Glycemic management in type 2 diabetes mellitus has become increasingly complex and, to some extent, controversial, with a widening array of pharmacological agents now available (1–5), mounting concerns about their potential adverse effects and new uncertainties regarding the benefits of intensive glycemic control on macrovascular complications (6–9). Many clinicians are therefore perplexed as to the optimal strategies for their patients. As a consequence, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) convened a joint task force to examine the evidence and develop recommendations for antihyperglycemic therapy in nonpregnant adults with type 2 diabetes. Several guideline documents have been developed by members of these two organizations (10) and by other societies and federations (2,11–15). However, an update was deemed necessary because of contemporary information on the benefits/risks of glycemic control, recent evidence concerning efficacy and safety of several new drug classes (16,17), the withdrawal/restriction of others, and increasing calls for a move toward more patient-centered care (18,19).
This statement has been written incorporating the best available evidence and, where solid support does not exist, using the experience and insight of the writing group, incorporating an extensive review by additional experts (acknowledged below). The document refers to glycemic control; yet this clearly needs to be pursued within a multifactorial risk reduction framework. This stems from the fact that patients with type 2 diabetes are at increased risk of cardiovascular morbidity and mortality; the aggressive management of cardiovascular risk factors (blood pressure and lipid therapy, antiplatelet treatment, and smoking cessation) is likely to have even greater benefits.
These recommendations should be considered within the context of the needs, preferences, and tolerances of each patient; individualization of treatment is the cornerstone of success. Our recommendations are less prescriptive than and not as algorithmic as prior guidelines. This follows from the general lack of comparative-effectiveness research in this area. Our intent is therefore to encourage an appreciation of the variable and progressive nature of type 2 diabetes, the specific role of each drug, the patient and disease factors that drive clinical decision making (20–23), and the constraints imposed by age and comorbidity (4,6). The implementation of these guidelines will require thoughtful clinicians to integrate current evidence with other constraints and imperatives in the context of patient-specific factors.
PATIENT-CENTERED APPROACH
Evidence-based advice depends on the existence of primary source evidence. This emerges only from clinical trial results in highly selected patients, using limited strategies. It does not address the range of choices available, or the order of use of additional therapies. Even if such evidence were available, the data would show median responses and not address the vital question of who responded to which therapy and why (24). Patient-centered care is defined as an approach to “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions” (25). This should be the organizing principle underlying health care for individuals with any chronic disease, but given our uncertainties in terms of choice or sequence of therapy, it is particularly appropriate in type 2 diabetes. Ultimately, it is patients who make the final decisions regarding their lifestyle choices and, to some degree, the pharmaceutical interventions they use; their implementation occurs in the context of the patients’ real lives and relies on the consumption of resources (both public and private).
Patient involvement in the medical decision making constitutes one of the core principles of evidence-based medicine, which mandates the synthesis of best available evidence from the literature with the clinician's expertise and patient's own inclinations (26). During the clinical encounter, the patient's preferred level of involvement should be gauged and therapeutic choices explored, potentially with the utilization of decision aids (21). In a shared decision-making approach, clinician and patient act as partners, mutually exchanging information and deliberating on options, in order to reach a consensus on the therapeutic course of action (27). There is good evidence supporting the effectiveness of this approach (28). Importantly, engaging patients in health care decisions may enhance adherence to therapy.
BACKGROUND
Epidemiology and health care impact
Both the prevalence and incidence of type 2 diabetes are increasing worldwide, particularly in developing countries, in conjunction with increased obesity rates and westernization of lifestyle. The attendant economic burden for health care systems is skyrocketing, owing to the costs associated with treatment and diabetes complications. Type 2 diabetes remains a leading cause of cardiovascular disorders, blindness, end-stage renal failure, amputations, and hospitalizations. It is also associated with increased risk of cancer, serious psychiatric illness, cognitive decline, chronic liver disease, accelerated arthritis, and other disabling or deadly conditions. Effective management strategies are of obvious importance.
Relationship of glycemic control to outcomes
It is well established that the risk of microvascular and macrovascular complications is related to glycemia, as measured by HbA1c; this remains a major focus of therapy (29). Prospective randomized trials have documented reduced rates of microvascular complications in type 2 diabetic patients treated to lower glycemic targets. In the UK Prospective Diabetes Study (UKPDS) (30,31), patients with newly diagnosed type 2 diabetes were randomized to two treatment policies. In the standard group, lifestyle intervention was the mainstay with pharmacological therapy used only if hyperglycemia became severe. In the more intensive treatment arm, patients were randomly assigned to either a sulfonylurea or insulin, with a subset of overweight patients randomized to metformin. The overall HbA1c achieved was 0.9% lower in the intensive policy group compared with the conventional policy arm (7.0% vs. 7.9%). Associated with this difference in glycemic control was a reduction in the risk of microvascular complications (retinopathy, nephropathy, neuropathy) with intensive therapy. A trend toward reduced rates of myocardial infarction in this group did not reach statistical significance (30). By contrast, substantially fewer metformin-treated patients experienced myocardial infarction, diabetes-related and all-cause mortality (32), despite a mean HbA1c only 0.6% lower than the conventional policy group. The UKPDS 10-year follow-up demonstrated that the relative benefit of having been in the intensive management policy group was maintained over a decade, resulting in the emergence of statistically significant benefits on cardiovascular disease (CVD) end points and total mortality in those initially assigned to sulfonylurea/insulin, and persistence of CVD benefits with metformin (33), in spite of the fact that the mean HbA1c levels between the groups converged soon after the randomized component of the trial had concluded.
In 2008, three shorter-term studies [Action to Control Cardiovascular Risk in Diabetes (ACCORD) (34), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) (35), Veterans Affairs Diabetes Trial (VADT) (36)] reported the effects of two levels of glycemic control on cardiovascular end points in middle-aged and older individuals with well-established type 2 diabetes at high risk for cardiovascular events. ACCORD and VADT aimed for an HbA1c <6.0% using complex combinations of oral agents and insulin. ADVANCE aimed for an HbA1c ≤6.5% using a less intensive approach based on the sulfonylurea gliclazide. None of the trials demonstrated a statistically significant reduction in the primary combined cardiovascular end points. Indeed, in ACCORD, a 22% increase in total mortality with intensive therapy was observed, mainly driven by cardiovascular mortality. An explanation for this finding has remained elusive, although rates of hypoglycemia were threefold higher with intensive treatment. It remains unclear, however, if hypoglycemia was responsible for the adverse outcomes, or if other factors, such as more weight gain, or simply the greater complexity of therapy, contributed. There were suggestions in these trials that patients without overt CVD, with shorter duration of disease, and lower baseline HbA1c, benefited from the more intensive strategies. Modest improvements in some microvascular end points in the studies were likewise demonstrated. Finally, a meta-analysis of cardiovascular outcomes in these trials suggested that every HbA1c reduction of ∼1% may be associated with a 15% relative risk reduction in nonfatal myocardial infarction, but without benefits on stroke or all-cause mortality (36).
Overview of the pathogenesis of type 2 diabetes
Any rise in glycemia is the net result of glucose influx exceeding glucose outflow from the plasma compartment. In the fasting state, hyperglycemia is directly related to increased hepatic glucose production. In the postprandial state, further glucose excursions result from the combination of insufficient suppression of this glucose output and defective insulin stimulation of glucose disposal in target tissues, mainly skeletal muscle. Once the renal tubular transport maximum for glucose is exceeded, glycosuria curbs, though does not prevent, further hyperglycemia.
Abnormal islet cell function is a key and requisite feature of type 2 diabetes. In early disease stages, insulin production is normal or increased in absolute terms, but disproportionately low for the degree of insulin sensitivity, which is typically reduced. However, insulin kinetics, such as the ability of the pancreatic β-cell to release adequate hormone in phase with rising glycemia, are profoundly compromised. This functional islet incompetence is the main quantitative determinant of hyperglycemia (37) and progresses over time. In addition, in type 2 diabetes, pancreatic α-cells hypersecrete glucagon, further promoting hepatic glucose production (38). Importantly, islet dysfunction is not necessarily irreversible. Enhancing insulin action relieves β-cell secretory burden, and any intervention that improves glycemia—from energy restriction to, most strikingly, bariatric surgery—can ameliorate β-cell dysfunction to an extent (39). More recently recognized abnormalities in the incretin system (represented by the gut hormones, glucagon-like peptide 1 [GLP-1], and glucose-dependent insulinotropic peptide [GIP]) are also found in type 2 diabetes, but it remains unclear whether these constitute primary or secondary defects (40). In most patients with type 2 diabetes, especially the obese, insulin resistance in target tissues (liver, muscle, adipose tissue, myocardium) is a prominent feature. This results in both glucose overproduction and underutilization. Moreover, an increased delivery of fatty acids to the liver favors their oxidation, which contributes to increased gluconeogenesis, whereas the absolute overabundance of lipids promotes hepatosteatosis (41).
