Abstract
OBJECTIVE
The risk factors for middle-age onset of type 2 diabetes are well known. However, information is scant regarding the age onset of type 2 diabetes and its correlates in community-based black and white relatively young adults.
RESEARCH DESIGN AND METHODS
This prospective cohort study consisted of normoglycemic (n = 2,459) and type 2 diabetic (n = 144) adults aged 18–50 years who were followed for an average of 16 years.
RESULTS
The incidence rate of the onset of type 2 diabetes was 1.6, 4.3, 3.9, and 3.4 per 1,000 person-years for age-groups 18–29, 30–39, and 40–50 and total sample, respectively. Incidences of diabetes increased with age by race and sex groups (P for trend ≤0.01); higher in black females versus white females and blacks versus whites in total sample (P < 0.05). In a multivariable Cox model, baseline parental diabetes (hazard ratio [HR] 5.24) and plasma insulin were significantly associated with diabetes incidence at the youngest age (18–29 years); black race, BMI, and glucose at age 30–39 years; female sex, parental diabetes (HR 2.44), BMI, ratio of triglycerides and HDL cholesterol (TG/HDL-C ratio), and glucose at age 40–50 years; and black race, parental diabetes (HR 2.44), BMI, TG/HDL-C ratio, and glucose in whole cohort. Further, patients with diabetes, regardless of age onset, displayed a significantly higher prevalence of maternal history of diabetes at baseline (P < 0.01).
CONCLUSIONS
In relatively young adults, predictability of baseline cardiometabolic risk factors along with race, sex, and parental history of diabetes for the onset of type 2 diabetes varied by age-group. These findings have implications for early prevention and intervention in relatively young adults.
Earlier national survey data portend that the prevalence and incidence of diabetes are rising in the United States (1,2). Impaired glucose homeostasis has become one of the most common causes of death in the U.S. (2). The progressive global epidemic of obesity has resulted in obesity being a major causal factor detected in prediabetes and type 2 diabetes (1).
A number of studies have indicated that hyperinsulinemia/insulin resistance is associated with cardiometabolic risk factors including obesity, dyslipidemia, and hypertension, a constellation of disorder characteristics of the metabolic syndrome commonly found in diabetes (3–8). Further, the impaired glucose homeostasis among offspring of young-age onset, maternal type 2 patients with diabetes has been attributed to perinatal exposures and related increase in diabetes risk (9). The optimal strategy for preventing the onset of type 2 diabetes postulates the knowledge of its modifiable cardiometabolic risk factors (8). However, most studies have been performed on the prevalence of type 2 diabetes (3,4,6,9) with single, baseline measurements at middle and older age (1,5,7,9–11). Information is lacking on the correlates among relatively young adults in a community on the age-onset of type 2 diabetes. The present analysis examines the occurrence of diabetes at increasing ages as part of the Bogalusa Heart Study, a biracial (black and white), community-based investigation of the evolution of cardiovascular disease risk beginning in childhood (12).
RESEARCH DESIGN AND METHODS
Study population
The Bogalusa Heart Study is being conducted in a biracial (65% white and 35% black) community of Bogalusa, LA. A panel design, based on repeated (at ∼3–4 years) cross-sectional surveys of school-aged children and adults who participated in earlier surveys as children, resulted in the formation of a prospective longitudinal cohort with serial observations from child- to adulthood. This study includes subjects (N = 2,603; young to middle-aged adults; 34% black and 57% females) who participated in their baseline and follow-up examinations during 1979–2011 and had fasting blood samples on both examinations. At the baseline examination, individuals with a history of diabetes or who had a fasting glucose level ≥126 mg/dL (7 mmol/L) were excluded. These subjects were 4–44 years of age (mean ± SD, 17.1 ± 7.0) at baseline and 18–50 at follow-up (33.2 ± 9.3). The study subjects were followed on average for 16 years (mean ± SD, 16.2 ± 7.5). With respect to age, race, sex, overall adiposity (BMI), and lipid, glucose, and insulin profile, the baseline characteristics of the study cohort, which represented 26% of the overall original ascertained baseline population, were similar to the characteristics of the subjects who did not participate in the follow-up survey as young adults (data not shown).
