Abstract
OBJECTIVE
Misdiagnosis of maturity-onset diabetes of the young (MODY) remains widespread, despite the benefits of optimized management. This cross-sectional study examined diagnostic misclassification of MODY in subjects with clinically labeled young adult-onset type 1 and type 2 diabetes by extending genetic testing beyond current guidelines.
RESEARCH DESIGN AND METHODS
Individuals were selected for diagnostic sequencing if they displayed features atypical for their diagnostic label. From 247 case subjects with clinically labeled type 1 diabetes, we sequenced hepatocyte nuclear factor 1 α (HNF1A) and hepatocyte nuclear factor 4 α (HNF4A) in 20 with residual β-cell function ≥3 years from diagnosis (random or glucagon-stimulated C-peptide ≥0.2 nmol/L). From 322 with clinically labeled type 2 diabetes, we sequenced HNF1A and HNF4A in 80 with diabetes diagnosed ≤30 years and/or diabetes diagnosed ≤45 years without metabolic syndrome. We also sequenced the glucokinase (GCK) in 40 subjects with mild fasting hyperglycemia.
RESULTS
In the type 1 diabetic group, two HNF1A mutations were found (0.8% prevalence). In type 2 diabetic subjects, 10 HNF1A, two HNF4A, and one GCK mutation were identified (4.0%). Only 47% of MODY case subjects identified met current guidelines for diagnostic sequencing. Follow-up revealed a further 12 mutation carriers among relatives. Twenty-seven percent of newly identified MODY subjects changed treatment, all with improved glycemic control (HbA1c 8.8 vs. 7.3% at 3 months; P = 0.02).
CONCLUSIONS
The systematic use of widened diagnostic testing criteria doubled the numbers of MODY case subjects identified compared with current clinical practice. The yield was greatest in young adult-onset type 2 diabetes. We recommend that all patients diagnosed before age 30 and with presence of C-peptide at 3 years' duration are considered for molecular diagnostic analysis.
Clinicians who manage diabetes arising in young adults are faced with a wide range of underlying etiologies, which includes type 2 diabetes, autoimmune diabetes, and a large number of less common causes (1). Despite the clinical benefits of assigning an accurate diagnostic label, detailed etiological assessment, a key part of the diagnostic process, is frequently neglected.
The best described less common subtypes of diabetes are the monogenic β-cell disorders known as maturity-onset diabetes of the young (MODY) (2). This heterogeneous group of disorders is characterized by autosomal dominant inheritance, young age of onset (usually in the 2nd–4th decade), and continued secretion of endogenous insulin. The most frequent causes are mutations in genes encoding the transcription factors hepatocyte nuclear factor 1 α (HNF1A) and hepatocyte nuclear factor 4 α (HNF4A) (accounting for 52 and 10% of U.K. case subjects) and the glucokinase (GCK) enzyme (32% of U.K. case subjects) (3). Mutations in a number of other genes can also present with a MODY phenotype but these are rare in clinical practice (2).
A confirmed molecular diagnosis has important implications since this facilitates tailored management specific to the diabetes subtype (2). GCK-MODY is associated with nonprogressive, mild fasting hyperglycemia, which is rarely associated with microvascular complications and can usually be managed without pharmacological intervention (2,4). Diabetes associated with HNF1A-MODY and HNF4A-MODY is progressive, and complications occur with suboptimal glycemic control. It is noteworthy that patients with HNF1A/4A-MODY show particular sensitivity to treatment with low-dose sulfonylureas (5), and good glycemic control can be maintained for many years on these agents (6). Thus, sulfonylureas should be used as first-line treatment in these patients, an important distinction from both type 1 and type 2 diabetes. A further benefit of definitive molecular diagnosis is that diagnostic or predictive genetic testing can be offered to relatives.