Antihyperglycemic agents are directed at one or more of the pathophysiological defects of type 2 diabetes, or modify physiological processes relating to appetite or to nutrient absorption or excretion. Ultimately, type 2 diabetes is a disease that is heterogeneous in both pathogenesis and in clinical manifestation—a point to be considered when determining the optimal therapeutic strategy for individual patients.
ANTIHYPERGLYCEMIC THERAPY
Glycemic targets
The ADA's “Standards of Medical Care in Diabetes” recommends lowering HbA1c to <7.0% in most patients to reduce the incidence of microvascular disease (42). This can be achieved with a mean plasma glucose of ∼8.3–8.9 mmol/L (∼150–160 mg/dL); ideally, fasting and premeal glucose should be maintained at <7.2 mmol/L (<130 mg/dL) and the postprandial glucose at <10 mmol/L (<180 mg/dL). More stringent HbA1c targets (e.g., 6.0–6.5%) might be considered in selected patients (with short disease duration, long life expectancy, no significant CVD) if this can be achieved without significant hypoglycemia or other adverse effects of treatment (20,43). Conversely, less stringent HbA1c goals—e.g., 7.5–8.0% or even slightly higher—are appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced complications, extensive comorbid conditions and those in whom the target is difficult to attain despite intensive self-management education, repeated counseling, and effective doses of multiple glucose-lowering agents, including insulin (20,44).
The accumulated results from the aforementioned type 2 diabetes cardiovascular trials suggest that not everyone benefits from aggressive glucose management. It follows that it is important to individualize treatment targets (5,34–36). The elements that may guide the clinician in choosing an HbA1c target for a specific patient are shown in Fig. 1. As mentioned earlier, the desires and values of the patient should also be considered, since the achievement of any degree of glucose control requires active participation and commitment (19,23,45,46). Indeed, any target could reflect an agreement between patient and clinician. An important related concept is that the ease with which more intensive targets are reached influences treatment decisions; logically, lower targets are attractive if they can be achieved with less complex regimens and no or minimal adverse effects. Importantly, utilizing the percentage of diabetic patients who are achieving an HbA1c <7.0% as a quality indicator, as promulgated by various health care organizations, is inconsistent with the emphasis on individualization of treatment goals.
Figure 1.
Depiction of the elements of decision making used to determine appropriate efforts to achieve glycemic targets. Greater concerns about a particular domain are represented by increasing height of the ramp. Thus, characteristics/predicaments toward the left justify more stringent efforts to lower HbA1c, whereas those toward the right are compatible with less stringent efforts. Where possible, such decisions should be made in conjunction with the patient, reflecting his or her preferences, needs, and values. This “scale” is not designed to be applied rigidly but to be used as a broad construct to help guide clinical decisions. Adapted with permission from Ismail-Beigi et al. (20).
Therapeutic options
Lifestyle.
Interventions designed to impact an individual's physical activity levels and food intake are critical parts of type 2 diabetes management (47,48). All patients should receive standardized general diabetes education (individual or group, preferably using an approved curriculum), with a specific focus on dietary interventions and the importance of increasing physical activity. While encouraging therapeutic lifestyle change is important at diagnosis, periodic counseling should also be integrated into the treatment program.
Weight reduction, achieved through dietary means alone or with adjunctive medical or surgical intervention, improves glycemic control and other cardiovascular risk factors. Modest weight loss (5–10%) contributes meaningfully to achieving improved glucose control. Accordingly, establishing a goal of weight reduction, or at least weight maintenance, is recommended.
Dietary advice must be personalized (49). Patients should be encouraged to eat healthy foods that are consistent with the prevailing population-wide dietary recommendations and with an individual's preferences and culture. Foods high in fiber (such as vegetables, fruits, whole grains, and legumes), low-fat dairy products, and fresh fish should be emphasized. High-energy foods, including those rich in saturated fats, and sweet desserts and snacks should be eaten less frequently and in lower amounts (50–52). Patients who eventually lose and keep weight off usually do so after numerous cycles of weight loss and relapse. The health care team should remain nonjudgmental but persistent, revisiting and encouraging therapeutic lifestyle changes frequently, if needed.
As much physical activity as possible should be promoted, ideally aiming for at least 150 min/week of moderate activity including aerobic, resistance, and flexibility training (53). In older individuals, or those with mobility challenges, so long as tolerated from a cardiovascular standpoint, any increase in activity level is advantageous.
At diagnosis, highly motivated patients with HbA1c already near target (e.g., <7.5%) could be given the opportunity to engage in lifestyle change for a period of 3–6 months before embarking on pharmacotherapy (usually metformin). Those with moderate hyperglycemia or in whom lifestyle changes are anticipated to be unsuccessful should be promptly started on an antihyperglycemic agent (also usually metformin) at diagnosis, which can later be modified or possibly discontinued if lifestyle changes are successful.
Oral agents and noninsulin injectables.
Important properties of antihyperglycemic agents that play a role in the choice of drug(s) in individual patients are summarized in Table 1. Ultimately, the aims of controlling glycemia are to avoid acute osmotic symptoms of hyperglycemia, to avoid instability in blood glucose over time, and to prevent/delay the development of diabetes complications without adversely affecting quality of life. Information on whether specific agents have this ability is incomplete; an answer to these questions requires long-term, large-scale clinical trials—not available for most drugs. Effects on surrogate measures for glycemic control (e.g., HbA1c) generally reflect changes in the probability of developing microvascular disease but not necessarily macrovascular complications. Particularly from a patient standpoint, stability of metabolic control over time may be another specific goal.
Table 1.
Properties of currently available glucose-lowering agents that may guide treatment choice in individual patients with type 2 diabetes mellitus
Metformin, a biguanide, remains the most widely used first-line type 2 diabetes drug; its mechanism of action predominately involves reducing hepatic glucose production (54,55). It is generally considered weight-neutral with chronic use and does not increase the risk of hypoglycemia. Metformin is associated with initial gastrointestinal side effects, and caution is advised to avoid its use in patients at risk for lactic acidosis (e.g., in advanced renal insufficiency, alcoholism), a rare complication of therapy. As noted earlier, there may be some cardiovascular benefits from this drug, but the clinical trial data are not robust.
The oldest oral agent class is the sulfonylurea insulin secretagogues. Through the closure of ATP-sensitive potassium channels on β-cells, these drugs stimulate insulin release (56). While effective in controlling glucose levels, their use is associated with modest weight gain and risk of hypoglycemia. In addition, studies have demonstrated a secondary failure rate that may exceed other drugs, ascribed to an exacerbation of islet dysfunction (57). Shorter-acting secretagogues, the meglitinides (or glinides), stimulate insulin release through similar mechanisms but may be associated with less hypoglycemia (58). They require more frequent dosing, however.
Thiazolidinediones (TZDs) are peroxisome proliferator–activated receptor γ activators (59) that improve insulin sensitivity in skeletal muscle and reduce hepatic glucose production (54,55). They do not increase the risk of hypoglycemia and may be more durable in their effectiveness than sulfonylureas and metformin (57). Pioglitazone appeared to have a modest benefit on cardiovascular events as a secondary outcome in one large trial involving patients with overt macrovascular disease (60). Another agent of this class, rosiglitazone, is no longer widely available owing to concerns of increased myocardial infarction risk (61). Pioglitazone has recently been associated with a possible increased risk of bladder cancer (62). Recognized side effects of TZDs include weight gain, fluid retention leading to edema and/or heart failure in predisposed individuals, and increased risk of bone fractures (57,60).
Drugs focused on the incretin system have been introduced more recently (63). The injectable GLP-1 receptor agonists mimic the effects of endogenous GLP-1, thereby stimulating pancreatic insulin secretion in a glucose-dependent fashion, suppressing pancreatic glucagon output, slowing gastric emptying, and decreasing appetite. Their main advantage is weight loss, which is modest in most patients but can be significant in some. A limiting side effect is nausea and vomiting, particularly early in the course of treatment. Concerns regarding an increased risk of pancreatitis remain unresolved. The oral dipeptidyl peptidase 4 (DPP-4) inhibitors enhance circulating concentrations of active GLP-1 and GIP (64). Their major effect appears to be in the regulation of insulin and glucagon secretion; they are weight neutral. Typically, neither of the incretin-based classes cause hypoglycemia by themselves.
Two agents that are used infrequently in the U.S. and Europe are the α-glucosidase inhibitors (AGIs), which retard gut carbohydrate absorption (65), and colesevelam, a bile acid sequestrant whose mechanism of glucose-lowering action remains poorly understood and whose major additional benefit is LDL-cholesterol reduction (66). Both have gastrointestinal effects, mainly flatulence with AGIs and constipation with colesevelam. The dopamine agonist bromocriptine is only available in the U.S. as an antihyperglycemic agent (67). Its mechanism of action and precise role are unclear. The amylin agonist, pramlintide, is typically reserved for patients treated with intensive insulin therapy, usually in type 1 diabetes mellitus; it decreases postprandial glucose excursions by inhibiting glucagon secretion and slowing gastric emptying (68).