The follow-up, cross-sectional examination date at which diabetes was identified was used as the date of diagnosis; otherwise, follow-up was censored at the last follow-up. According to the American Diabetes Association criteria (13), follow-up adult subjects were classified as nondiabetic (n = 2,459) if they had a fasting glucose level <126 mg/dL (7.0 mmol/L) and diabetic (n = 144) if the fasting glucose level was ≥126 mg/dL (7 mmol/L) or if they were on medication for diabetes. Informed consent was obtained from all participants, and the study was approved by the institutional review board of the Tulane University Health Sciences Center.
General examination
Standardized protocols were used by trained examiners across all surveys (14). Participants were instructed to fast for 12 h before the venipuncture, and compliance was ascertained by an interview on the day of examination. Information on personal health history (e.g., hypertension, dyslipidemia, or diabetes and medical treatment for these conditions) was obtained by questionnaires. Anthropometric and blood pressure measurements were made in replicate and mean values were used. BMI (kg/m2, weight in kilograms divided by the square of height in meters) was used as a measure of overall adiposity. Right upper arm length and circumference were used to select the cuff size for blood pressure measurements with mercury sphygmomanometers. Two randomly assigned trained nurses or observers measured blood pressure (three replicates each) on the right arm while subjects were in a relaxed, sitting position. Systolic and diastolic blood pressures were recorded at the first and fourth (children) or fifth (adults) Korotkoff, respectively. Mean arterial pressure (MAP), calculated as diastolic blood pressure plus one-third pulse pressure, was used in the analysis. Participants were characterized as having a parental history of type 2 diabetes if one or both natural parents reported having the disease at baseline.
Laboratory analyses
Cholesterol and triglyceride (TG) levels were initially measured using chemical procedures on a Technicon Autoanalyzer II (Technicon Instrument) according to the laboratory manual of the Lipid Research Clinics Program. Later, these variables were determined by enzymatic procedures on an Abbott VP instrument (Abbott Laboratories) between 1987 and 1996 and on a Hitachi 902 Automatic Analyzer (Roche Diagnostics) afterward. Both chemical and enzymatic procedures met the performance requirements of the Lipid Standardization Program of the Centers for Disease Control and Prevention (CDC), which routinely monitors the precision and accuracy of total cholesterol, TGs, and HDL cholesterol (HDL-C) measurements since the beginning of this study. Serum lipoprotein cholesterols were analyzed by using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures (15).The intraclass correlation coefficients between the blind duplicate (10% random sample) values ranged from 0.86 to 0.98 for HDL-C, 0.86 to 0.98 for LDL cholesterol (LDL-C), and 0.88 to 0.99 for TGs (12,14).
From 1976 to 1991, plasma glucose was measured initially by a glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments). Since then, it has been measured enzymatically as part of a multichemistry (SMA20) profile. Plasma-immunoreactive insulin levels were measured by a commercial radioimmunoassay kit (Phadebas; Pharmacia Diagnostics). The intraclass correlation coefficients between blind duplicate values ranged from 0.94 to 0.98 for insulin and 0.86 to 0.98 for glucose. In addition, an index of insulin resistance was calculated according to the homeostasis model assessment formula HOMA-IR = (insulin [μU/mL] × glucose [mmol/L]/22.5).
Statistical analysis
All of the statistical analyses were performed with SAS version 9.2 (SAS Institute). Continuous variables were tested for normality using a Kolmogorov-Smirnov test. Values of TGs, ratio of TGs and HDL-C (TG/HDL-C ratio), glucose, insulin, and HOMA-IR variables used in the analyses were log transformed. General linear models were used to examine the baseline cardiometabolic risk factor variables, including parental history of diabetes, by status of follow-up young adult diabetes (nondiabetes/diabetes) and age-onset diabetes group, adjusted for age, race, and sex. The trends of incidence of diabetes in age onset, stratified by race and sex, were examined using the Cochran-Armitage trend test. Logistic regression models, adjusted for age, race, and sex, were used to examine whether the maternal diabetes measured at the initial survey (baseline) predicted diabetes at follow-up by age-onset type 2 diabetes group.
Models assessing the independent relations between baseline cardiometabolic risk factor variables and young-onset type 2 diabetes by age-onset group were constructed using a backward elimination multivariate Cox proportional hazards model with the years of follow-up as the time scale that was used to estimate the hazard ratios (HRs) and 95% CIs. The baseline independent variables initially included in these models were age, race, sex, race by sex interaction, parental (or maternal) history of diabetes (yes/no), BMI, MAP, TG/HDL-C ratio, and fasting plasma glucose and insulin. Nonsignificant terms (P > 0.05) were removed from the model by backward stepwise procedure. Before fitting the model, the assumptions of the Cox proportional hazards regression model were checked. The Schoenfeld residual goodness-of-fit test for each independent variable included in the Cox model was performed. Because there was no interaction effect between baseline race (or sex) and glucose (or insulin and HOMA-IR) levels, the race-sex groups were combined to increase statistical power and to simplify the presentation.
