Abstract
OBJECTIVE
To evaluate the effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in type 2 diabetes mellitus inadequately controlled with metformin monotherapy.
RESEARCH DESIGN AND METHODS
This was a double-blind, placebo-controlled, parallel-group, multicenter, dose-ranging study in 451 subjects randomized to canagliflozin 50, 100, 200, or 300 mg once daily (QD) or 300 mg twice daily (BID), sitagliptin 100 mg QD, or placebo. Primary end point was change in A1C from baseline through week 12. Secondary end points included change in fasting plasma glucose (FPG), body weight, and overnight urinary glucose-to-creatinine ratio. Safety and tolerability were also assessed.
RESULTS
Canagliflozin was associated with significant reductions in A1C from baseline (7.6–8.0%) to week 12: −0.79, −0.76, −0.70, −0.92, and −0.95% for canagliflozin 50, 100, 200, 300 mg QD and 300 mg BID, respectively, versus −0.22% for placebo (all P < 0.001) and −0.74% for sitagliptin. FPG was reduced by −16 to −27 mg/dL, and body weight was reduced by −2.3 to −3.4%, with significant increases in urinary glucose-to-creatinine ratio. Adverse events were transient, mild to moderate, and balanced across arms except for a non–dose-dependent increase in symptomatic genital infections with canagliflozin (3–8%) versus placebo and sitagliptin (2%). Urinary tract infections were reported without dose dependency in 3–9% of canagliflozin, 6% of placebo, and 2% of sitagliptin arms. Overall incidence of hypoglycemia was low.
CONCLUSIONS
Canagliflozin added onto metformin significantly improved glycemic control in type 2 diabetes and was associated with low incidence of hypoglycemia and significant weight loss. The safety/tolerability profile of canagliflozin was favorable except for increased frequency of genital infections in females.
More than 40% of adults with type 2 diabetes mellitus in the U.S. do not have glycemic control at recommended goal levels (1). Additional therapeutic options with mechanisms of action that complement existing therapies may help achieve and maintain better glycemic control. Agents that can improve glycemic control without increasing hypoglycemia while promoting weight loss and improving β-cell function are desirable, and sodium-glucose cotransporter 2 (SGLT2) inhibitors may prove to be such agents (2).
SGLT2 is expressed primarily in the early proximal renal tubule and is responsible for most of the glucose reabsorption in the kidneys (2,3). Inhibition of SGLT2 decreases glucose reabsorption in the renal tubule and increases glucose excretion (3,4). Partitioning of glucose out of the body through increased urinary glucose excretion (UGE) directly reduces elevated glucose concentrations and, by loss of calories (since each gram of glucose lost is equivalent to 4 calories), tends to lead to weight loss.
Canagliflozin, an SGLT2 inhibitor currently in phase 3 development for the treatment of type 2 diabetes, has been shown to reduce the renal threshold for glucose reabsorption, increase UGE, reduce plasma glucose, and lead to weight loss in a short-term study (5). Following this, the objective of the current study was to determine the dose-response efficacy and safety of canagliflozin during a 12-week period in subjects with type 2 diabetes inadequately controlled with metformin monotherapy.
RESEARCH DESIGN AND METHODS
This was a randomized, double-blind, placebo-controlled, parallel-group, multicenter, dose-ranging study. Subjects were randomized to one of seven treatment groups: canagliflozin at doses of 50, 100, 200, or 300 mg once daily (QD) or 300 mg twice daily (BID); sitagliptin 100 mg QD, or placebo. Sitagliptin was included as an active-reference treatment group to provide clinical perspective. The study periods included a 3- to 4-week pretreatment screening phase, a 12-week double-blind treatment phase, and a 2-week posttreatment phase (Supplementary Fig. 1).