The glucose-lowering effectiveness of noninsulin pharmacological agents is said to be high for metformin, sulfonylureas, TZDs, and GLP-1 agonists (expected HbA1c reduction ∼1.0–1.5%) (1,69,70), and generally lower for meglitinides, DPP-4 inhibitors, AGIs, colesevelam, and bromocriptine (∼0.5–1.0%). However, older drugs have typically been tested in clinical trial participants with higher baseline HbA1c, which is itself associated with greater treatment emergent glycemic reductions, irrespective of therapy type. In head-to-head studies, any differential effects on glucose control are small. So agent- and patient-specific properties, such as dosing frequency, side-effect profiles, cost, and other benefits often guide their selection.
Insulin.
Due to the progressive β-cell dysfunction that characterizes type 2 diabetes, insulin replacement therapy is frequently required (71). Importantly, most patients maintain some endogenous insulin secretion even in late stages of disease. Accordingly, the more complex and intensive strategies of type 1 diabetes are not typically necessary (72).
Ideally, the principle of insulin use is the creation of as normal a glycemic profile as possible without unacceptable weight gain or hypoglycemia (73). As initial therapy, unless the patient is markedly hyperglycemic and/or symptomatic, a “basal” insulin alone is typically added (74). Basal insulin provides relatively uniform insulin coverage throughout the day and night, mainly to control blood glucose by suppressing hepatic glucose production in between meals and during sleep. Either intermediate-acting (neutral protamine Hagedorn [NPH]) or long-acting (insulin glargine [A21Gly,B31Arg,B32Arg human insulin] or insulin detemir [B29Lys(ε-tetradecanoyl),desB30 human insulin]) formulations may be used. The latter two are associated with modestly less overnight hypoglycemia (insulin glargine, insulin detemir) than NPH and possibly slightly less weight gain (insulin detemir), but are more expensive (75,76). Of note, the dosing of these basal insulin analogs may differ, with most comparative trials showing a higher average unit requirement with insulin detemir (77).
Although the majority of patients with type 2 diabetes requiring insulin therapy can be successfully treated with basal insulin alone, some, because of progressive diminution in their insulin secretory capacity, will require prandial insulin therapy with shorter-acting insulins. This is typically provided in the form of the rapid insulin analogs, insulin lispro (B28Lys,B29Pro human insulin), insulin aspart (B28Asp human insulin), or insulin glulisine (B3Lys,B29Glu human insulin), which may be dosed just before the meal. They result in better postprandial glucose control than the less costly human regular insulin, whose pharmacokinetic profile makes it less attractive in this setting.
Ideally, an insulin treatment program should be designed specifically for an individual patient, to match the supply of insulin to his or her dietary/exercise habits and prevailing glucose trends, as revealed through self-monitoring. Anticipated glucose-lowering effects should be balanced with the convenience of the regimen, in the context of an individual's specific therapy goals (Fig. 1).
Proper patient education regarding glucose monitoring, insulin injection technique, insulin storage, recognition/treatment of hypoglycemia, and “sick day” rules is imperative. Where available, certified diabetes educators can be invaluable in guiding the patient through this process.
KEY POINTS.
Glycemic targets and glucose-lowering therapies must be individualized.
Diet, exercise, and education remain the foundation of any type 2 diabetes treatment program.
Unless there are prevalent contraindications, metformin is the optimal first-line drug.
After metformin, there are limited data to guide us. Combination therapy with an additional 1–2 oral or injectable agents is reasonable, aiming to minimize side effects where possible.
Ultimately, many patients will require insulin therapy alone or in combination with other agents to maintain glucose control.
All treatment decisions, where possible, should be made in conjunction with the patient, focusing on his/her preferences, needs, and values.
Comprehensive cardiovascular risk reduction must be a major focus of therapy.
Implementation strategies
Initial drug therapy.
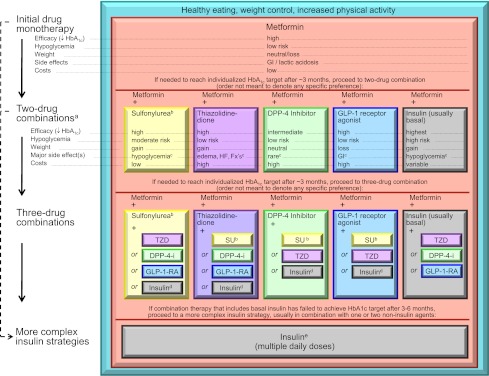
It is generally agreed that metformin, if not contraindicated and if tolerated, is the preferred and most cost-effective first agent (42) (Fig. 2 and Supplementary Figs.). It is initiated at, or soon after, diagnosis, especially in patients in whom lifestyle intervention alone has not achieved, or is unlikely to achieve, HbA1c goals. Because of frequent gastrointestinal side effects, it should be started at a low dose with gradual titration. Patients with a high baseline HbA1c (e.g., ≥9.0%) have a low probability of achieving a near-normal target with monotherapy. It may therefore be justified to start directly with a combination of two noninsulin agents or with insulin itself in this circumstance (78). If a patient presents with significant hyperglycemic symptoms and/or has dramatically elevated plasma glucose concentrations (e.g., >16.7–19.4 mmol/L [>300–350 mg/dL]) or HbA1c (e.g., ≥10.0–12.0%), insulin therapy should be strongly considered from the outset. Such treatment is mandatory when catabolic features are exhibited or, of course, if ketonuria is demonstrated, the latter reflecting profound insulin deficiency. Importantly, unless there is evidence of type 1 diabetes, once symptoms are relieved, glucotoxicity resolved, and the metabolic state stabilized, it may be possible to taper insulin partially or entirely, transferring to noninsulin antihyperglycemic agents, perhaps in combination.
Figure 2.
Antihyperglycemic therapy in type 2 diabetes: general recommendations. Moving from the top to the bottom of the figure, potential sequences of antihyperglycemic therapy. In most patients, begin with lifestyle changes; metformin monotherapy is added at, or soon after, diagnosis (unless there are explicit contraindications). If the HbA1c target is not achieved after ∼3 months, consider one of the five treatment options combined with metformin: a sulfonylurea, TZD, DPP-4 inhibitor, GLP-1 receptor agonist, or basal insulin. (The order in the chart is determined by historical introduction and route of administration and is not meant to denote any specific preference.) Choice is based on patient and drug characteristics, with the over-riding goal of improving glycemic control while minimizing side effects. Shared decision making with the patient may help in the selection of therapeutic options. The figure displays drugs commonly used both in the U.S. and/or Europe. Rapid-acting secretagogues (meglitinides) may be used in place of sulfonylureas. Other drugs not shown (α-glucosidase inhibitors, colesevelam, dopamine agonists, pramlintide) may be used where available in selected patients but have modest efficacy and/or limiting side effects. In patients intolerant of, or with contraindications for, metformin, select initial drug from other classes depicted and proceed accordingly. In this circumstance, while published trials are generally lacking, it is reasonable to consider three-drug combinations other than metformin. Insulin is likely to be more effective than most other agents as a third-line therapy, especially when HbA1c is very high (e.g., ≥9.0%). The therapeutic regimen should include some basal insulin before moving to more complex insulin strategies (Fig. 3). Dashed arrow line on the left-hand side of the figure denotes the option of a more rapid progression from a two-drug combination directly to multiple daily insulin doses, in those patients with severe hyperglycemia (e.g., HbA1c ≥10.0–12.0%). DPP-4-i, DPP-4 inhibitor; Fx's, bone fractures; GI, gastrointestinal; GLP-1-RA, GLP-1 receptor agonist; HF, heart failure; SU, sulfonylurea. aConsider beginning at this stage in patients with very high HbA1c (e.g., ≥9%). bConsider rapid-acting, nonsulfonylurea secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on sulfonylureas. cSee Table 1 for additional potential adverse effects and risks, under “Disadvantages.” dUsually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents. eCertain noninsulin agents may be continued with insulin (see text). Refer to Fig. 3 for details on regimens. Consider beginning at this stage if patient presents with severe hyperglycemia (≥16.7–19.4 mmol/L [≥300–350 mg/dL]; HbA1c ≥10.0–12.0%) with or without catabolic features (weight loss, ketosis, etc.).
If metformin cannot be used, another oral agent could be chosen, such as a sulfonylurea/glinide, pioglitazone, or a DPP-4 inhibitor; in occasional cases where weight loss is seen as an essential aspect of therapy, initial treatment with a GLP-1 receptor agonist might be useful. Where available, less commonly used drugs (AGIs, colesevelam, bromocriptine) might also be considered in selected patients, but their modest glycemic effects and side-effect profiles make them less attractive candidates. Specific patient preferences, characteristics, susceptibilities to side effects, potential for weight gain and hypoglycemia should play a major role in drug selection (20,21). (See Supplementary Figs. for adaptations of Fig. 2 that address specific patient scenarios.)