Unfortunately, the advantages of establishing a diagnosis of MODY are not consistently translated into clinical practice and it is estimated that most MODY patients are mislabeled as type 1 or type 2 diabetes (3). Education of clinicians and cost of testing are important factors, but a major challenge is the clinical differentiation of the relatively small number of patients with monogenic diabetes from the larger numbers of patients with type 1 or type 2 diabetes. Clinical features overlap between MODY and common forms of diabetes: type 1 diabetes and MODY both present in lean individuals at a young age, whereas type 2 diabetes and MODY both retain endogenous insulin secretion and are not typically associated with β-cell antibodies or high-risk HLA haplotypes (7,8). Current guidelines for selecting case subjects for molecular testing include age of diabetes diagnosis before 25 years, parental history of diabetes, and noninsulin dependence (8). Strict adherence to these guidelines confers high specificity but low sensitivity to identify MODY subjects since less than half of those with a confirmed genetic diagnosis of MODY in European countries meet these testing criteria (3,9). Previous studies indicate age of diagnosis and parental diabetes are poor discriminators of HNF1A-MODY compared with both type 1 and young-onset type 2 diabetes (7,10).
Encouraging wider use of molecular testing to identify the majority of case subjects would be beneficial to patients, but requires a broader evidence base on which to establish diagnostic pathways. The Young Diabetes in Oxford (YDX) study was established to examine the etiological basis of diabetes diagnosed in early adulthood, where differential diagnosis is greatest and the potential for lifelong diagnostic misclassification is most marked. The study aims to elucidate the proportion of misdiagnosed MODY in young adults with clinically labeled type 1 or type 2 diabetes, to define additional clinical features that can be used to select subjects for genetic testing and to produce recommendations to guide clinical practice.
RESEARCH DESIGN AND METHODS
The YDX study comprises subjects diagnosed with diabetes up to 45 years of age. This is a cross-sectional study of subjects ascertained from a survey of 12 GP surgeries in Oxfordshire, U.K. and a search of the hospital clinic database. All those diagnosed with diabetes up to age 45 and currently aged ≥18 years were invited to take part. We report here on 569 individuals whose medical records at the time of recruitment indicated either a clinical label of type 1 diabetes (n = 247) or type 2 diabetes (n = 322). The study was approved by the Oxfordshire Local Research Ethics Committee, and all subjects gave informed consent.
Subjects who satisfied current genetic testing guidelines for MODY (i.e., age of diabetes diagnosis ≤25 years, a family history of diabetes and some evidence of noninsulin dependence for HNF1A/4A-MODY, and these features plus fasting glucose 5.5–8 mmol/L and HbA1c ≤8% for GCK-MODY) were identified (8). Those who had not undergone definitive molecular genetic testing were selected for resequencing of the HNF1A, HNF4A, and GCK genes. Furthermore, to explore the value of diagnostic testing in individuals not meeting current testing guidelines, we attempted to reach a definitive molecular diagnosis in those satisfying extended criteria for genetic testing. These criteria were based on the individual having clinical features that were atypical for their existing clinical classification, but consistent with a diagnosis of MODY (e.g., young-onset, C-peptide–positive diabetes with a predominantly β-cell defect).
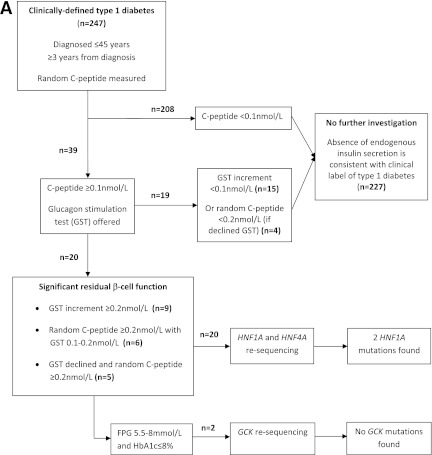
For individuals with clinically labeled type 1 diabetes, the extended testing was performed on those with persistent β-cell function outside the honeymoon period (≥3 years from diagnosis). Clinical data and anthropometry were collected. Initial investigations included a random C-peptide and glucose level (Fig. 1A). Random C-peptide was repeated if the glucose level was ≤4 mmol/L. GAD antibodies were also measured in all subjects by a radioimmunoassay using 35S-labeled full-length GAD65, and results were expressed in World Health Organization (WHO) units per milliliter derived from a standard curve calibrated from international reference material (National Institute for Biological Standards and Control code 97/550). A positive level was defined as >14 WHO units per milliliter (97.5th percentile of healthy children) (11). Those with random C-peptide ≥0.1 nmol/L were offered further assessment with a glucagon stimulation test (GST). In the GST, plasma C-peptide and glucose were measured at baseline and 6 min after intravenous administration of 1 mg glucagon (Novo Nordisk, Copenhagen). A C-peptide increment on stimulation of ≥0.2 nmol/L generally indicates significant residual β-cell function (12). To make the study as inclusive as possible we defined significant residual β-cell function as: 1) GST increment ≥0.2 nmol/L, 2) GST increment 0.1–0.2 nmol/L with random C-peptide ≥0.2 nmol/L, and 3) random C-peptide ≥0.2 nmol/L in those not having GST. All those with residual β-cell function underwent sequencing of HNF1A and HNF4A. In addition those with residual β-cell function and other features consistent with GCK-MODY, i.e., fasting glucose 5.5–8 mmol/L, HbA1c ≤8%, or a 1.5 anhydroglucitol level ≥10 μg/mL (13) underwent sequencing of GCK.