Study population
Eligible subjects were men and women 18–65 years of age who were diagnosed with type 2 diabetes for at least 3 months, had an A1C level ≥7% and ≤10.5%, were on metformin monotherapy at a stable (≥3 months) dose of ≥1,500 mg/day, had a stable body weight and BMI 25–45 kg/m2 (24–45 kg/m2 for those of Asian descent), and had serum creatinine levels <1.5 mg/dL for men and <1.4 mg/dL for women.
The study protocol was reviewed and approved by institutional review boards and independent ethics committees. The study was conducted in accordance with the principles in the Declaration of Helsinki and was consistent with good clinical practices and applicable regulatory requirements. All study participants gave written consent prior to screening for this study. This trial is registered on ClinicalTrials.gov under the identifier NCT00642278.
Study end points
The primary end point was change in A1C from baseline to week 12. Secondary end points included change from baseline to week 12 in fasting plasma glucose (FPG), overnight urinary glucose-to-creatinine (UGlucose-to-UCreatinine) ratio, and body weight, as well as a change in the percentage of subjects with A1C <7.0% and <6.5% after 12 weeks of treatment. Other end points included change in fasting serum lipids (triglycerides, HDL cholesterol, LDL cholesterol, total cholesterol, and total cholesterol–to–HDL cholesterol ratio). β-Cell function was indirectly assessed by changes in homeostasis model assessment 2 (HOMA2) index of β-cell function (HOMA2-%B). The safety and tolerability of canagliflozin was assessed based on end points described in the next section.
Study assessments
Key efficacy parameters were evaluated at baseline and at several time points during the 12-week double-blind treatment period. To account for differences in plasma glucose and glomerular filtration rate, the overnight renal threshold for glucose excretion (RTG) was calculated from UGE, plasma glucose, and estimated glomerular filtration rate using an approach similar to that used to calculate the renal threshold for phosphate (6) as previously described (7). β-Cell function was assessed using HOMA2-%B, HOMA2 calculator version 2.2 (Oxford, U.K.), with fasting glucose and C-peptide values at baseline and at week 12.
Safety and tolerability assessments included collection of adverse event (AE) reports, vital signs, 12-lead electrocardiograms, physical examinations, and safety laboratory assessments. Self-administered vaginal swabs for Candida culture and urine cultures were to be obtained from all subjects at baseline and week 12. Vaginal swabs for Candida culture were also to be obtained at the time of a vulvovaginal AE (VVAE). Subjects were instructed on how to recognize the signs and symptoms of hypoglycemia.
Statistical methods
The study was powered at 80% to detect a mean difference of 0.55% in change of A1C at week 12 between the canagliflozin groups and placebo, assuming a 1.0% SD. Efficacy analyses were based on the intent-to-treat analysis set (all randomized subjects), and safety analyses included all intent-to-treat subjects who received at least one dose of the study medication. The primary efficacy analysis of canagliflozin and placebo was based on an ANCOVA model that included terms for treatment, the baseline value as a covariate, and the stratification factor of whether the subject participated in the mixed-meal tolerance test. The between-group differences were assessed by testing the difference in the least squares mean change from baseline at week 12 versus placebo, and Dunnett procedure was used to adjust for the multiple treatment comparisons. Similar models were used to analyze the continuous secondary end points. No statistical comparisons were made with canagliflozin versus sitagliptin, which was used to provide a benchmark clinical perspective. Missing values in the efficacy end points were imputed using the last observation carried forward. Safety and tolerability were assessed by a review of safety parameters.
RESULTS
Subject disposition and baseline characteristics
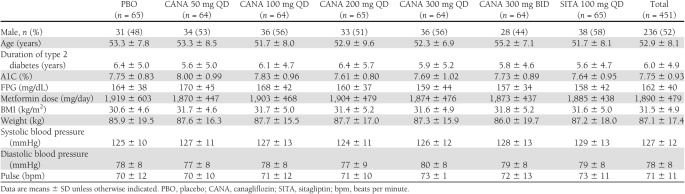
The intent-to-treat analysis set included 451 subjects randomized at 85 study sites in 12 countries. Demographic and baseline characteristics were balanced across treatment groups (Table 1). Mean age was 52.9 years, and 52% of the subjects were male. Mean BMI was 31.5 kg/m2, and 56% of the subjects were classified as obese (BMI ≥30 kg/m2). Baseline glycemic control reflected mild to moderate hyperglycemia, with baseline A1C in the 7.6–8.0% range across treatment groups. A total of 49 (11%) subjects discontinued before study completion, with a similar proportion discontinuing across treatment groups (Supplementary Fig. 2).