To evaluate the discriminatory capability of the models using the area under the ROC curve (C statistic), the multivariate C statistic logistic regressions were performed on the association of the selected baseline variables (parental or maternal history of diabetes, BMI, TG/HDL-C ratio, glucose, and insulin) with incident diabetes status at the follow-up in young adulthood adjusted for age, race, sex, and MAP. ROCs (C value and its 95% CI) were tested for equality by pairwise comparison of each model with the rest. In addition, to assess model discrimination, increment in C statistic in a model with traditional risk factor (age, race, sex, and MAP) and a selected baseline variable compared with traditional risk factor alone was calculated.
RESULTS
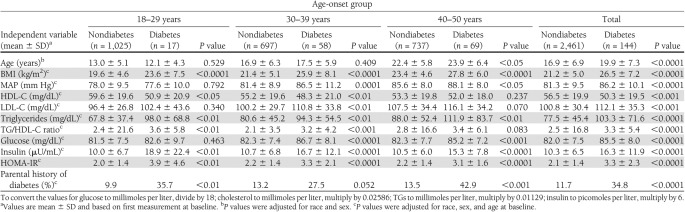
The characteristics of the study cohort at baseline by onset-diabetes status (yes/no) and age-onset group are shown in Table 1. Comparisons were made after adjustment for race, sex, and age at baseline. The overall increase with age showed an increased occurrence of diabetes beginning with 17 cases at age 18–29 years. At baseline, the diabetes group versus nondiabetes group showed higher levels of BMI, TGs, insulin, and HOMA index and greater parental history of diabetes across all four groups; higher levels of glucose (except for 18–29 age-group); lower levels of HDL-C (except for 40–50 age-group) and higher levels of MAP (except for 18–29 age-group) and TG/HDL-C ratio (except for 40–50 age-group); higher levels of LDL-C (30–39 age-group and total sample only); and higher age (40–50 age-group and total sample only).
Table 1.
Cardiometabolic risk factor variables at baseline according to age onset of type 2 diabetes in young adults: the Bogalusa Heart Study
Supplementary Fig. 1 illustrates the incidence (percent) of onset of type 2 diabetes by race, sex, and age-onset group over an average of 16 years. The incidence of onset of diabetes increased with age, regardless of race and sex (P for trend ≤0.01). Overall, the incidences of type 2 diabetes were 1.6% (17 of 1,044) at age 18–29 years, 7.7% (58 of 755) at age 30–39 years, and 8.6% (69 of 806) at age 40–50 years. Overall incidence of type 2 diabetes was 5.5% in the total sample. The incidence was higher in black women (7.2%) than in white women (4.7%) (P < 0.05). No such difference was detected at age 18–29 years (P = 0.411). In the total sample, blacks (7.1%) were significantly more incident than whites (4.8%), as expected (P < 0.05). No significant sex difference in the progression of type 2 diabetes was observed (data not shown).
Among 2,603 participants in the study cohort, there were 144 new diabetes cases (events) over an average of 16 years. The sum of person-time (year) of the population at risk was 10,949, 13,531, 17,653, and 42,133 person-years for age-groups 18–29, 30–39, 40–50, and total sample, respectively. The person-year incidence rate of the onset of diabetes was 1.6 at age 18–29 years, 4.3 at age 30–39 years, and 3.9 at age 40–50 years per 1,000 person-years. The overall incidence rate was 3.4 per 1,000 person-years in the total sample (data not shown).
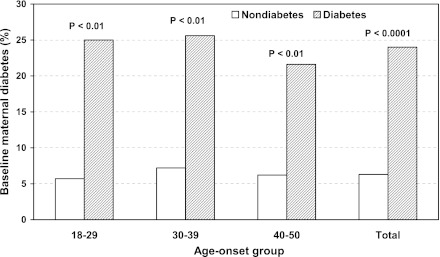
Figure 1 displays the prevalence (percent) of baseline maternal diabetes by follow-up diabetes status and age-onset group after 16 years. The prevalence of baseline maternal diabetes at ages 18–29, 30–39, and 40–50 years and in total sample was 6.1, 8.6, 7.5, and 7.2%, respectively. The prevalence of maternal diabetes was greater among patients with diabetes than those without diabetes, regardless of age-group (P < 0.01). Further, the prevalence of maternal diabetes occurred 1.5-fold more frequently than paternal diabetes among the different age-groups (P < 0.01). No significant difference in the prevalence of baseline paternal diabetes between the two groups was detected in all age-groups. At follow-up, maternal diabetes consistently showed much greater prevalence among all age-groups and in the total sample, whereas paternal diabetes showed only more prevalence at age-group 30–39 and in the total sample (P < 0.01) (data not shown).