Advancing to dual combination therapy.
Figure 2 (and Supplementary Figs.) also depicts potential sequences of escalating glucose-lowering therapy beyond metformin. If monotherapy alone does not achieve/maintain an HbA1c target over ∼3 months, the next step would be to add a second oral agent, a GLP-1 receptor agonist, or basal insulin (5,10). Notably, the higher the HbA1c, the more likely insulin will be required. On average, any second agent is typically associated with an approximate further reduction in HbA1c of ∼1% (70,79). If no clinically meaningful glycemic reduction (i.e., “nonresponder”) is demonstrated, then, adherence having been investigated, that agent should be discontinued, and another with a different mechanism of action substituted. With a distinct paucity of long-term comparative-effectiveness trials available, uniform recommendations on the best agent to be combined with metformin cannot be made (80). Thus, advantages and disadvantages of specific drugs for each patient should be considered (Table 1).
Some antihyperglycemic medications lead to weight gain. This may be associated with worsening markers of insulin resistance and cardiovascular risk. One exception may be TZDs (57); weight gain associated with this class occurs in association with decreased insulin resistance. Although there is no uniform evidence that increases in weight in the range observed with certain therapies translate into a substantially increased cardiovascular risk, it remains important to avoid unnecessary weight gain by optimal medication selection and dose titration.
For all medications, consideration should also be given to overall tolerability. Even occasional hypoglycemia may be devastating, if severe, or merely irritating, if mild (81). Gastrointestinal side effects may be tolerated by some, but not others. Fluid retention may pose a clinical or merely an aesthetic problem (82). The risk of bone fractures may be a specific concern in postmenopausal women (57).
It must be acknowledged that costs are a critical issue driving the selection of glucose-lowering agents in many environments. For resource-limited settings, less expensive agents should be chosen. However, due consideration should be also given to side effects and any necessary monitoring, with their own cost implications. Moreover, prevention of morbid long-term complications will likely reduce long-term expenses attributed to the disease.
Advancing to triple combination therapy.
Some studies have shown advantages of adding a third noninsulin agent to a two-drug combination that is not yet or no longer achieving the glycemic target (83–86). Not surprisingly, however, at this juncture, the most robust response will usually be with insulin. Indeed, since diabetes is associated with progressive β-cell loss, many patients, especially those with long-standing disease, will eventually need to be transitioned to insulin, which should be favored in circumstances where the degree of hyperglycemia (e.g., ≥8.5%) makes it unlikely that another drug will be of sufficient benefit (87). If triple combination therapy exclusive of insulin is tried, the patient should be monitored closely, with the approach promptly reconsidered if it proves to be unsuccessful. Many months of uncontrolled hyperglycemia should specifically be avoided.
In using triple combinations the essential consideration is obviously to use agents with complementary mechanisms of action (Fig. 2 and Supplementary Figs.). Increasing the number of drugs heightens the potential for side effects and drug–drug interactions, raises costs, and negatively impacts patient adherence. The rationale, benefits, and side effects of each new medication should be discussed with the patient. The clinical characteristics of patients more or less likely to respond to specific combinations are, unfortunately, not well defined.
Transitions to and titrations of insulin.
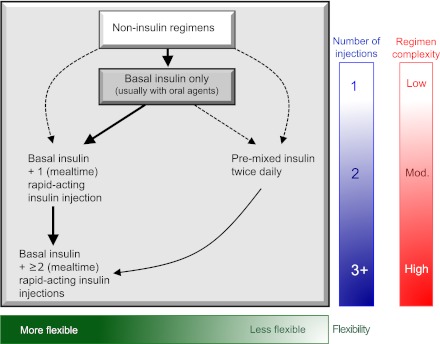
Most patients express reluctance to beginning injectable therapy, but, if the practitioner feels that such a transition is important, encouragement and education can usually overcome such reticence. Insulin is typically begun at a low dose (e.g., 0.1–0.2 U kg−1 day−1), although larger amounts (0.3–0.4 U kg−1 day−1) are reasonable in the more severely hyperglycemic. The most convenient strategy is with a single injection of a basal insulin, with the timing of administration dependent on the patient's schedule and overall glucose profile (Fig. 3).
Figure 3.
Sequential insulin strategies in type 2 diabetes. Basal insulin alone is usually the optimal initial regimen, beginning at 0.1–0.2 units/kg body weight, depending on the degree of hyperglycemia. It is usually prescribed in conjunction with one to two noninsulin agents. In patients willing to take more than one injection and who have higher HbA1c levels (≥9.0%), twice-daily premixed insulin or a more advanced basal plus mealtime insulin regimen could also be considered (curved dashed arrow lines). When basal insulin has been titrated to an acceptable fasting glucose but HbA1c remains above target, consider proceeding to basal plus mealtime insulin, consisting of one to three injections of rapid-acting analogs (see text for details). A less studied alternative—progression from basal insulin to a twice-daily premixed insulin—could be also considered (straight dashed arrow line); if this is unsuccessful, move to basal plus mealtime insulin. The figure describes the number of injections required at each stage, together with the relative complexity and flexibility. Once a strategy is initiated, titration of the insulin dose is important, with dose adjustments made based on the prevailing glucose levels as reported by the patient. Noninsulin agents may be continued, although insulin secretagogues (sulfonylureas, meglitinides) are typically stopped once more complex regimens beyond basal insulin are utilized. Comprehensive education regarding self-monitoring of blood glucose, diet, exercise, and the avoidance of, and response to, hypoglycemia are critical in any patient on insulin therapy. Mod., moderate.
Although extensive dosing instructions for insulin are beyond the scope of this statement, most patients can be taught to uptitrate their own insulin dose based on several algorithms, each essentially involving the addition of a small dose increase if hyperglycemia persists (74,76,88). For example, the addition of 1–2 units (or, in those already on higher doses, increments of 5–10%) to the daily dose once or twice weekly if the fasting glucose levels are above the preagreed target is a reasonable approach (89). As the target is neared, dosage adjustments should be more modest and occur less frequently. Downward adjustment is advisable if any hypoglycemia occurs. During self-titration, frequent contact (telephone, e-mail) with the clinician may be necessary. Practitioners themselves can, of course, also titrate basal insulin, but this would involve more intensive contact with the patient than typically available in routine clinical practice. Daily self-monitoring of blood glucose is of obvious importance during this phase. After the insulin dose is stabilized, the frequency of monitoring should be reviewed (90).
Consideration should be given to the addition of prandial or mealtime insulin coverage when significant postprandial glucose excursions (e.g., to >10.0 mmol/L [>180 mg/dL]) occur. This is suggested when the fasting glucose is at target but the HbA1c remains above goal after 3–6 months of basal insulin titration (91). The same would apply if large drops in glucose occur during overnight hours or in between meals, as the basal insulin dose is increased. In this scenario, the basal insulin dose would obviously need to be simultaneously decreased as prandial insulin is initiated. Although basal insulin is titrated primarily against the fasting glucose, generally irrespective of the total dose, practitioners should be aware that the need for prandial insulin therapy will become likely the more the daily dose exceeds 0.5 U kg−1 day−1, especially as it approaches 1 U kg−1 day−1. The aim with mealtime insulin is to blunt postprandial glycemic excursions, which can be extreme in some individuals, resulting in poor control during the day. Such coverage may be provided by one of two methods.
The most precise and flexible prandial coverage is possible with “basal-bolus” therapy, involving the addition of premeal rapid-acting insulin analog to ongoing basal insulin. One graduated approach is to add prandial insulin before the meal responsible for the largest glucose excursion—typically that with the greatest carbohydrate content, often, but not always, the evening meal (92). Subsequently, a second injection can be administered before the meal with the next largest excursion (often breakfast). Ultimately, a third injection may be added before the smallest meal (often lunch) (93). The actual glycemic benefits of these more advanced regimens after basal insulin are generally modest in typical patients (92). So, again, individualization of therapy is key, incorporating the degree of hyperglycemia needing to be addressed and the overall capacities of the patient. Importantly, data trends from self-monitoring may be particularly helpful in titrating insulins and their doses within these more advanced regimens to optimize control.
A second, perhaps more convenient but less adaptable method involves “premixed” insulin, consisting of a fixed combination of an intermediate insulin with regular insulin or a rapid analog. Traditionally, this is administered twice daily, before morning and evening meals. In general, when compared with basal insulin alone, premixed regimens tend to lower HbA1c to a larger degree, but often at the expense of slightly more hypoglycemia and weight gain (94). Disadvantages include the inability to titrate the shorter- from the longer-acting component of these formulations. Therefore, this strategy is somewhat inflexible but may be appropriate for certain patients who eat regularly and may be in need of a simplified approach beyond basal insulin (92,93). (An older and less commonly used variation of this two-injection strategy is known as “split-mixed,” involving a fixed amount of intermediate insulin mixed by the patient with a variable amount of regular insulin or a rapid analog. This allows for greater flexibility in dosing.)