Table 1.
Baseline demographics and clinical characteristics
Efficacy
A1C lowering.
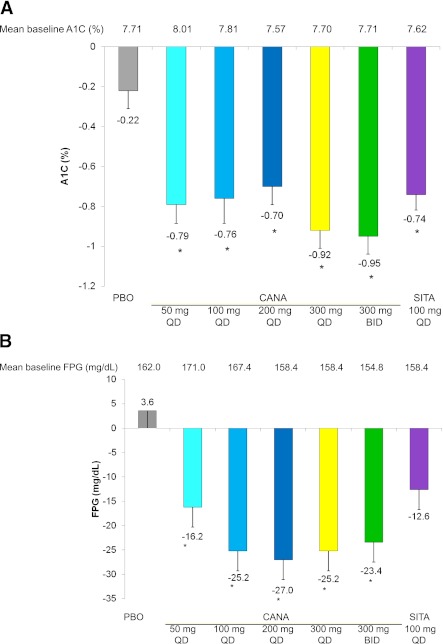
For the primary efficacy end point, change in A1C from baseline to week 12, there was a significant reduction in all canagliflozin doses relative to placebo (P < 0.001) (Fig. 1A).
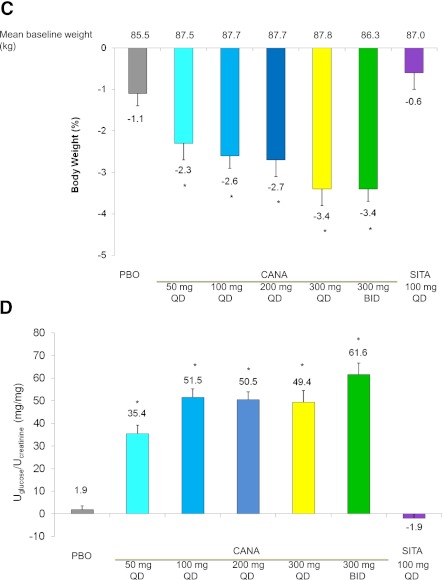
Figure 1.
Effects of canagliflozin from baseline to week 12 in subjects with type 2 diabetes on metformin. Mean change in A1C (A), FPG (B), body weight (C), and UGlucose-to-UCreatinine ratio (D) from baseline to week 12. Data are observed mean changes from baseline. Error bars show SE of the mean (last observation carried forward). *P < 0.001 vs. placebo calculated using least squares means. PBO, placebo; CANA, canagliflozin; SITA, sitagliptin.
A1C reductions from a baseline of 7.6–8.0% with canagliflozin ranged from 0.70 to 0.95%, with the greatest reductions observed in the 300-mg QD and BID treatment groups (−0.79, −0.76, −0.70, −0.92, −0, and 0.95% for canagliflozin 50, 100, 200, and 300 mg QD and 300 mg BID, respectively, vs. −0.22% for placebo). The difference was statistically significant (P < 0.001) compared with placebo across all canagliflozin treatment groups after adjustment using Dunnett procedure. Sitagliptin reduced A1C by 0.74% (P < 0.001). A greater proportion of subjects (P < 0.05 based on logistic regression) achieved the target of A1C <7.0% at week 12 with canagliflozin doses of 100 mg QD and above (53–72%) and with sitagliptin (65%) compared with placebo (34%). In addition, significantly more subjects achieved A1C <6.5% with canagliflozin at 100 and 300 mg QD and at 300 mg BID (27, 42, and 32%, respectively), and sitagliptin (45%) compared with placebo (13%).
