues (flipping x coordinates) were identified as the individualized ROIs.
Results
Behavioral Results
Accuracy on the numerical tasks was above 90% for all conditions (Table 3). RTs differed across four conditions (F(1.23, 23.44) = 128.176, p < .001, repeated measured ANOVA with Greenhouse–Geisser correction). A contrast analysis showed faster RT for shape matching than number matching (F(1, 19) = 101.920, p < .001), faster RT for number matching than addition (F(1, 19) = 81.991, p < .001), and faster RT for addition than subtraction (F(1, 19) = 115.280, p < .001).
Table 3.
Behavioral Results of the Numerical Tasks Performed in the Scanner (n = 20)
| Number Matching | Shape Matching | Addition | Subtraction | |
|---|---|---|---|---|
| Accuracy (%) | 96.1 (2.94) | 95.5 (3.21) | 94.8 (3.97) | 92.6 (4.99) |
| Reaction time (msec) | 796.88 (94.87) | 684.02 (89.01) | 1054.88 (177.36) | 1252.62 (210.53) |
Mean accuracy and RT for the correct trials were measured for each subject, and the average (SD) of these scores across subjects are reported.
Co-lateralization Analysis
The primary purpose of Experiment 2 was to test whether lateralization of number recognition in ventral visual cortex is related to lateralization of higher-level numerical processing in parietal cortex. More specifically, we correlated the visual number LI (as well as the visual letter LI) from Experiment 1 with the parietal numerical LI from Experiment 2. First, we observed individual differences in the lateralization of higher-level numerical processing in parietal cortex. Across subjects, the parietal numerical LI ranged from −0.510 to 0.365 with a median of −0.184. Positive LI represents left lateralization and negative LI represents right lateralization. As hypothesized, the visual number LI, defined from the ROIs constructed at the group level, was significantly correlated with the parietal numerical LI (r = 0.782, p < .001; Figure 7). That is, subjects who exhibited greater right laterality for visual number processing in the ventral visual cortex exhibited greater right laterality for numerical processing in the parietal area. On the other hand, the visual letter LI showed nonsignificant correlation with the parietal numerical area LI (r = −0.315, p = .189). The results were qualitatively the same when the LIs were computed from the ROIs constructed at the individual level: the parietal numerical LI, ranging from −0.489 to 0.293 with a median of −0.139, was positively correlated with the visual number LI (r = 0.439, p = .053) but not with the visual letter LI (r = −0.033, p = .892).
One might speculate that visual number activity and parietal numerical activity are primarily right-lateralized in which case variations in the neural activities in the right hemisphere alone could produce such co-lateralization results. However, further analysis showed that there was negligible correlation between the LI measure and the associated QR measure in the visual number ROI (r = 0.051, p = .830) and the parietal numerical ROI (r = −0.131, p = .580), suggesting that the variance in LI measures cannot be explained solely by the amount of activity in the right hemisphere. Moreover, QR in the visual number LI calculation showed no correlation with QR in the parietal numerical LI calculation (r = 0.093, p = .695), suggesting that the variability in the right hemisphere activity alone does not explain the present co-lateralization results. Additionally, the visual number LI and the parietal numerical LI were still correlated with each other after controlling for the linear effect of RT (r = 0.704, p = .001), suggesting that the present co-lateralization results still hold even with RT effects excluded.
Note that the parietal activity was first defined by contrasting neural activity for all numerical tasks (addition, subtraction, and number matching) versus a control task (shape matching). We tested if the co-lateralization of the visual number activity and the parietal numerical activity would hold true for each of the numerical tasks. That is, within the bilateral ROI the laterality of each individual’s parietal activity was computed from other contrasts, namely, addition versus shape matching, subtraction versus shape matching, and number matching versus shape matching.
The mean (± standard deviation) parietal LIs from the number matching, addition, and subtraction conditions were −0.316 (± 0.291), −0.122 (± 0.190), and −0.029 (± 0.297), respectively. A within-subject ANOVA indicated a significant linear effect of LIs across the three conditions (F(1, 19) = 15.286, p = .001), showing more right-lateralized activity for number matching than addition or subtraction. However, these LIs were moderately correlated with each other across individuals (correlation between addition LI and subtraction LI, r = 0.662, p = .001; correlation between addition LI and number matching LI, r = 0.453, p = .044; correlation between addition LI and subtraction LI, r = 0.382, p = .096). More importantly, the visual number LI showed strong positive correlations with all of the parietal activity LIs (addition vs. shape matching LI, r = 0.680, p < .001; subtraction vs. shape matching LI, r = 0.712, p < .001; number matching vs. shape matching LI, r = 0.420, p = .065). The results were qualitatively the same when the LIs were computed within individually defined ROIs (addition vs. shape matching LI, r = 0.622, p < .001; subtraction vs. shape matching LI, r = 0.589, p = .010; number matching vs. shape matching LI, r = 0.481, p = .038).