The key messages from dozens of comparative insulin trials in type 2 diabetes include the following:
-
1.
Any insulin will lower glucose and HbA1c.
-
2.
All insulins are associated with some weight gain and some risk of hypoglycemia.
-
3.
The larger the doses and the more aggressive the titration, the lower the HbA1c, but often with a greater likelihood of adverse effects.
-
4.
Generally, long-acting insulin analogs reduce the incidence of overnight hypoglycemia, and rapid-acting insulin analogs reduce postprandial glucose excursions as compared with corresponding human insulins (NPH, Regular), but they generally do not result in clinically significantly lower HbA1c.
Metformin is often continued when basal insulin is added, with studies demonstrating less weight gain when the two are used together (95). Insulin secretagogues do not seem to provide for additional HbA1c reduction or prevention of hypoglycemia or weight gain after insulin is started, especially after the dose is titrated and stabilized. When basal insulin is used, continuing the secretagogue may minimize initial deterioration of glycemic control. However, secretagogues should be avoided once prandial insulin regimens are employed. TZDs should be reduced in dose (or stopped) to avoid edema and excessive weight gain, although in certain individuals with large insulin requirements from severe insulin resistance, these insulin sensitizers may be very helpful in lowering HbA1c and minimizing the required insulin dose (96). Data concerning the glycemic benefits of incretin-based therapy combined with basal insulin are accumulating; combination with GLP-1 receptor agonists may be helpful in some patients (97,98). Once again, the costs of these more elaborate combined regimens must be carefully considered.
OTHER CONSIDERATIONS
Age
Older adults (>65–70 years) often have a higher atherosclerotic disease burden, reduced renal function, and more comorbidities (99,100). Many are at risk for adverse events from polypharmacy and may be both socially and economically disadvantaged. Life expectancy is reduced, especially in the presence of long-term complications. They are also more likely to be compromised by hypoglycemia; for example, unsteadiness may result in falls and fractures (101), and a tenuous cardiac status may deteriorate into catastrophic events. It follows that glycemic targets for elderly with long-standing or more complicated disease should be less ambitious than for the younger, healthier individuals (20). If lower targets cannot be achieved with simple interventions, an HbA1c of <7.5–8.0% may be acceptable, transitioning upward as age increases and capacity for self-care, cognitive, psychological and economic status, and support systems decline.
While lifestyle modification can be successfully implemented across all age-groups, in the aged, the choice of antihyperglycemic agent should focus on drug safety, especially protecting against hypoglycemia, heart failure, renal dysfunction, bone fractures, and drug–drug interactions. Strategies specifically minimizing the risk of low blood glucose may be preferred.
In contrast, healthier patients with long life expectancy accrue risk for vascular complications over time. Therefore, lower glycemic targets (e.g., an HbA1c <6.5–7.0%) and tighter control of body weight, blood pressure, and circulating lipids should be achieved to prevent or delay such complications. This usually requires combination therapy, the early institution of which may have the best chance of modifying the disease process and preserving quality of life.
Weight
The majority of individuals with type 2 diabetes are overweight or obese (∼80%) (102). In these, intensive lifestyle intervention can improve fitness, glycemic control, and cardiovascular risk factors for relatively small changes in body weight (103). Although insulin resistance is thought of as the predominate driver of diabetes in obese patients, they actually have a similar degree of islet dysfunction to leaner patients (37). Perhaps as a result, the obese may be more likely to require combination drug therapy (20,104). While common practice has favored metformin in heavier patients, because of weight loss/weight neutrality, this drug is as efficacious in lean individuals (75). TZDs, on the other hand, appear to be more effective in those with higher BMIs, although their associated weight gain makes them, paradoxically, a less attractive option here. GLP-1 receptor agonists are associated with weight reduction (38), which in some patients may be substantial.
Bariatric surgery is an increasingly popular option in severe obesity. Type 2 diabetes frequently resolves rapidly after these procedures. The majority of patients are able to stop some, or even all, of their antihyperglycemic medications, although the durability of this effect is not known (105).
In lean patients, consideration should be given to the possibility of latent autoimmune diabetes in adults (LADA), a slowly progressive form of type 1 diabetes. These individuals, while presenting with mild hyperglycemia, often responsive to oral agents, eventually develop more severe hyperglycemia and require intensive insulin regimens (106). Measuring titres of islet-associated autoantibodies (e.g., anti-GAD) may aid their identification, encouraging a more rapid transition to insulin therapy.
Sex/racial/ethnic/genetic differences
While certain racial/ethnic features that increase the risk of diabetes are well recognized [greater insulin resistance in Latinos (107), more β-cell dysfunction in East Asians (108)], using this information to craft optimal therapeutic strategies is in its infancy. This is not surprising given the polygenic inheritance pattern of the disease. Indeed, while matching a drug's mechanism of action to the underlying causes of hyperglycemia in a specific patient seems logical, there are few data that compare strategies based on this approach (109). There are few exceptions, mainly involving diabetes monogenic variants often confused with type 2 diabetes, such as maturity-onset diabetes of the young (MODY), several forms of which respond preferentially to sulfonylureas (110). While there are no prominent sex differences in the response to various antihyperglycemic drugs, certain side effects (e.g., bone loss with TZDs) may be of greater concern in women.
Comorbidities
Coronary artery disease.
Given the frequency with which type 2 diabetic patients develop atherosclerosis, optimal management strategies for those with or at high risk for coronary artery disease (CAD) are important. Since hypoglycemia may exacerbate myocardial ischemia and may cause dysrhythmias (111), it follows that medications that predispose patients to this adverse effect should be avoided, if possible. If they are required, however, to achieve glycemic targets, patients should be educated to minimize risk. Because of possible effects on potassium channels in the heart, certain sulfonylureas have been proposed to aggravate myocardial ischemia through effects on ischemic preconditioning (112), but the actual clinical relevance of this remains unproven. Metformin may have some cardiovascular benefits and would appear to be a useful drug in the setting of CAD, barring prevalent contraindications (32). In a single study, pioglitazone was shown to reduce modestly major adverse cardiovascular events in patients with established macrovascular disease. It may therefore also be considered, unless heart failure is present (60). In very preliminary reports, therapy with GLP-1 receptor agonists and DPP-4 inhibitors has been associated with improvement in either cardiovascular risk or risk factors, but there are no long-term data regarding clinical outcomes (113). There are very limited data suggesting that AGIs (114) and bromocriptine (115) may reduce cardiovascular events.
Heart failure.
With an aging population and recent decreases in mortality after myocardial infarction, the diabetic patient with progressive heart failure is an increasingly common scenario (116). This population presents unique challenges given their polypharmacy, frequent hospitalizations, and contraindications to various agents. TZDs should be avoided (117,118). Metformin, previously contraindicated in heart failure, can now be used if the ventricular dysfunction is not severe, if patient's cardiovascular status is stable, and if renal function is normal (119). As mentioned, cardiovascular effects of incretin-based therapies, including those on ventricular function, are currently under investigation (120).
Chronic kidney disease.
Kidney disease is highly prevalent in type 2 diabetes, and moderate to severe renal functional impairment (eGFR <60 mL/min) occurs in approximately 20–30% of patients (121,122). The individual with progressive renal dysfunction is at increased risk for hypoglycemia, which is multifactorial. Insulin and, to some degree, the incretin hormones are eliminated more slowly, as are antihyperglycemic drugs with renal excretion. Thus, dose reduction may be necessary, contraindications need to be observed, and consequences (hypoglycemia, fluid retention, etc.) require careful evaluation.
Current U.S. prescribing guidelines warn against the use of metformin in patients with a serum creatinine ≥133 mmol/L (≥1.5 mg/dL) in men or 124 mmol/L (≥1.4 mg/dL) in women. Metformin is eliminated renally, and cases of lactic acidosis have been described in patients with renal failure (123). There is an ongoing debate, however, as to whether these thresholds are too restrictive and that those with mild–moderate renal impairment would gain more benefit than harm from using metformin (124,125). In the U.K., the National Institute for Health and Clinical Excellence (NICE) guidelines are less proscriptive and more evidence-based than those in the U.S., generally allowing use down to a GFR of 30 mL/min, with dose reduction advised at 45 mL/min (14). Given the current widespread reporting of estimated GFR, these guidelines appear very reasonable.
Most insulin secretagogues undergo significant renal clearance (exceptions include repaglinide and nateglinide) and the risk of hypoglycemia is therefore higher in patients with chronic kidney disease (CKD). For most of these agents, extreme caution is imperative at more severe degrees of renal dysfunction. Glyburide (known as glibenclamide in Europe), which has a prolonged duration of action and active metabolites, should be specifically avoided in this group. Pioglitazone is not eliminated renally, and therefore there are no restrictions for use in CKD. Fluid retention may be a concern, however. Among the DPP-4 inhibitors, sitagliptin, vildagliptin, and saxagliptin share prominent renal elimination. In the face of advanced CKD, dose reduction is necessary. One exception is linagliptin, which is predominantly eliminated enterohepatically. For the GLP-1 receptor agonists exenatide is contraindicated in stage 4–5 CKD (GFR <30 mL/min) as it is renally eliminated; the safety of liraglutide is not established in CKD though pharmacokinetic studies suggest that drug levels are unaffected as it does not require renal function for clearance.