FPG.
Significantly greater mean reductions in FPG were observed with all doses of canagliflozin (−16.2 to −27.0 mg/dL) compared with placebo (3.6 mg/dL). Sitagliptin also reduced FPG (−12.6 mg/dL) (Fig. 1B). The FPG reduction with canagliflozin appeared maximal at doses of 200 mg QD and above. Reduction in FPG was maximal by the first double-blind treatment period visit at week 3 and was maintained through week 12.
Body weight.
Body weight reductions were seen in all canagliflozin groups relative to placebo (Fig. 1C). Canagliflozin was associated with reductions in body weight from baseline; these reductions were −2.3 to −3.4% (−2.0 to −2.9 kg) at week 12. Reductions observed in the placebo and sitagliptin treatment groups were −1.1% (−0.8 kg) and −0.6% (−0.4 kg) from baseline, respectively. Weight loss appeared to be greatest in subjects in the canagliflozin 300-mg QD and BID groups. Weight loss was progressive during the 12-week treatment period without apparently reaching a plateau (Supplementary Fig. 3). At week 12, last observation carried forward, at least a 5% body weight loss from baseline was observed in 16, 12, 22, 21, and 32% of subjects in the canagliflozin 50-, 100-, 200-, and 300-mg QD and the 300-mg BID dose groups, respectively, compared with 5% in the placebo group and 6% in the sitagliptin group.
UGE.
All doses of canagliflozin increased the overnight UGlucose-to-UCreatinine ratio (Fig. 1D). The increase in UGlucose-to-UCreatinine ratio was similar with 100- to 300-mg QD doses and modestly greater than observed with the 50-mg QD dose; an additional increase in UGE with 300 mg BID was also noted. The increase in UGE observed at week 3 persisted without evident attenuation through week 12. Canagliflozin lowered overnight calculated RTG in a dose-dependent fashion (Supplementary Fig. 4). RTG (mean ± SD) was lowered to 80.3 ± 27.1 and 77.1 ± 23.1 mg/dL with 300-mg QD and 300-mg BID treatments, respectively, at week 12.
Fasting lipids.
There was an increase in HDL cholesterol (significant with canagliflozin 300 mg BID, P = 0.001), a slight reduction in the ratio of total cholesterol to HDL cholesterol, and a significant reduction in triglycerides with canagliflozin 300-mg QD and BID doses compared with placebo (P = 0.025 and 0.001, respectively). Compared with placebo, there were slight increases in LDL cholesterol with canagliflozin 300 mg BID, with no notable changes observed at the once-daily doses of canagliflozin (Supplementary Table 1).
β-Cell function.
There was a significant improvement in β-cell function as indirectly assessed by HOMA2-%B with canagliflozin at doses of 100 mg QD and above and with sitagliptin at week 12 relative to placebo (Supplementary Table 2).
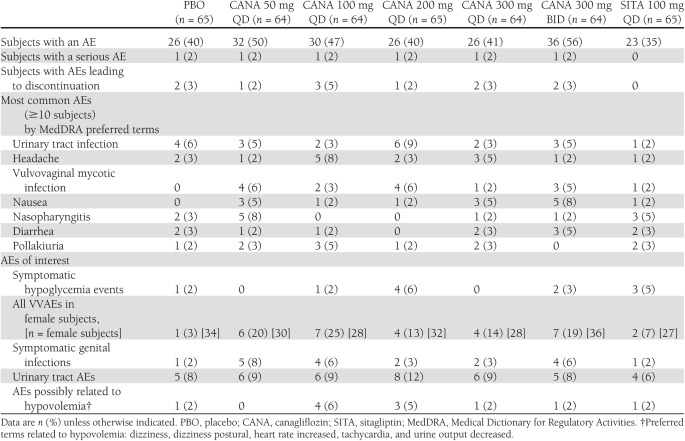
Safety
The incidence of AEs was generally similar across once-daily canagliflozin treatment groups and placebo, with a slightly higher incidence observed in the canagliflozin 300-mg BID treatment group (Table 2). The majority of AEs were evaluated as mild to moderate in intensity by the investigators. The incidence of serious AEs was low and similar across treatment groups (Table 2). Discontinuation because of AEs occurred in 11 subjects overall (2%) and in 9 subjects in the canagliflozin groups (3%) without apparent relation to dose (Table 2); most discontinuations were for gastrointestinal disorders (5 subjects [1 placebo, 4 canagliflozin], 1%). The incidence of symptomatic hypoglycemia was low and generally similar across active and placebo treatment groups (Table 2), with no severe or serious events.