Discussion
In Experiment 2, we asked whether individual differences in the laterality of visual number activity could be explained by individual differences in the laterality of parietal numerical activity. We found that they could. The co-lateralization analysis showed that subjects with more right-lateralized visual number-preferred activity had more right-lateralized parietal numerical activity. These results cannot be explained by task-independent hemispheric dominance because the visual letter LI was not correlated with the parietal numerical LI nor by the variability in right-hemisphere activity alone. Moreover, the co-lateralization results still held when the effects of RT were removed. We also verified that the results held true for parietal activity defined from different contrasts (addition, subtraction, or counting), suggesting that the co-lateralization of the visual number activity and the parietal numerical activity generalizes across numerical processes.
We found that numerical activity in both parietal and ventral visual cortex was somewhat right-lateralized in most participants, but some previous studies have reported left-lateralized activity for some numerical tasks. One potential explanation for this discrepancy is based on the triple code model of number processing (Dehaene & Cohen, 1995; Dehaene, 1992). According to that model, numerical tasks that make high demands on verbal processes, such as retrieving memorized multiplication tables, elicit activation primarily in left perisylvian areas (e.g., Prado et al., 2011; Chochon, Cohen, van de Moortele, & Dehaene, 1999). On the other hand, tasks that put more demands on analog magnitude representations, such as approximate arithmetic, subtraction, number comparison, or even passive adaptation, rely more on bilateral parietal cortex (Prado et al., 2011; Eger et al., 2009; Piazza, Izard, Pinel, Le Bihan, & Dehaene, 2004; Chochon et al., 1999; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999). Our task was designed to make few demands on verbal fact retrieval. Although our task involved addition and subtraction, we reasoned that calculation of nonsymbolic numbers in small ranges relies more on analog magnitude representations. These kinds of tasks can be performed by nonhuman primates (Hauser, MacNeilage, & Ware, 1996; Rumbaugh, Savagerumbaugh, & Hegel, 1987), preverbal infants (Wynn, 1992), preschool children (Barth, La Mont, Lipton, & Spelke, 2005), brain-damaged patients with impaired exact calculation (Lemer, Dehaene, Spelke, & Cohen, 2003), and adults in cultures without large number words (Pica, Lemer, Izard, & Dehaene, 2004) and, therefore, presumably do not rely on verbal processes. Therefore, the results of our experiment should be considered in the context of other studies that report more bilateral or even right-lateralized (see e.g., Prado et al., 2011; Chochon et al., 1999) activations for number processing.
GENERAL DISCUSSION
In this study, we investigated the neural representation underlying the visual recognition of letters and numbers by directly contrasting neural activation patterns elicited by letters and numbers. In Experiment 1, we found letter-preferred activity in the left occipito-temporal cortex and number-preferred activity in the right occipito-temporal cortex at the group level (Figure 3). In Experiment 2, we demonstrated that individual differences in the cerebral lateralization of number-preferred activity in visual cortex could be explained by individual differences in the lateralization of numerical processing in parietal cortex (Figure 7).
Left-lateralized letter-preferred activity in ventral visual cortex is consistent with reports showing robust and reproducible neural activation in left occipito-temporal cortex in response to words and letters (Polk et al., 2002; Cohen et al., 2000; Polk & Farah, 1998; Puce et al., 1996; Petersen, Fox, Snyder, & Raichle, 1990). What is more novel in the results from Experiment 1 is the neural dissociation of numbers from letters: The number versus letter contrast produced significant activation in right lateral occipital cortex at the group level.