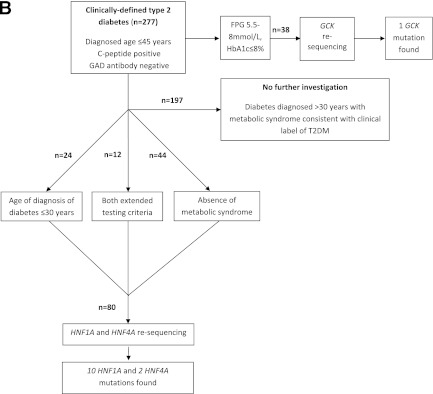
Figure 1.
A: Flowchart for investigation of individuals with clinically labeled type 1 diabetes. B: Flowchart for investigation of individuals with clinically labeled type 2 diabetes. FPG, fasting plasma glucose.
For those individuals with clinically labeled type 2 diabetes, clinical and anthropometric data were collected. Fasting blood was taken for measurement of HbA1c, glucose, lipid profile, C-peptide, and GAD antibodies. Testing was performed on two groups of GAD− subjects: 1) all those diagnosed up to 30 years and 2) those without metabolic syndrome (using International Diabetes Federation [IDF] criteria [14]) diagnosed up to 45 years (Fig. 1B). These individuals underwent sequencing of HNF1A and HNF4A. Subjects for GCK sequencing were selected using the criteria outlined above.
Seven clinically labeled type 1 diabetic subjects and 45 clinically labeled type 2 diabetic subjects were of non-European ethnicity (18 Asian, 25 Black, 1 Chinese, and 8 mixed or other).
All genetic testing was performed in the CPA-accredited Molecular Genetics Laboratory at the Royal Devon and Exeter National Health Service (NHS) Foundation Trust. Semiautomated unidirectional sequencing of HNF1A exons 1–10, HNF4A promoter P2, exons 1a and 2–10 and GCK promoter and exons 1–10 was performed on an ABI 3730 capillary sequencer (Carlsbad, CA) and analyzed using Mutation Surveyor v3.24 (SoftGenetics, State College, Pennsylvania). This method has >99% sensitivity to detect heterozygous base substitutions (15).
When MODY mutations were identified, all first-degree relatives were offered screening to ascertain glycemic and mutation status. The pathogenicity of novel missense mutations was determined by family studies looking for cosegregation of the mutation with dysglycemia, presence of typical MODY phenotype, and evidence from SIFT and PolyPHEN analysis. A trial of sulfonylurea was considered in all individuals with HNF1A or HNF4A mutations (probands and relatives); this was performed as detailed in the Supplementary Data. Successful transfer was defined as maintenance or improvement in HbA1c at 3 months compared with HbA1c at mutation confirmation.
All statistical analysis was performed in SPSS v17, and P < 0.05 was assumed to be significant. No adjustment for multiple comparisons was made.