Table 2.
Summary of AEs
On the basis of the mechanism of action of canagliflozin to increase UGE, several AEs were prespecified for additional analysis, including VVAEs, urinary tract infections, AEs potentially reflecting an osmotic diuresis (e.g., polyuria), and AEs reflecting volume depletion (e.g., orthostatic hypotension).
The incidence of symptomatic and asymptomatic (i.e., positive vaginal cultures only) genital infections in female subjects treated with canagliflozin increased without apparent relation to dose. Genital infections were reported in 13–25% of female subjects across canagliflozin treatment groups (3–8% of all subjects) and in 3% of female placebo subjects (2% of all placebo subjects) (Table 2). One genital AE (balanoposthitis) occurred in a male subject treated with canagliflozin. The most common VVAEs reported were vulvovaginal mycotic infection and vulvovaginal candidiasis. VVAEs were assessed by the investigators as mild in intensity and responded to a standard course of oral (n = 7), topical (n = 5), or a combination of oral and topical (n = 5) azole antifungal agents; none led to discontinuation. Subjects’ self-administered vaginal swabs were collected at baseline and at the final study visit. At baseline, 12% (23 of 198 had a vaginal swab result available) were positive for a Candida species, which predicted the occurrence of VVAEs in women treated with canagliflozin (odds ratio 9.1 [95% CI 2.4–34.0]) (8). Among women with swab results available at baseline and end study, and with negative swab cultures at baseline, 35 of 114 (31%) converted to a positive Candida culture in the canagliflozin treatment groups (pooling the treatment groups, since there was no dose dependency to conversion), and 14% of subjects in the pooled placebo and sitagliptin treatment groups converted to positive (pooling, since rates of conversion were similar in these groups). Nine of the 16 subjects in the pooled canagliflozin groups with VVAEs had a vaginal culture at the time of the AEs, and all of these 9 were positive for Candida (8).
Urinary tract AEs seemed to occur with a generally similar incidence across placebo (8%) and canagliflozin treatment groups (8–12%). Urinary tract infections apparently occurred at similar rates in placebo (6%) and canagliflozin (3–9%) treatment groups. Polyuria and pollakiuria were reported at low and similar rates in placebo (0 and 2%, respectively) and canagliflozin (0–5 and 0–5%, respectively). These generally occurred early in the double-blind treatment period, were evaluated as mild in severity, and did not lead to discontinuation. A low rate of AEs possibly related to hypovolemia was observed in the canagliflozin treatment groups (0–6%), apparently similar to the incidence observed in the placebo group (2%) (Table 2). There was a trend toward slight systolic blood pressure reductions with canagliflozin, which were not accompanied by changes in pulse (Supplementary Table 3).
Small increases in blood urea nitrogen (0.79–2.00 mg/dL), serum magnesium (0.10–0.20 mg/dL), hemoglobin (3.2–8.1 g/L), and hematocrit (1.1–2.6%) and a decrease in serum uric acid (−0.53 to −0.96 mg/dL) were observed in canagliflozin treatment groups (Supplementary Table 4). Except for a modest increase in serum collagen type 1 β-carboxy-terminal telopeptide (0.06–0.11 ng/mL), a marker of bone resorption, with canagliflozin treatment, no changes were seen in markers of either bone formation or resorption in canagliflozin treatment groups relative to placebo (Supplementary Table 4).