The identification of this neural double dissociation and the right-lateralized number-preferred activity are important for at least three reasons. First, the double dissociation rules out alternative explanations that assume that the observed letter-preferred or number-preferred neural activity is an artifact of difficulty or effort. Second, the neural dissociation between letters and numbers is consistent with previously reported behavioral double dissociations (Hamilton et al., 2006; Jonides & Gleitman, 1972), providing further evidence of experience-dependent changes in the neural architecture underlying visual recognition. Third, the fact that number-preferred activity was localized in the right occipito-temporal region is problematic for the “interhemispheric differences hypothesis,” which assumes that letter and word recognition is localized in the left hemisphere because of that hemisphere’s superiority in processing fine-grained and local visual features (see Robertson & Lamb, 1991, for a review). That hypothesis would predict that numbers should also be processed primarily in the left hemisphere given that they also involve processing fine-grained and local visual features.
We also observed individual differences in the lateralization of neural representations for visual and numerical processing, which is a topic that has not yet received much attention. A few studies have reported different patterns of functional cerebral asymmetries between right- and left-handed subjects in the domain of vision. For example, as described in the Introduction, Cai et al. (2008) studied the relationship between cerebral lateralization of VWFA and anterior language processing sites in right- and left-handed subjects. A recent neuroimaging study has also reported that cerebral lateralization for the fusiform face area and the extrastriate body area was coupled with handedness (Willems, Peelen, & Hagoort, 2010). Of course, all of our participants were right-handed, so the individual differences in lateralization that we observed cannot be attributed to differences in handedness.
The visual processing of numbers has also received relatively little attention in the literature. Besides a previous study by Polk et al. (2002), Arabic digits have typically been used as control stimuli when looking for letter- and word-specific neural activity (Reinke et al., 2008; Baker et al., 2007; James et al., 2005) or have been compared with verbal numerals when looking for notation effects in number processing (Pinel, Dehaene, Riviere, & LeBihan, 2001; Pinel et al., 1999; Dehaene, 1996). Here, we extended the work by Polk et al. (2002) and found significant neural activation in response to numbers compared with letters in right visual cortex.
The observed neural dissociation between letters and numbers is noteworthy given that the distinction between letters and numbers is culturally defined and in some sense arbitrary. How might such a dissociation emerge? Polk and Farah (1995, 1998) proposed a bottom–up model on the basis of a co-occurrence hypothesis. According to this model, letter and number recognition become differentiated because letters tend to co-occur with letters (and numbers with numbers) in the environment. Correlation-based learning mechanisms in the brain are assumed to pick up on these co-occurrence patterns to lead to neural segregation (Polk & Farah, 1995). Although this purely bottom–up hypothesis is plausible for the neural dissociation of letters and numbers, it does not predict why the VWFA consistently forms in the left hemisphere rather than the right (or why number recognition tends to be right-lateralized).
According to the neuronal recycling hypothesis (Dehaene & Cohen, 2007), one of the reasons that VWFA tends to develop in the left occipito-temporal cortex is because this area has relatively direct connections to and from anterior language processing sites in the left hemisphere (McCandliss et al., 2003). Consistent with this view, the pattern of activation in VWFA is closely related to components of language-related functions. For instance, it is invariant to letter case (Dehaene et al., 2004; Polk & Farah, 2002; Dehaene et al., 2001) and is greater when the orthographic stimuli are familiar to subjects than when they are unfamiliar (i.e., Hebrew to Hebrew readers vs. to non-Hebrew readers; Baker et al., 2007). Activation in VWFA is hierarchically organized so that the strength of activation increases with orthographic regularities (Vinckier et al., 2007) and bigram frequency (Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006). As noted, it has been shown that VWFA is right-lateralized in a subsample of left-handed subjects who showed right-lateralized language sites (Cai et al., 2008).
Our findings support another aspect of this hypothesis in the numerical cognition framework. We found that individual differences in the lateralization of numerical processing in the parietal cortex predicted the lateralization of the visual Arabic number form processing in visual cortex. These results are consistent with the hypothesis that top–down influences from parietal numerical activity play an important role in the neural localization of number recognition in ventral visual cortex.2
To conclude, the current findings demonstrate a neural double dissociation between letter and number recognition and suggest that top–down influences play an important role in experience-dependent neural reorganization.
Acknowledgments
This study was supported by grant 3R37AG006265 from the National Institute on Aging to D. C. P.
Footnotes
Responses to false fonts in these ROIs were also examined. Like the previous report (Vinckier et al., 2007), false fonts activated more than letters and numbers in both the posterior letter area (t(19) = 6.558, p < .001) and the posterior number area (t(19) = 6.662, p < .001), perhaps because they were more demanding and required significantly more time to process.