RESULTS
Investigation of those with clinically labeled type 1 diabetes
From 247 subjects with clinically labeled type 1 diabetes, 39 (15.8%) subjects had random C-peptide ≥0.1 nmol/L. Thirty-one patients agreed to further assessment with GST. Of these, nine had a C-peptide increment at 6 min ≥0.2 nmol/L and six had random C-peptide ≥0.2 nmol/L with GST 0.1–0.2 nmol/L. Five of eight patients who declined GST had random C-peptide ≥0.2 nmol/L. Random C-peptide was correlated with fasting C-peptide (Pearson coefficient, r = 0.81, P < 3 × 10−8), homeostasis model assessment-B (r = 0.78, P < 3 × 10−7), stimulated C-peptide (r = 0.70, P < 8 × 10−7), and C-peptide increment in the GST (r = 0.66, P < 6 × 10−5).
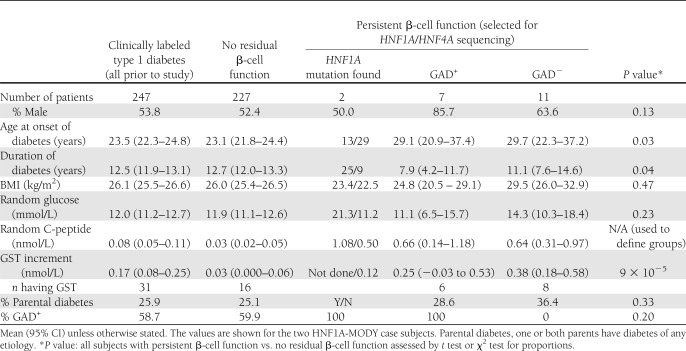
Thus 20 (8.1%) individuals with apparent type 1 diabetes had significant residual β-cell function and underwent resequencing of HNF1A and HNF4A (Table 1). They were diagnosed with diabetes at an older age (28.7 vs. 23.1 years; P = 0.031), had shorter duration of diabetes (10.4 vs. 12.7 years; P = 0.036), and had lower doses of insulin replacement (0.74 vs. 0.81 units/kg/day; P = 0.007) compared with the rest of the group, all features described previously as associated with persistent β-cell function (12). There was no difference in GAD positivity, BMI, or parental history of diabetes. Only 3 of these 20 individuals satisfied current guidelines for MODY testing (8).
Table 1.
Characteristics of subjects with clinically labeled type 1 diabetes before and after investigation
Of these 20, 2 individuals were found to have HNF1A mutations (10% of those resequenced), 1 of whom met current criteria for MODY testing. Two individuals underwent resequencing of the GCK gene, and no mutations were identified.
Family pedigrees for the MODY case subjects are shown in Supplementary Fig. 1. Proband 1 had a random C-peptide of 1.08 nmol/L, declined GST, and was heterozygous for a G31D c.92G>A missense mutation in HNF1A exon 1. The mutation-carrying sister of proband 1 is not diabetic at the age of 43. G31D has been reported previously >30 times in MODY case subjects but there is some evidence penetrance may be decreased ([16], Ellard unpublished). Before genetic diagnosis, proband 1 was treated with basal-bolus insulin. This was changed to gliclazide plus reduced basal insulin with improved glycemic control at 3 months (HbA1c from 10.5 to 8.6%). Proband 2, with a random C-peptide of 0.50 nmol/L and GST increment of 0.12 nmol/L, was heterozygous for a novel missense mutation (M490R c.1469T>G) in exon 8 of HNF1A. M490R is at a highly conserved residue and has not been identified in 400 normal chromosomes. This patient safely transferred from basal-bolus insulin to gliclazide and then nateglinide. However, after 2 months basal-bolus insulin was recommenced as a result of raised postprandial sugars. In addition to HNF1A mutations, both probands have GAD+ antibodies (564 and 118 WHO units, respectively). Insulinoma-associated protein 2 (IA2) antibodies were not detected. The overall prevalence of MODY in apparent type 1 diabetes was 0.8% (2 of 247).
We examined the features of the remaining 18 patients with residual β-cell function who did not have MODY mutations, dividing them into GAD+ and GAD− case subjects (Table 1). The GAD− subjects were diagnosed at a mean age of 29.7 years and were overweight (mean BMI 29.5 kg/m2). This suggests further diagnostic misclassification within the group; for instance, some of these subjects in reality had type 2 diabetes.