CONCLUSIONS
This dose-ranging trial demonstrates that canagliflozin, a novel oral antihyperglycemic agent, at all doses studied, significantly improved glycemic control without an increased occurrence of hypoglycemia in subjects with type 2 diabetes who had inadequate glycemic control with metformin. The extent of improvement in A1C observed with canagliflozin is particularly notable given the mild baseline hyperglycemia in the subject population studied. Improvements in glycemic control, with reductions in A1C and FPG, were observed at 100 mg QD with further improvement seen up to a 300-mg QD dose, as well as modestly greater weight reduction at the top once-daily canagliflozin dose. Notable in this observation is the fact that UGE (ratio of UGlucose to UCreatinine) did not appear to be substantively greater at the 300-mg relative to the 100-mg QD doses, similar to observations in other studies of canagliflozin, suggesting that the higher dose may provide an additional mechanism of glucose lowering. Perhaps an effect in slowing the rate of glucose absorption after a meal (through inhibition of luminal SGLT1 in the proximal small intestine) as has been suggested at higher canagliflozin doses, including at 300 mg, may explain the tendency for greater effects with these higher doses (5). However, this mechanism remains speculative and is under active study. Whether 300 mg QD provides greater efficacy compared with 100 mg, as well as the mechanisms responsible for the greater efficacy, requires further dosage studies of canagliflozin.
Canagliflozin treatment provided significant and clinically meaningful weight loss at all doses studied. The most likely mechanism of weight loss is through the increased UGE partitioning out calorie equivalents: each gram of glucose excreted translates to a loss of 4 kcal. Early studies in subjects with type 2 diabetes showed UGE of ∼60–100 g/day—equivalent to ∼240–400 kcal/day (5). Longer-term treatment studies are necessary to determine whether the weight loss observed in this study will persist beyond the short treatment period examined here.
In this study, canagliflozin improved a fasting indirect measure of β-cell function (HOMA2-%B). Canagliflozin-induced improvements in β-cell function have also been previously observed after a 2-week treatment period in subjects with type 2 diabetes (9). Weight loss has been shown to improve β-cell function, likely by reducing insulin resistance. In a similar manner, reduced β-cell demand, such as through pharmacologic interventions, also improves β-cell function (10). The increased glucose excretion with canagliflozin both reduced weight and likely directly reduced demand for insulin secretion and may explain the β-cell functional improvements we observed. Reversal of glucotoxicity may also have contributed to the observed improvement, which cannot be excluded without a concurrent active control that does not directly enhance insulin secretion (11). Additional studies of canagliflozin, with direct measures of insulin secretion, are necessary to understand the observation of improved β-cell function and whether this provides long-term benefits, such as improved glucose control durability.
Canagliflozin was well tolerated in this study, with no evident increase in overall AE incidence compared with placebo, across the once-daily treatment groups except for increased incidence of genital infections in female subjects. This included both symptomatic and asymptomatic reports (the latter reporting positive vaginal Candida cultures). The symptomatic infections were reported as mild and responded to standard oral and topical antifungal treatments. All women with a vaginal culture at the time of the VVAE were positive for yeast (8). The symptomatic infections were reported as mild and responded to standard oral and topical antifungal treatments. While baseline A1C, FPG, and BMI were not significant predictors of VVAEs in the pooled canagliflozin groups in a logistic regression analysis, positive cultures at baseline as well as location in the North American region predicted the occurrence of VVAEs in women treated with canagliflozin (odds ratios 9.1 [95% CI 2.4–34.0] and 4.2 [1.2–14.4], respectively) (8). By increasing UGE, SGLT2 inhibitors increase vaginal colonization with Candida species, and the increase in colonization may be the etiology for the increased VVAEs seen in women treated with canagliflozin.