An alternative explanation is that right-lateralized activity in ventral visual cortex influences the laterality of parietal activity for higher-order number processing (or that a third factor influences the lateralization in both ventral visual and parietal cortex). Given that the processing of numbers may be evolutionarily older than reading (Butterworth, Reeve, Reynolds, & Lloyd, 2008; Pica et al., 2004; Brannon & Terrace, 1998; Gallistel & Gelman, 1992) and that numerical competence develops before recognizing symbols for numbers (Gebuis, Herfs, Kenemans, de Haan, & van der Smagt, 2009; Xu & Spelke, 2000; Wynn, 1992), we prefer the hypothesis that parietal organization influences ventral visual processes top–down. Our data do not rule out the alternatives however.
References
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio AR, Damasio H. Troubled letters but not numbers—Domain specific cognitive impairments following focal damage in frontal-cortex. Brain. 1990;113:749–766. doi: 10.1093/brain/113.3.749. [DOI] [PubMed] [Google Scholar]
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proceedings of the National Academy of Sciences, USA. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth H, La Mont K, Lipton J, Spelke ES. Abstract number and arithmetic in preschool children. Proceedings of the National Academy of Sciences, USA. 2005;102:14116–14121. doi: 10.1073/pnas.0505512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Terrace HS. Ordering of the numerosities 1 to 9 by monkeys. Science. 1998;282:746–749. doi: 10.1126/science.282.5389.746. [DOI] [PubMed] [Google Scholar]
- Butterworth B, Reeve R, Reynolds F, Lloyd D. Numerical thought with and without words: Evidence from indigenous Australian children. Proceedings of the National Academy of Sciences, USA. 2008;105:13179–13184. doi: 10.1073/pnas.0806045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Lavidor M, Brysbaert M, Paulignan Y, Nazir TA. Cerebral lateralization of frontal lobe language processes and lateralization of the posterior visual word processing system. Journal of Cognitive Neuroscience. 2008;20:672–681. doi: 10.1162/jocn.2008.20043. [DOI] [PubMed] [Google Scholar]
- Chochon F, Cohen L, van de Moortele PF, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Warrington EK, Butterworth B. Selective impairment in manipulating Arabic numerals. Cortex. 1995;31:73–86. doi: 10.1016/s0010-9452(13)80106-2. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, et al. The visual word form area—Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Varieties of numerical abilities. Cognition. 1992;44:1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The organization of brain activations in number comparison: Event-related potentials and the additive-factors method. Journal of Cognitive Neuroscience. 1996;8:47–68. doi: 10.1162/jocn.1996.8.1.47. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Towards an anatomical and functional model of number processing. Mathematical Cognition. 1995;1:83–120. [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, et al. Letter binding and invariant recognition of masked words - Behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin JF, Poline JB, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Eger E, Michel V, Thirion B, Amadon A, Dehaene S, Kleinschmidt A. Deciphering cortical number coding from human brain activity patterns. Current Biology. 2009;19:1608–1615. doi: 10.1016/j.cub.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, et al. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21:829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gelman R. Preverbal and verbal counting and computation. Cognition. 1992;44:43–74. doi: 10.1016/0010-0277(92)90050-r. [DOI] [PubMed] [Google Scholar]
- Gebuis T, Herfs IK, Kenemans JL, de Haan EHF, van der Smagt MJ. The development of automated access to symbolic and nonsymbolic number knowledge in children: An ERP study. European Journal of Neuroscience. 2009;30:1999–2008. doi: 10.1111/j.1460-9568.2009.06994.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Mirkin M, Polk TA. Category-level contributions to the alphanumeric category effect in visual search. Psychonomic Bulletin & Review. 2006;13:1074–1077. doi: 10.3758/bf03213928. [DOI] [PubMed] [Google Scholar]
- Hauser MD, MacNeilage P, Ware M. Numerical representations in primates. Proceedings of the National Academy of Sciences, USA. 1996;93:1514–1517. doi: 10.1073/pnas.93.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles JL, Eskes GA. A comparison of letter and digit processing in letter-by-letter reading. Journal of the International Neuropsychological Society. 2008;14:164–173. doi: 10.1017/S1355617708080119. [DOI] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong ACN, Gauthier I. Letter processing in the visual system: Different activation patterns for single letters and strings. Cognitive Affective & Behavioral Neuroscience. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jonides J, Gleitman H. Conceptual category effect in visual search—O as letter or as digit. Perception & Psychophysics. 1972;12:457–460. [Google Scholar]
- Joseph JE, Cerullo MA, Farley AB, Steinmetz NA, Mier CR. fMRI correlates of cortical specialization and generalization for letter processing. Neuroimage. 2006;32:806–820. doi: 10.1016/j.neuroimage.2006.04.175. [DOI] [PubMed] [Google Scholar]
- Lemer C, Dehaene S, Spelke E, Cohen L. Approximate quantities and exact number words: Dissociable systems. Neuropsychologia. 2003;41:1942–1958. doi: 10.1016/s0028-3932(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Nasti M, Zorzi M. Selective impairment for reading numbers and number words: A single case study. Neuropsychologia. 2004;42:997–1006. doi: 10.1016/j.neuropsychologia.2004.01.004. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An on-line orthographic database of the English language. 2005 Retrieved from www.neuro.mcw.edu/mcword/ on 5 September 2008.