Figure 1.
Prevalence of maternal diabetes at baseline by follow-up age-group and diabetes status. P values were compared between the diabetes groups, adjusted for race, sex, and age at baseline.
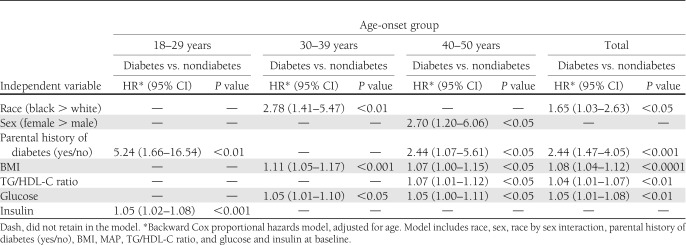
Table 2 shows the results of a multivariable adjusted Cox proportional hazards model that included race, sex, race by sex interaction, parental history of diabetes, BMI, MAP, TG/HDL-C ratio, glucose, and insulin at baseline. After adjusting for age at baseline, race (black versus white) showed significant HRs of 2.78 and 1.65 of being young-onset type 2 diabetes after 16 years for age-group 30–39 and total sample, respectively; parental history of diabetes (yes/no) displayed HRs of 5.24, 2.44, and 2.44 for age-groups 18–29 and 40–50 and total sample, respectively; BMI showed HRs of 1.11, 1.07, and 1.08 for age-groups 30–39 and 40–50 and total sample, respectively; TG/HDL-C ratio showed HRs of 1.07 and 1.04 for age-group 40–50 and total sample, respectively; glucose showed HRs of 1.05 for age-groups 30–39 and 40–50 and total sample; and insulin showed a HR of 1.05 for age-group 18–29. Further, alternate multivariate analyses using maternal diabetes, instead of parental diabetes, gave essentially identical results (data not shown).
Table 2.
Baseline predictors of follow-up type 2 diabetes by age-onset status: the Bogalusa Heart Study
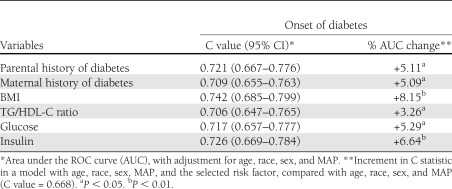
In terms of discriminative values of different selected baseline cardiometabolic variables (parental or maternal history of diabetes, BMI, TG/HDL-C ratio, glucose, and insulin) (Table 3), the predictive models produced C values ranging from 0.706 to 0.742 for onset of type 2 diabetes for these measures, which were relatively similar to each other in magnitude. Compared with the traditional model including age, race, sex, and MAP, the diabetic model that added the selected baseline variable above had a significant increment in C statistic for BMI, insulin, glucose, parental and maternal history of diabetes, and TG/HDL-C ratio, in that order.
Table 3.
Discriminatory value of selected cardiometabolic risk factor variables in predicting onset of type 2 diabetes: the Bogalusa Heart Study
CONCLUSIONS
The current study explores the natural history of type 2 diabetes in a biracial community–based population of relatively young adults, free from a selection bias, and monitored longitudinally over a period of 16 years. The results show that, after adjusting for race and sex, adiposity (as depicted by BMI), TGs, TG/HDL-C ratio, fasting plasma glucose (except for age-group 18–29 at baseline), insulin, HOMA index, and parental history of diabetes measured at baseline were consistently and significantly different between the nondiabetes and diabetes groups across the age-onset groups and in the total sample size. The incidences of onset of diabetes increased with age when stratifying by race and sex groups (P for trend ≤0.01), greater in black versus white females and blacks versus whites in the total cohort (P < 0.05). In a multivariable Cox proportional hazards model adjusted for age, baseline parental history of diabetes and fasting plasma insulin were significantly associated with type 2 diabetes in the 16-year follow-up of young adults in the age-onset group 18–29; whereas race (black versus white), BMI, and glucose were associated with diabetes in the older age-group with onset, 30–39 years; sex (female versus male), parental history of diabetes, BMI, TG/HDL-C ratio, and glucose were associated with diabetes in age-group 40–50; and race (black versus white), parental history of diabetes, BMI, TG/HDL-C ratio, and glucose were associated with diabetes in the total sample. In terms of discriminative values of different selected baseline variables (parental and maternal history of diabetes, BMI, TG/HDL-C ratio, glucose, and insulin), the predictive models produced C values ranging from 0.706 to 0.742 for onset of type 2 diabetes for these measures.