There appears to be no higher rate of urinary tract infections with canagliflozin treatment. These observations may suggest that an increase in UGE raises the risk of genital mycotic infections but apparently not of urinary tract infections. This is consistent with the observation that the incidence of mycotic genital infections is higher with poorly controlled diabetes (especially vulvovaginal candidiasis and balanoposthitis) but without a clear increase in bacterial or candidal urinary tract infections (12,13). However, evidence from the phase 3 dapagliflozin development program indicated an increase in events suggestive of urinary tract infections with dapagliflozin versus comparators (14). Larger and longer studies will be needed to better define the incidence of both types of infections and the impact, if any, on renal function in patients treated with canagliflozin.
RTG was lowered with canagliflozin (maximal reduction of 77 mg/dL) but remained above the hypoglycemic threshold (usually considered to be 60–70 mg/dL). Since minimal UGE occurs below the RTG, hypoglycemia would not be expected, and was not observed, above the rate reported for placebo in this study.
Although the increase in UGE would be expected to lead to osmotic diuresis, no evidence of clinically important volume depletion was observed; in particular, the incidence of AEs suggestive of volume depletion (e.g., orthostatic dizziness and hypotension) was not increased, and only a small reduction in blood pressure—with no change in heart rate—was observed. In a similar manner, few AEs suggestive of osmotic diuresis (such as polyuria and pollakiuria) were reported. These observations may relate to the fact that the extent of osmotic load is relatively modest, not leading to more marked changes in volume or urinary output, with compensatory mechanisms contributing to maintaining normal volume. Consistent with a slight decrease in intravascular volume was a modest increase in blood urea nitrogen, hemoglobin, and hematocrit in the canagliflozin treatment groups relative to the placebo group. There was a reduction in serum urate concentrations similar to what has been described with other SGLT2 inhibitors (15,16). The SGLT2 inhibitor–induced decreases in serum urate concentrations may be mediated by glucosuria facilitating urate efflux into the tubular lumen by the high-capacity urate transporter SLC2A9 (GLUT9) (17).
In conclusion, canagliflozin added onto metformin monotherapy provides clinically valuable improvements in glycemic control associated with weight loss and low hypoglycemia risk. An increase in genital infections in women (particularly VVAEs suggestive of vulvovaginal candidiasis) was seen, but these appeared to be generally mild to moderate and responded to usual antifungal therapies. Future long-term studies examining the efficacy of canagliflozin with other antihyperglycemic medications will be needed; however, the unique and distinct mechanism of glucose lowering with canagliflozin suggests that combination efficacy may be observed when this agent is added to other classes of agents.
Phase 3 studies of canagliflozin are now under way to better define the efficacy profile across multiple uses, including in monotherapy and various combinations, and to better understand the longer-term safety and efficacy profile of this agent. The profile of effective glucose lowering, weight loss, improved β-cell function, and low risk of hypoglycemia suggest that canagliflozin may be a clinically useful new antihyperglycemic agent.
Acknowledgments
This study was funded by Janssen Global Services, LLC. Editorial assistance was provided by Susan DePetris, PhD, Phase Five Communications, Inc., supported by Janssen Global Services, LLC. Canagliflozin is being developed by Janssen Global Services, LLC in collaboration with Mitsubishi Tanabe Pharma Corporation.
J.R. has served on scientific advisory boards and received honoraria or consulting fees from Pfizer, Roche, sanofi-aventis, Novo Nordisk, Eli Lilly, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Johnson & Johnson, Novartis, Boehringer Ingelheim, Lexicon, and Amylin and also has received grants/research support from Merck, Pfizer, sanofi-aventis, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly, Forest, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, Johnson & Johnson, Daiichi Sankyo, MannKind, Lexicon, and Boehinger Ingelheim. D.P., Y.Z., K.U., G.C., and W.C. are employees of Janssen Global Services, LLC. D.A. was employed at Johnson & Johnson Pharmaceutical Research & Development, LLC during the conduct of this study. No other potential conflicts of interest relevant to this article were reported.