More severe renal functional impairment is associated with slower elimination of all insulins. Thus doses need to be titrated carefully, with some awareness for the potential for more prolonged activity profiles.
Liver dysfunction.
Individuals with type 2 diabetes frequently have hepatosteatosis as well as other types of liver disease (126). There is preliminary evidence that patients with fatty liver may benefit from treatment with pioglitazone (45,127,128). It should not be used in an individual with active liver disease or an alanine transaminase level above 2.5 times the upper limit of normal. In those with steatosis but milder liver test abnormalities, this insulin sensitizer may be advantageous. Sulfonylureas can rarely cause abnormalities in liver tests but are not specifically contraindicated; meglitinides can also be used. If hepatic disease is severe, secretagogues should be avoided because of the increased risk of hypoglycemia. In patients with mild hepatic disease, incretin-based drugs can be prescribed, except if there is a coexisting history of pancreatitis. Insulin has no restrictions for use in patients with liver impairment and is indeed the preferred choice in those with advanced disease.
Hypoglycemia.
Hypoglycemia in type 2 diabetes was long thought to be a trivial issue, as it occurs less commonly than in type 1 diabetes. However, there is emerging concern based mainly on the results of recent clinical trials and some cross-sectional evidence of increased risk of brain dysfunction in those with repeated episodes. In the ACCORD trial, the frequency of both minor and major hypoglycemia was high in intensively managed patients—threefold that associated with conventional therapy (129). It remains unknown whether hypoglycemia was the cause of the increased mortality in the intensive group (130,131). Clearly, however, hypoglycemia is more dangerous in the elderly and occurs consistently more often as glycemic targets are lowered. Hypoglycemia may lead to dysrhythmias, but can also lead to accidents and falls (which are more likely to be dangerous in the elderly) (132), dizziness (leading to falls), confusion (so other therapies may not be taken or taken incorrectly), or infection (such as aspiration during sleep, leading to pneumonia). Hypoglycemia may be systematically under-reported as a cause of death, so the true incidence may not be fully appreciated. Perhaps just as importantly, additional consequences of frequent hypoglycemia include work disability and erosion of the confidence of the patient (and that of family or caregivers) to live independently. Accordingly, in at-risk individuals, drug selection should favor agents that do not precipitate such events and, in general, blood glucose targets may need to be moderated.
FUTURE DIRECTIONS/RESEARCH NEEDS
For antihyperglycemic management of type 2 diabetes, the comparative evidence basis to date is relatively lean, especially beyond metformin monotherapy (70). There is a significant need for high-quality comparative-effectiveness research, not only regarding glycemic control, but also costs and those outcomes that matter most to patients—quality of life and the avoidance of morbid and life-limiting complications, especially CVD (19,23,70). Another issue about which more data are needed is the concept of durability of effectiveness (often ascribed to β-cell preservation), which would serve to stabilize metabolic control and decrease the future treatment burden for patients. Pharmacogenetics may very well inform treatment decisions in the future, guiding the clinician to recommend a therapy for an individual patient based on predictors of response and susceptibility to adverse effects. We need more clinical data on how phenotype and other patient/disease characteristics should drive drug choices. As new medications are introduced to the type 2 diabetes pharmacopeia, their benefit and safety should be demonstrated in studies versus best current treatment, substantial enough both in size and duration to provide meaningful data on meaningful outcomes. It is appreciated, however, that head-to-head comparisons of all combinations and permutations would be impossibly large (133). Informed judgment and the expertise of experienced clinicians will therefore always be necessary.
Acknowledgments
This position statement was written by joint request of the ADA and the EASD Executive Committees, which have approved the final document. The process involved wide literature review, three face-to-face meetings of the Writing Group, several teleconferences, and multiple revisions via e-mail communications.
We gratefully acknowledge the following experts who provided critical review of a draft of this statement: James Best, Melbourne Medical School, The University of Melbourne, Melbourne, Australia; Henk Bilo, Isala Clinics, Zwolle, the Netherlands; John Boltri, Wayne State University School of Medicine, Detroit, MI; Thomas Buchanan, Keck School of Medicine, University of Southern California, Los Angeles, CA; Paul Callaway, University of Kansas School of Medicine-Wichita, Wichita, KS; Bernard Charbonnel, University of Nantes, Nantes, France; Stephen Colagiuri, The University of Sydney, Sydney, Australia; Samuel Dagogo-Jack, The University of Tennessee Health Science Center, Memphis, TN; Margo Farber, Detroit Medical Center, Detroit, MI; Cynthia Fritschi, College of Nursing, University of Illinois at Chicago, Chicago, IL; Rowan Hillson, The Hillingdon Hospital, Uxbridge, U.K.; Faramarz Ismail-Beigi, Case Western Reserve University School of Medicine/Cleveland VA Medical Center, Cleveland, OH; Devan Kansagara, Oregon Health & Science University/Portland VA Medical Center, Portland, OR; Ilias Migdalis, NIMTS Hospital, Athens, Greece; Donna Miller, Keck School of Medicine, University of Southern California, Los Angeles, CA; Robert Ratner, MedStar Health Research Institute/Georgetown University School of Medicine, Washington, DC; Julio Rosenstock, Dallas Diabetes and Endocrine Center at Medical City, Dallas, TX; Guntram Schernthaner, Rudolfstiftung Hospital, Vienna, Austria; Robert Sherwin, Yale University School of Medicine, New Haven, CT; Jay Skyler, Miller School of Medicine, University of Miami, Miami, FL; Geralyn Spollett, Yale University School of Nursing, New Haven, CT; Ellie Strock, International Diabetes Center, Minneapolis, MN; Agathocles Tsatsoulis, University of Ioannina, Ioannina, Greece; Andrew Wolf, University of Virginia School of Medicine, Charlottesville, VA; Bernard Zinman, Mount Sinai Hospital/University of Toronto, Toronto, ON, Canada. The American Association of Diabetes Educators, American College of Physicians, and The Endocrine Society, and several other organizations who wished to remain anonymous nominated reviewers who provided input on the final draft. Such feedback does not constitute endorsement by these groups or these individuals. The final draft was also peer reviewed and approved by the Professional Practice Committee of the ADA and the Panel for Overseeing Guidelines and Statements of the EASD. We are indebted to Dr. Sue Kirkman of the ADA for her guidance and support during this process. We also thank Carol Hill and Mary Merkin for providing administrative assistance.
Funding
The three face-to-face meetings and the travel of some of the writing group were supported by the EASD and ADA. D.R. Matthews acknowledges support from the National Institute for Health Research.
Duality of interest
During the past 12 months, the following relationships with companies whose products or services directly relate to the subject matter in this document are declared:
R.M. Bergenstal: membership of scientific advisory boards and consultation for or clinical research support with Abbott Diabetes Care, Amylin, Bayer, Becton Dickinson, Boehringer Ingelheim, Calibra, DexCom, Eli Lilly, Halozyme, Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, NIH, Novo Nordisk, Roche, Sanofi, and Takeda (all under contracts with his employer). Inherited stock in Merck (held by family)
J.B. Buse: research and consulting with Amylin Pharmaceuticals, Inc.; AstraZeneca; Biodel Inc.; Boehringer Ingelheim; Bristol-Myers Squibb Company; Diartis Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd; Halozyme Therapeutics; Johnson & Johnson; Medtronic MiniMed; Merck & Co., Inc.; Novo Nordisk; Pfizer Inc.; Sanofi; and TransPharma Medical Ltd (all under contracts with his employer)
M. Diamant: member of advisory boards of Abbott Diabetes Care, Eli Lilly, Merck Sharp & Dohme (MSD), Novo Nordisk, Poxel Pharma. Consultancy for: Astra-BMS, Sanofi. Speaker engagements: Eli Lilly, MSD, Novo Nordisk. Through Dr. Diamant, the VU University receives research grants from Amylin/Eli Lilly, MSE, Novo Nordisk, Sanofi (all under contracts with the Institutional Research Foundation)
E. Ferrannini: membership on scientific advisory boards or speaking engagements for: Merck Sharp & Dohme, Boehringer Ingelheim, GlaxoSmithKline, BMS/AstraZeneca, Eli Lilly & Co., Novartis, Sanofi. Research grant support from: Eli Lilly & Co. and Boehringer Ingelheim
S.E. Inzucchi: advisor/consultant to: Merck, Takeda, Boehringer Ingelheim. Research funding or supplies to Yale University: Eli Lilly, Takeda. Participation in medical educational projects, for which unrestricted funding from Amylin, Eli Lilly, Boehringer Ingelheim, Merck, Novo Nordisk, and Takeda was received by Yale University
D.R. Matthews: has received advisory board consulting fees or honoraria from Novo Nordisk, GlaxoSmithKline, Novartis, Eli Lilly, Johnson & Johnson, and Servier. He has research support from Johnson & Johnson and Merck Sharp & Dohme. He has lectured for Novo Nordisk, Servier, and Novartis
M. Nauck: has received research grants (to his institution) from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck Sharp & Dohme, Novartis Pharma, GlaxoSmithKline, Novo Nordisk, Roche, and Tolerx. He has received consulting and travel fees or honoraria for speaking from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Diartis, Eli Lilly & Co., F. Hoffmann-La Roche Ltd, Intarcia Therapeutics, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis Pharma, and Versartis
A.L. Peters: has received lecturing fees and/or fees for ad hoc consulting from Amylin, Lilly, Novo Nordisk, Sanofi, Takeda, Boehringer Ingelheim
A. Tsapas: has received travel grant, educational grant, research grant and lecture fees from Merck Serono, Novo Nordisk, and Novartis, respectively
R. Wender: declares he has no duality of interest
Contribution statement
All the named writing group authors contributed substantially to the document including each writing part of the text. They were at the face-to-face meetings and teleconferences. All authors supplied detailed input and approved the final version. S.E. Inzucchi and D.R. Matthews directed, chaired, and coordinated the input with multiple e-mail exchanges between all participants.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0413/-/DC1.