Investigation of those with clinically labeled type 2 diabetes
Forty-five individuals from 322 subjects with clinically labeled young adult-onset type 2 diabetes had GAD+ antibodies and so were reclassified as latent autoimmune diabetes of adulthood (LADA). The clinical and biochemical characteristics of the LADA subjects are highlighted in Supplementary Table 1.
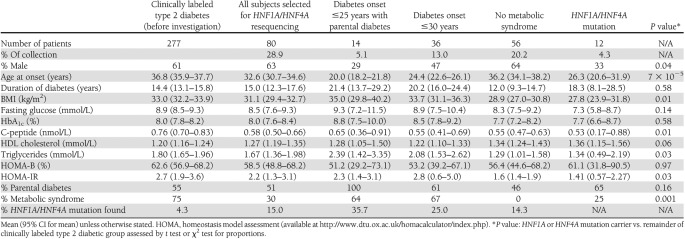
In the remaining 277 GAD− subjects, 14 were diagnosed with diabetes before age 25 years with parental diabetes (i.e., satisfied current testing guidelines), 36 individuals were diagnosed with diabetes ≤30 years, and 56 individuals were diagnosed with diabetes ≤45 years with no features of metabolic syndrome. The characteristics of these three groups are detailed in Table 2. Twelve individuals met both extended criteria (Supplementary Fig. 2). HNF1A and HNF4A were thus resequenced in 80 individuals (28.9% of group; Table 2). Ten HNF1A and two HNF4A mutations were found (15% of those tested; Supplementary Fig. 1; families 3 to 14). Five of the HNF1A/4A mutations identified were in patients meeting current guidelines for MODY testing. Eight of the HNF1A/HNF4A mutations have been described previously in association with MODY: HNF1A K150N [16], HNF1A c.872delC P291fs [16], HNF1A del exon 1 [17], HNF1A P291T c.871C>A [18], HNF1A R229× [16], and HNF4A F112 L [19]. Two subjects had novel truncating mutations highly likely to result in haploinsufficiency: HNF1A 9QX and HNF1A L377fsdelC. The other two individuals were heterozygous for novel missense mutations: HNF1A G606S and HNF4A H214R. The individual with HNF1A G606S had a two-generation family history of young-onset type 2 diabetes with long periods of control on sulfonylureas. HNF4A H214R showed cosegregation with IGT in the father and brother of the proband. Neither mutation had been identified in >400 normal chromosomes, and the amino acids concerned were highly conserved across species.
Table 2.
Subjects with clinically labeled young adult-onset type 2 diabetes showing characteristics of the subgroups selected for sequencing of HNF1A and HNF4A
Supplementary Fig. 2 shows the overlaps between the groups and the number of mutations identified for each testing criteria. Nine HNF1A/4A mutations were found in subjects diagnosed with diabetes up to 30 years (25.0% of those tested), and eight HNF1A/4A mutations were found in subjects with no features of metabolic syndrome (14.3% of those tested). Five of 12 individuals meeting both extended diagnostic testing criteria had HNF1A or HNF4A mutations (41.6%).
Resequencing for GCK-MODY was performed on 38 individuals, and one previously reported mutation (G299R [20]) was identified (Supplementary Fig. 1; family 15). This represented 2.6% of those tested and <0.5% of the type 2 diabetic group. This patient had a clinical phenotype entirely consistent with GCK-MODY and was on dietary treatment only. The overall prevalence of MODY in apparent young adult-onset type 2 diabetes was 4.0% (15 of 322).