J.R. contributed to the design and conduct of the study and the acquisition, analysis, and interpretation of data and developed, reviewed, and approved the manuscript. N.A. contributed to the acquisition, analysis, and interpretation of data and reviewed and approved the manuscript. D.P. contributed to the analysis and interpretation of data and reviewed and approved the manuscript. Y.Z. and G.C. contributed to the design of the study and the analysis and interpretation of data and reviewed and approved the manuscript. D.A., K.U., and W.C. contributed to the design and conduct of the study and the analysis and interpretation of data and developed, reviewed, and approved the manuscript. J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010, and at the 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
The authors acknowledge Sue Sha, MD, PhD, Janssen Global Services, LLC, for her assistance in data analysis and her contributions to the discussion.
Footnotes
Clinical trial reg. no. NCT00642278, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1926/-/DC1.
†Deceased.
A complete list of the Canagliflozin DIA 2001 Study Group may be found in the Supplementary Data online.
References
- 1.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009;122:443–453 [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009;75:1272–1277 [DOI] [PubMed] [Google Scholar]
- 3.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007;261:32–43 [DOI] [PubMed] [Google Scholar]
- 4.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520–526 [DOI] [PubMed] [Google Scholar]
- 5.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672 [DOI] [PubMed] [Google Scholar]
- 6.Bagga A, Bajpai A, Menon S. Approach to renal tubular disorders. Indian J Pediatr 2005;72:771–776 [DOI] [PubMed] [Google Scholar]
- 7.Polidori D, Sha S, Devineni D, Rothenberg P. The renal glucose threshold is increased in patients with type 2 diabetes: results from a novel method for measuring the renal threshold. Late-breaking abstract presented at the 70th Scientific Sessions of the American Diabetes Association, 25–29 June 2010, Orlando, Florida [Google Scholar]
- 8.Nyirjesy P, Zhao Y, Ways K, Usiskin K. Effects of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor on vulvovaginal candidal colonization in patients with type 2 diabetes mellitus (T2DM). Late-breaking abstract presented at the 71st Scientific Sessions of the American Diabetes Association, 24–27 June 2011, San Diego, California [Google Scholar]
- 9.Polidori D, Zhao Y, Sha S, Canovatchel W. Canagliflozin treatment improves beta cell function in subjects with type 2 diabetes. Late-breaking abstract presented at the 70th Scientific Sessions of the American Diabetes Association, 25–29 June 2010, Orlando, Florida [Google Scholar]
- 10.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbiner B, Polonsky KS, Beltz WF, et al. Effects of weight loss and reduced hyperglycemia on the kinetics of insulin secretion in obese non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab 1990;70:1594–1602 [DOI] [PubMed] [Google Scholar]
- 12.Geerlings SE, Stolk RP, Camps MJL, Netten PM, Collet TJ, Hoepelman AI, Diabetes Women Asymptomatic Bacteriuria Utrecht Study Group Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care 2000;23:1737–1741 [DOI] [PubMed] [Google Scholar]
- 13.Goswami R, Dadhwal V, Tejaswi S, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J Infect 2000;41:162–166 [DOI] [PubMed] [Google Scholar]
- 14.US Food & Drug Administration Endocrinologic & Metabolic Advisory Committee. Background document on dapagliflozin (BMS-512148 NDA 202293) [article online], 13 June 2011. Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262996.pdf Accessed 4 August 2011
- 15.Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors. Systematic review and meta-analysis of randomized trials. Ann Med. 15 April 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E. Sodium-glucose transporter-2 inhibition as an antidiabetic therapy. Nephrol Dial Transplant 2010;25:2041–2043 [DOI] [PubMed] [Google Scholar]
- 17.Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 2008;5:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]