See accompanying editorial, p. 1201.
S.E. Inzucchi and D.R. Matthews were co-chairs for the Position Statement Writing Group. R.M. Bergenstal, J.B. Buse, A.L. Peters, and R. Wender were the Writing Group for the ADA. M. Diamant, E. Ferrannini, M. Nauck, and A. Tsapas were the Writing Group for the EASD.
This article is being simultaneously published in 2012 in Diabetes Care and Diabetologia by the American Diabetes Association and the European Association for the Study of Diabetes.
References
- 1.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–399 [DOI] [PubMed] [Google Scholar]
- 2.Bergenstal RM, Bailey CJ, Kendall DM. Type 2 diabetes: assessing the relative risks and benefits of glucose-lowering medications. Am J Med 2010;123:374.e9–374.e18 [DOI] [PubMed]
- 3.Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011;60:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan JJ. Consensus guidelines, algorithms and care of the individual patient with type 2 diabetes. Diabetologia 2010;53:1247–1249 [DOI] [PubMed] [Google Scholar]
- 5.Blonde L. Current antihyperglycemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am J Med 2010;123(Suppl.):S12–S18 [DOI] [PubMed] [Google Scholar]
- 6.Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med 2009;151:854–860 [DOI] [PubMed] [Google Scholar]
- 7.Matthews DR, Tsapas A. Four decades of uncertainty: landmark trials in glycaemic control and cardiovascular outcome in type 2 diabetes. Diab Vasc Dis Res 2008;5:216–218 [DOI] [PubMed] [Google Scholar]
- 8.Skyler JS, Bergenstal R, Bonow RO, et al. ; American Diabetes Association; American College of Cardiology Foundation; American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudkin JS, Richter B, Gale EA. Intensified glucose control in type 2 diabetes—whose agenda? Lancet 2011;377:1220–1222 [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 11.IDF Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes. Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 12.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 13.Berard LD, Booth G, Capes S, Quinn K, Woo V. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Canadian Journal of Diabetes 2008;32:S1–S201 [Google Scholar]
- 14.NICE Type 2 Diabetes: The Management of Type 2 Diabetes: NICE Clinical Guideline 87. National Institute for Health and Clinical Excellence, 2009 [Google Scholar]
- 15.Home P, Mant J, Diaz J, Turner C; Guideline Development Group Management of type 2 diabetes: summary of updated NICE guidance. BMJ 2008;336:1306–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson JA. Incorporating incretin-based therapies into clinical practice: differences between glucagon-like peptide 1 receptor agonists and dipeptidyl peptidase 4 inhibitors. Mayo Clin Proc 2010;85(Suppl.):S27–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA. Current issues in the treatment of type 2 diabetes. Overview of newer agents: where treatment is going. Am J Med 2010;123(Suppl.):S38–S48 [DOI] [PubMed] [Google Scholar]
- 18.Murad MH, Shah ND, Van Houten HK, et al. Individuals with diabetes preferred that future trials use patient-important outcomes and provide pragmatic inferences. J Clin Epidemiol 2011;64:743–748 [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Peeples M, Skovlund SE. Where is the patient in diabetes performance measures? The case for including patient-centered and self-management measures. Diabetes Care 2008;31:1046–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 21.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med 2009;169:1560–1568 [DOI] [PubMed] [Google Scholar]
- 22.Schernthaner G, Barnett AH, Betteridge DJ, et al. Is the ADA/EASD algorithm for the management of type 2 diabetes (January 2009) based on evidence or opinion? A critical analysis. Diabetologia 2010;53:1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi GY, Murad MH, Fujiyoshi A, et al. Patient-important outcomes in registered diabetes trials. JAMA 2008;299:2543–2549 [DOI] [PubMed] [Google Scholar]
- 24.Smith RJ, Nathan DM, Arslanian SA, Groop L, Rizza RA, Rotter JI. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: what we know and what we need to know. J Clin Endocrinol Metab 2010;95:1566–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on Quality of Health Care in America: Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, The National Academies Press, 2001 [Google Scholar]
- 26.Guyatt GH, Haynes RB, Jaeschke RZ, et al. ; Evidence-Based Medicine Working Group Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users’ Guides to patient care. JAMA 2000;284:1290–1296 [DOI] [PubMed] [Google Scholar]
- 27.Tsapas A, Matthews DR. N of 1 trials in diabetes: making individual therapeutic decisions. Diabetologia 2008;51:921–925 [DOI] [PubMed] [Google Scholar]
- 28.Shah ND, Mullan RJ, Breslin M, Yawn BP, Ting HH, Montori VM. Translating comparative effectiveness into practice: the case of diabetes medications. Med Care 2010;48(Suppl.):S153–S158 [DOI] [PubMed] [Google Scholar]
- 29.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner RC, Holman RR, Cull CA, et al. ; UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 31.UKPDS Group UK Prospective Diabetes Study VIII: study design, progress and performance. Diabetologia 1991;34:877–890 [PubMed] [Google Scholar]
- 32.Turner RC, Holman RR, Cull CA, et al. ; UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 33.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 34.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 36.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298. Erratum 52:2470. [DOI] [PubMed]
- 37.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 38.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011;124(Suppl.):S3–S18 [DOI] [PubMed] [Google Scholar]
- 39.Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 40.Nauck MA. Unraveling the science of incretin biology. Am J Med 2009;122(Suppl.):S3–S10 [DOI] [PubMed] [Google Scholar]
- 41.Groop LC, Ferrannini E. Insulin action and substrate competition. Baillieres Clin Endocrinol Metab 1993;7:1007–1032 [DOI] [PubMed] [Google Scholar]
- 42.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akalin S, Berntorp K, Ceriello A, et al. ; Global Task Force on Glycaemic Control Intensive glucose therapy and clinical implications of recent data: a consensus statement from the Global Task Force on Glycaemic Control. Int J Clin Pract 2009;63:1421–1425 [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Eng C. Goals of glycemic control in frail older patients with diabetes. JAMA 2011;305:1350–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab 2009;11:188–195 [DOI] [PubMed] [Google Scholar]
- 46.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339:b2803. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003;22:331–339 [DOI] [PubMed] [Google Scholar]
- 48.Klein S, Sheard NF, Pi-Sunyer X, et al. ; American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004;27:2067–2073 [DOI] [PubMed] [Google Scholar]
- 49.Bantle JP, Wylie-Rosett J, Albright AL, et al. ; American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 50.Elmer PJ, Obarzanek E, Vollmer WM, et al. ; PREMIER Collaborative Research Group Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med 2006;144:485–495 [DOI] [PubMed] [Google Scholar]
- 51.Gordon NF, Salmon RD, Franklin BA, et al. Effectiveness of therapeutic lifestyle changes in patients with hypertension, hyperlipidemia, and/or hyperglycemia. Am J Cardiol 2004;94:1558–1561 [DOI] [PubMed] [Google Scholar]
- 52.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–1571 [DOI] [PubMed] [Google Scholar]
- 53.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286:1218–1227 [DOI] [PubMed] [Google Scholar]
- 54.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574–579 [DOI] [PubMed] [Google Scholar]
- 55.Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2011;13:221–228 [DOI] [PubMed] [Google Scholar]
- 56.Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des 2005;11:2699–2716 [DOI] [PubMed] [Google Scholar]
- 57.Kahn SE, Haffner SM, Heise MA, et al. ; ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 58.Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA. PRESERVE-β: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care 2005;28:2093–2099 [DOI] [PubMed] [Google Scholar]
- 59.Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–1118 [DOI] [PubMed] [Google Scholar]
- 60.Dormandy JA, Charbonnel B, Eckland DJ, et al. ; PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 61.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 2010;170:1191–1201 [DOI] [PubMed] [Google Scholar]
- 62.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 64.Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 2011;13:7–18 [DOI] [PubMed] [Google Scholar]
- 65.Van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, de Grauw WJ. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev 2006;(Issue 4)CD005061. DOI: 10.1002/14651858.CD005061.pub2 [DOI] [PubMed] [Google Scholar]