Clinical features of the HNF1A/4A-MODY subjects
Within the two groups of subjects with either clinically labeled type 1 or type 2 diabetes (n = 569), 14 subjects were found to have HNF1A/4A-MODY. The mean duration of diabetes before genetic diagnosis was 17.8 (range 9.0–26.6) years (Supplementary Table 1). Thirteen subjects are white, and one is South Asian. All mutation carriers have detectable fasting C-peptide levels (range 0.15–1.58 nmol/L). Three (21%) have GAD+ antibodies but undetectable IA2 antibodies. Six (43%) have metabolic syndrome (IDF criteria). Nine (64%) have at least one first-degree relative with diabetes. Family screening detected 12 relatives who were mutation carriers: eight with diabetes including two newly diagnosed in the study, three with impaired glucose tolerance (1 new), and 1 normoglycemic. Two relatives with diabetes did not have MODY mutations (Supplementary Fig. 1). Mean (95% CI) HbA1c at 3 months in the HNF1A/4A-MODY individuals undergoing treatment changes (n = 6) improved from 8.8% (6.7–10.8%) to 7.3% (6.1–8.5%) at 3 months (P = 0.02) (Supplementary Fig. 3).
CONCLUSIONS
This study confirms that MODY is misdiagnosed as both type 1 and type 2 diabetes and that an accurate molecular diagnosis is often delayed for many years. Until falling costs for diagnostic resequencing allow more comprehensive investigation of MODY genes in all patients with young-onset diabetes, using much wider selection criteria than present, based on simple clinical features, can be used to identify individuals at high risk of having MODY. This is the widest and most extensive study of its kind to date and led to high positive rates of HNF1A/HNF4A-MODY (10–25% of those tested), particularly within the young adult-onset type 2 diabetic subjects. These results of course require validation in other populations, particularly non-European.
By extending MODY diagnostic testing beyond current guidelines, we identified MODY subjects with clinical features not expected to be present in MODY. These included raised BMI, presence of metabolic syndrome, GAD antibody-positivity, and absence of family history of diabetes. Furthermore, additional more atypical MODY cases cannot be excluded in the untested individuals. This emphasizes that the presence of these features should not preclude genetic testing where there is a high clinical suspicion. Given our findings, the decision to exclude subjects with LADA from genetic investigation may have been premature. There is a lack of consensus on the prevalence of positive β-cell antibodies in MODY patients: a very low prevalence was observed in U.K. MODY case subjects (21), whereas positive β-cell antibodies were found in 20% of children and adolescents from Germany with proven MODY mutations (22). Our results were in keeping with the U.K. dataset (21) where no MODY case subjects were reported with IA2 antibodies. Widening selection criteria are likely to lead to more MODY mutation carriers with mixed phenotypes, and in these case subjects, it will be increasingly difficult to assess the pathogenicity of novel mutations.
We identified only one patient with GCK-MODY out of 40 patients tested. This is comparable with another study indicating a low proportion of GCK-MODY in older populations (>35 years) in contrast with children or adolescents with the same biochemical features (23). Mild hyperglycemia in adults is more likely to reflect type 2 diabetes than MODY as a result of a GCK mutation. Moreover, the majority of the study subjects were recruited from secondary care, and it is likely that individuals with undiagnosed GCK-MODY will be managed in primary care (as a result of good glycemic control without development of complications).
Persistent β-cell function outside the honeymoon period in those with clinical label of type 1 diabetes was used to identify candidates for MODY testing. It is known that a proportion of patients assumed to have type 1 diabetes continue to secrete C-peptide for many years after initial diagnosis (12,24). Such individuals probably represent a mixture of some with alternative etiologies and some with a true slowly progressive autoimmune process. Subjects assumed to have type 1 diabetes arguably have the most to gain from an early diagnosis of MODY since they have the best chance of successful transfer from insulin to sulfonylureas. In this study only two individuals (<1% of the type 1 group) were found to have MODY, and although both showed some response to sulfonylureas, neither was able to stop insulin. Both were GAD antibody positive, so it is possible that autoimmunity is modulating their clinical phenotype. Of note, several MODY patients had been identified locally after finding them to be C-peptide positive after long durations of clinically labeled type 1 diabetes. This may have contributed to underestimated MODY prevalence in the type 1 group. In other centers, where less investigation for MODY has been performed as part of routine practice, this approach may have higher success rates. Because performing formal stimulation testing of β-cell function is not feasible in a routine clinical setting, and random C-peptide was well correlated with fasting and stimulated measures, a random C-peptide, measured either in blood or urine (25) and preferably taken postprandially, is a practical alternative. A limitation of using C-peptide for one-step screening is that ideally the molecular diagnosis should be made shortly after diagnosis to allow the withdrawal of insulin, but most with type 1 diabetes will retain some insulin secretion at this point. Waiting 3 years after diagnosis would exclude most with type 1 diabetes but is less satisfactory. More specific biomarkers (e.g., high-sensitivity C-reactive protein for HNF1A-MODY [26]) may be required to differentiate MODY subtypes from recently diagnosed type 1 diabetic subtypes.