- Pernet C, Celsis P, Demonet JF. Selective response to letter categorization within the left fusiform gyrus. Neuroimage. 2005;28:738–744. doi: 10.1016/j.neuroimage.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Pica P, Lemer C, Izard W, Dehaene S. Exact and approximate arithmetic in an Amazonian indigene group. Science. 2004;306:499–503. doi: 10.1126/science.1102085. [DOI] [PubMed] [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Pinel P, Le Clec’H G, van de Moortele PF, Naccache L, Le Bihan D, Dehaene S. Event-related fMRI analysis of the cerebral circuit for number comparison. NeuroReport. 1999;10:1473–1479. doi: 10.1097/00001756-199905140-00015. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Brain localization for arbitrary stimulus categories: A simple account based on Hebbian learning. Proceedings of the National Academy of Sciences, USA. 1995;92:12370–12373. doi: 10.1073/pnas.92.26.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. The neural development and organization of letter recognition: Evidence from functional neuroimaging, computational modeling, and behavioral studies. Proceedings of the National Academy of Sciences, USA. 1998;95:847–852. doi: 10.1073/pnas.95.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah ML. Functional MRI evidence for an abstract, not perceptual, word-form area. Journal of Experimental Psychology: General. 2002;131:65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D’Esposito M, Detre JA, et al. Neural specialization for letter recognition. Journal of Cognitive Neuroscience. 2002;14:145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Prado J, Mutreja R, Zhang H, Mehta R, Desroches AS, Minas JE, et al. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Human Brain Mapping. 2011;32:1932–1947. doi: 10.1002/hbm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke K, Fernandes M, Schwindt G, O’Craven K, Grady CL. Functional specificity of the visual word form area: General activation for words and symbols but specific network activation for words. Brain and Language. 2008;104:180–189. doi: 10.1016/j.bandl.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part whole organization. Cognitive Psychology. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Roux FE, Lubrano V, Lauwers-Cances V, Giussani C, Demonet JF. Cortical areas involved in Arabic number reading. Neurology. 2008;70:210–217. doi: 10.1212/01.wnl.0000297194.14452.a0. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM, Savagerumbaugh S, Hegel MT. Summation in the chimpanzee (Pan Troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:107–115. [PubMed] [Google Scholar]
- Shallice T. From neuropsychology to mental structure. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Starrfelt R. Selective alexia and agraphia sparing numbers—A case study. Brain and Language. 2007;102:52–63. doi: 10.1016/j.bandl.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Suarez RO, Whalen S, Nelson AP, Tie Y, Meadows ME, Radmanesh A, et al. Threshold-independent functional MRI determination of language dominance: A validation study against clinical gold standards. Epilepsy & Behavior. 2009;16:288–297. doi: 10.1016/j.yebeh.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Willems RM, Peelen MV, Hagoort P. Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cerebral Cortex. 2010;20:1719–1725. doi: 10.1093/cercor/bhp234. [DOI] [PubMed] [Google Scholar]
- Wong AC, Gauthier I, Woroch B, DeBuse C, Curran T. An early electrophysiological response associated with expertise in letter perception. Cognitive, Affective & Behavioral Neuroscience. 2005;5:306–318. doi: 10.3758/cabn.5.3.306. [DOI] [PubMed] [Google Scholar]
- Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–750. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]