- 66.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab 2010;12:384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFronzo RA. Bromocriptine: a sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care 2011;34:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh-Franco D, Robles G, Gazze D. Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 2007;29:535–562 [DOI] [PubMed] [Google Scholar]
- 69.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med 2010;123(Suppl.):S28–S37 [DOI] [PubMed] [Google Scholar]
- 70.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 2011;154:602–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jabbour S. Primary care physicians and insulin initiation: multiple barriers, lack of knowledge or both? Int J Clin Pract 2008;62:845–847 [DOI] [PubMed] [Google Scholar]
- 72.Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 74.Holman RR, Farmer AJ, Davies MJ, et al. ; 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 75.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 76.Riddle MC. The Treat-to-Target Trial and related studies. Endocr Pract 2006;12(Suppl. 1):71–79 [DOI] [PubMed] [Google Scholar]
- 77.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simonson GD, Cuddihy RM, Reader D, Bergenstal RM. International Diabetes Center treatment of type 2 diabetes glucose algorithm. Diabetes Management 2011;1:175–189 [Google Scholar]
- 79.Gross JL, Kramer CK, Leitão CB, et al. ; Diabetes and Endocrinology Meta-analysis Group (DEMA) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011;154:672–679 [DOI] [PubMed] [Google Scholar]
- 80.Karagiannis T, Paschos P, Paletas P, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369. [DOI] [PubMed] [Google Scholar]
- 81.Cryer PE. Severe iatrogenic hypoglycemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2007;3:4–5 [DOI] [PubMed] [Google Scholar]
- 82.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ 2011;342:d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 84.Zinman B, Gerich J, Buse JB, et al. ; LEAD-4 Study Investigators Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberts VL, Stewart J, Issa M, Lake B, Melis R. Triple therapy with glimepiride in patients with type 2 diabetes mellitus inadequately controlled by metformin and a thiazolidinedione: results of a 30-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2005;27:1535–1547 [DOI] [PubMed] [Google Scholar]
- 86.Bell DS, Dharmalingam M, Kumar S, Sawakhande RB. Triple oral fixed-dose diabetes polypill versus insulin plus metformin efficacy demonstration study in the treatment of advanced type 2 diabetes (TrIED study-II). Diabetes Obes Metab 2011;13:800–805 [DOI] [PubMed] [Google Scholar]
- 87.Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care 2006;29:554–559 [DOI] [PubMed] [Google Scholar]
- 88.Yki-Järvinen H, Juurinen L, Alvarsson M, et al. Initiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care 2007;30:1364–1369 [DOI] [PubMed] [Google Scholar]
- 89.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R; ATLANTUS Study Group Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care 2005;28:1282–1288 [DOI] [PubMed] [Google Scholar]
- 90.Garber AJ. The importance of titrating starting insulin regimens in patients with type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 5):10–13 [DOI] [PubMed] [Google Scholar]
- 91.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract 2011;17:395–403 [DOI] [PubMed] [Google Scholar]
- 93.Raccah D. Options for the intensification of insulin therapy when basal insulin is not enough in type 2 diabetes mellitus. Diabetes Obes Metab 2008;10(Suppl. 2):76–82 [DOI] [PubMed] [Google Scholar]
- 94.Ilag LL, Kerr L, Malone JK, Tan MH. Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparison. Clin Ther 2007;29:1254–1270 [PubMed] [Google Scholar]
- 95.Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1999;131:182–188 [DOI] [PubMed] [Google Scholar]
- 96.Strowig SM, Raskin P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes Metab 2005;7:633–641 [DOI] [PubMed] [Google Scholar]
- 97.Buse JB. Type 2 diabetes mellitus in 2010: individualizing treatment targets in diabetes care. Nat Rev Endocrinol 2011;7:67–68 [DOI] [PubMed] [Google Scholar]
- 98.Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167–177 [DOI] [PubMed] [Google Scholar]
- 99.Del Prato S, Heine RJ, Keilson L, Guitard C, Shen SG, Emmons RP. Treatment of patients over 64 years of age with type 2 diabetes: experience from nateglinide pooled database retrospective analysis. Diabetes Care 2003;26:2075–2080 [DOI] [PubMed] [Google Scholar]
- 100.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36 [DOI] [PubMed] [Google Scholar]
- 101.Nelson JM, Dufraux K, Cook PF. The relationship between glycemic control and falls in older adults. J Am Geriatr Soc 2007;55:2041–2044 [DOI] [PubMed] [Google Scholar]
- 102.Sluik D, Boeing H, Montonen J, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol 2011;174:22–34 [DOI] [PubMed] [Google Scholar]
- 103.Unick JL, Beavers D, Jakicic JM, et al. ; Look AHEAD Research Group Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 2011;34:2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005;65:385–411 [DOI] [PubMed] [Google Scholar]
- 105.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed]
- 106.Davis TM, Wright AD, Mehta ZM, et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia 2005;48:695–702 [DOI] [PubMed] [Google Scholar]
- 107.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003;163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen KW, Boyko EJ, Bergstrom RW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care 1995;18:747–753 [DOI] [PubMed] [Google Scholar]
- 109.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 110.Malecki MT, Mlynarski W. Monogenic diabetes: implications for therapy of rare types of disease. Diabetes Obes Metab 2008;10:607–616 [DOI] [PubMed] [Google Scholar]
- 111.Nordin C. The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia 2010;53:1552–1561 [DOI] [PubMed] [Google Scholar]
- 112.Riveline JP, Danchin N, Ledru F, Varroud-Vial M, Charpentier G. Sulfonylureas and cardiovascular effects: from experimental data to clinical use. Available data in humans and clinical applications. Diabetes Metab 2003;29:207–222 [DOI] [PubMed] [Google Scholar]
- 113.Sulistio M, Carothers C, Mangat M, Lujan M, Oliveros R, Chilton R. GLP-1 agonist-based therapies: an emerging new class of antidiabetic drug with potential cardioprotective effects. Curr Atheroscler Rep 2009;11:93–99 [DOI] [PubMed] [Google Scholar]
- 114.Hanefeld M, Schaper F. Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther 2008;6:153–163 [DOI] [PubMed] [Google Scholar]
- 115.Gaziano JM, Cincotta AH, O'Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010;33:1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masoudi FA, Inzucchi SE. Diabetes mellitus and heart failure: epidemiology, mechanisms, and pharmacotherapy. Am J Cardiol 2007;99:113B–132B [DOI] [PubMed] [Google Scholar]
- 117.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129–1136 [DOI] [PubMed] [Google Scholar]
- 118.Chaggar PS, Shaw SM, Williams SG. Review article: Thiazolidinediones and heart failure. Diab Vasc Dis Res 2009;6:146–152 [DOI] [PubMed] [Google Scholar]
- 119.Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation 2008;117:574–584 [DOI] [PubMed] [Google Scholar]
- 121.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the Diabetes and Aging Study. Diabetes Care 2011;34:1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koro CE, Lee BH, Bowlin SJ. Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States. Clin Ther 2009;31:2608–2617 [DOI] [PubMed] [Google Scholar]
- 123.Holstein A, Stumvoll M. Contraindications can damage your health—is metformin a case in point? Diabetologia 2005;48:2454–2459 [DOI] [PubMed] [Google Scholar]
- 124.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nye HJ, Herrington WG. Metformin: the safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract 2011;118:c380–c383 [DOI] [PubMed] [Google Scholar]
- 126.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 2007;11:1–16, vii [DOI] [PubMed] [Google Scholar]
- 127.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–649 [DOI] [PubMed] [Google Scholar]
- 128.Tushuizen ME, Bunck MC, Pouwels PJ, van Waesberghe JH, Diamant M, Heine RJ. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int 2006;26:1015–1017 [DOI] [PubMed] [Google Scholar]
- 129.Gerstein HC, Miller ME, Genuth S, et al. ; ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Riddle MC. Counterpoint: Intensive glucose control and mortality in ACCORD—still looking for clues. Diabetes Care 2010;33:2722–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother 2010;44:712–717 [DOI] [PubMed] [Google Scholar]
- 133.Rodbard D. The combinatorics of medications precludes evidence-based algorithms for therapy. Diabetologia 2010;53:2456–2457 [DOI] [PubMed] [Google Scholar]
- 134.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486–494 [DOI] [PubMed] [Google Scholar]