We defined wider criteria to select subjects for genetic testing compared with current recommendations. The use of these extended diagnostic testing criteria more than doubled the number of transcription factor gene mutations identified, but at the expense of an approximate sixfold increase in the number of genetic tests performed compared with current practice. In this study, 9% of the subjects with type 1 diabetes and nearly 30% of subjects with type 2 diabetes diagnosed before 45 years had genetic sequencing. Health economic assessment will be an important component of implementing more wide-scale molecular investigation in diabetes into clinical practice.
Given the high prevalence of MODY (25%) we observed in subjects with apparent type 2 diabetes diagnosed before 30 years, we propose that all patients with C-peptide–positive diabetes (serum C-peptide ≥0.2 nmol/L, 3 years after diagnosis for those assumed to have type 1 diabetes) diagnosed up to the age of 30 years should be considered for resequencing of the HNF1A and HNF4A genes, regardless of the family history of diabetes, GAD positivity, or metabolic features consistent with insulin resistance. The majority of case subjects of MODY will remain misdiagnosed until such a systematic assessment of etiology is adopted for young-onset diabetes.
Acknowledgments
The study was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford; Diabetes UK; the European Community FP7 Programme CEED3 (HEALTH-F2-2008-223211); and the Oxford Hospitals Charitable Fund. The study was also supported by the NIHR Thames Valley Diabetes Local Research Network, part of the U.K. Clinical Research Network. S.E. is employed as a core member of staff within the NIHR-funded Peninsula Clinical Research Facility. A.J.F. is supported by the NIHR School for Primary Care Research and the Oxford NIHR Biomedical Research Centre. K.R.O. is an NIHR-funded Clinician Scientist.
No potential conflicts of interest relevant to this article were reported.
G.T. and A.P. researched and interpreted data and drafted and edited the manuscript. M.P.S., C.D., K.F., P.J.B., S.E., and A.J.F. researched data, contributed to the discussion, and edited the manuscript. M.I.M. designed the study, researched and interpreted the data, and drafted and edited the manuscript. K.R.O. designed the study, researched and interpreted the data, drafted and edited the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Diabetes UK Annual Professional Conference, Liverpool, U.K., 3–5 March 2010 and at the 46th Scientific Sessions of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
The authors thank their research nurses Jilly Grew, Beryl Barrow, Vanessa Loach, and Rachel Craven-Todd (all University of Oxford) and their Genetic Diabetes Specialist nurse Amanda Webster (Oxford Radcliffe NHS Trust); Tim James in the Department of Biochemistry, Oxford Radcliffe NHS Trust, for help and advice; and all patients and families involved in their research.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1243/-/DC1.
References
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molven A, Njølstad PR. Role of molecular genetics in transforming diagnosis of diabetes mellitus. Expert Rev Mol Diagn 2011;11:313–320 [DOI] [PubMed] [Google Scholar]
- 3.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–2508 10.1007/s00125-010-1799-4 [DOI] [PubMed] [Google Scholar]
- 4.Steele AM, Wensley KJ, Shields BM, Shepherd M, Ellard S, Colclough K, Hattersley AT. Microvascular complication risk in patients with 50 years of moderate hyperglycaemia: are target ranges for glycaemic control appropriate [Abstract]? Diabet Med 2011;28(Suppl. 1):28 [Google Scholar]
- 5.Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362:1275–1281 10.1016/S0140-6736(03)14571-0 [DOI] [PubMed] [Google Scholar]
- 6.Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med 2009;26:437–441 10.1111/j.1464-5491.2009.02690.x [DOI] [PubMed] [Google Scholar]
- 7.Lambert AP, Ellard S, Allen LI, et al. Identifying hepatic nuclear factor 1α mutations in children and young adults with a clinical diagnosis of type 1 diabetes. Diabetes Care 2003;26:333–337 10.2337/diacare.26.2.333 [DOI] [PubMed] [Google Scholar]
- 8.Ellard S, Bellanné-Chantelot C, Hattersley AT, European Molecular Genetics Quality Network (EMQN) MODY group Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia 2008;51:546–553 10.1007/s00125-008-0942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellanne-Chantelot C, Levy DJ, Carette C, et al. Clinical characteristics and diagnostic criteria of Maturity-Onset Diabetes of the Young (MODY) due to molecular anomalies of the HNF1A gene. J Clin Endocrinol Metab 2011;96:E1346–E1351. [DOI] [PubMed]
- 10.Owen KR, Shepherd M, Stride A, Ellard S, Hattersley AT. Heterogeneity in young adult onset diabetes: aetiology alters clinical characteristics. Diabet Med 2002;19:758–761 10.1046/j.1464-5491.2002.00766.x [DOI] [PubMed] [Google Scholar]
- 11.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 10.2337/diabetes.46.11.1701 [DOI] [PubMed] [Google Scholar]
- 12.Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J Clin Endocrinol Metab 1987;65:30–36 10.1210/jcem-65-1-30 [DOI] [PubMed] [Google Scholar]
- 13.Pal A, Farmer AJ, Dudley C, et al. Evaluation of serum 1,5 anhydroglucitol levels as a clinical test to differentiate subtypes of diabetes. Diabetes Care 2010;33:252–257 [DOI] [PMC free article] [PubMed]
- 14.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–480 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 15.Ellard S, Shields B, Tysoe C, et al. Semi-automated unidirectional sequence analysis for mutation detection in a clinical diagnostic setting. Genet Test Mol Biomarkers 2009;13:381–386 10.1089/gtmb.2008.0096 [DOI] [PubMed] [Google Scholar]
- 16.Ellard S, Colclough K. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha (HNF1A) and 4 alpha (HNF4A) in maturity-onset diabetes of the young. Hum Mutat 2006;27:854–869 10.1002/humu.20357 [DOI] [PubMed] [Google Scholar]
- 17.Ellard S, Thomas K, Edghill EL, et al. Partial and whole gene deletion mutations of the GCK and HNF1A genes in maturity-onset diabetes of the young. Diabetologia 2007;50:2313–2317 10.1007/s00125-007-0798-6 [DOI] [PubMed] [Google Scholar]
- 18.Bellanné-Chantelot C, Carette C, Riveline JP, et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2008;57:503–508 10.2337/db07-0859 [DOI] [PubMed] [Google Scholar]
- 19.Harries LW, Locke JM, Shields B, et al. The diabetic phenotype in HNF4A mutation carriers is moderated by the expression of HNF4A isoforms from the P1 promoter during fetal development. Diabetes 2008;57:1745–1752 10.2337/db07-1742 [DOI] [PubMed] [Google Scholar]
- 20.Osbak KK, Colclough K, Saint-Martin C, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat 2009;30:1512–1526 10.1002/humu.21110 [DOI] [PubMed] [Google Scholar]
- 21.McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med 2011;28:1028–1033 [DOI] [PubMed] [Google Scholar]
- 22.Schober E, Rami B, Grabert M, et al. DPV-Wiss Initiative of the German Working Group for Paediatric Diabetology and Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with Type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med 2009;26:466–473 10.1111/j.1464-5491.2009.02720.x [DOI] [PubMed] [Google Scholar]
- 23.Gloyn AL, van de Bunt M, Stratton IM, et al. Prevalence of GCK mutations in individuals screened for fasting hyperglycaemia. Diabetologia 2009;52:172–174 10.1007/s00125-008-1188-4 [DOI] [PubMed] [Google Scholar]
- 24.Liu EH, Digon BJ, 3rd, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia 2009;52:1369–1380 10.1007/s00125-009-1342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besser RE, Shepherd MH, McDonald TJ, et al. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-α/hepatocyte nuclear factor 4-α maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care 2011;34:286–291 10.2337/dc10-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen KR, Thanabalasingham G, James TJ, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care 2010;33:1919–1924 10.2337/dc10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]