In the current study, the overall incidence rate of type 2 diabetes was lower than that found previously (1,9–11). This difference might be explained by the lower average age in this overall cohort (1,9–11) and ethnic difference of our cohort, especially when compared with that in the study of Pima Indians (8). Nonetheless, the observed incidence rate of type 2 diabetes at ages 18–29 (1,16,17), 30–39 (17), and 40–50 years (5) did not differ from other previous findings. In the Bogalusa sample (4), as parents of a children’s study, they were younger and blacks were about twice more than whites.
The observed incidence rate of young-onset type 2 diabetes increased with age by race-sex group, as might be expected and is consistent with previous observations (1,10). The difference of race (black > white) in the current study cohort is also in agreement with the earlier reports (1,10,11,17). Further, compared with other race-sex groups, diabetes was less incident among white females (17). Aside from the traditional risk factors such as anthropometry, parental history of diabetes, and certain physiologic covariates, the differential distribution of a genetic polymorphism in black and white populations, the greater exposure to an adverse environment in early life, and the reduced availability of vitamin D in dark-skinned populations may contribute to the higher incidence of type 2 diabetes in blacks (11). As a whole and as in other studies, the incidence of diabetes increases among older people and in ethnic minority populations (1,10).
Having a parent with diabetes contributed strongly to diabetes risk in our present multivariate model, regardless of overall age and other risk factors. This is well known, as in previous cohort studies (4,6–9,11,17). The current study, consistent with earlier reports (9,18), demonstrated an excess maternal transmission of type 2 diabetes, and importantly, we noted this association was strongest at the young age of onset, 18–29 years. At the young age of children, parents may be too young to manifest type 2 diabetes. Indeed, in the current cohort, although parents were also at a younger age, the HR of being type 2 diabetes at age 18–29 years was twofold higher than that in other age-groups and in total sample. Adding paternal (or maternal) diabetes to the traditional model significantly improved discrimination of type 2 diabetes by at least 5% (area under the curve = 0.721). The importance of parental history at an earlier age suggests the important and more obvious role of genetics in the transmission of diabetes to offspring (19). This was also suggested in our earlier glucose tolerance study by parental history (4,6,20). As in other studies (16,17), our observations have indicated that parental (or maternal) diabetes, but not glucose or obesity, and fasting plasma insulin measured at the average childhood age at baseline were the independent predictors of young-onset type 2 diabetes at follow-up age of 18–29 years. Further, fasting plasma glucose, but not insulin or parental (or maternal) diabetes, along with black race and obesity at the baseline adolescence age were predictors of younger-onset diabetes at follow-up age of 30–39 years (17). In addition, environmental exposure (21) is increasingly involved in the risk for the development of diabetes currently being observed in the epidemic of obesity (1,2), and all conventional cardiometabolic risk factors beginning at an early age were predictors of the later (40–50 years) age-onset of diabetes. As a result, the simple and feasible composition of elementary office measurements, knowledge of parental diabetes, and childhood glucose and insulin may predict the young onset of type 2 diabetes (3,17).
Of interest, adiposity, as depicted by BMI, was a strong predictor of diabetes in this study and in many earlier observations (1,3,5,7,8,10). Indeed, in the current study, the addition of BMI to the traditional model maximally improved the C statistic by 8% as compared with other risk factors. As is well known, obesity is pathologically linked to insulin resistance/hyperinsulinemia and related to development of dysglycemia. This is consistent with our earlier studies in that obesity precedes childhood-detectable hyperinsulinemia/insulin resistance or metabolic syndrome (22). Moreover, excess central adiposity augments the expression of proinflammatory adipocytokines, including tumor necrosis factor-α, and reduces the expression of insulin-sensitizing and anti-inflammatory adiponectin, which causes an increase in insulin resistance (23). Excess fat and related insulin resistance/hyperinsulinemia increase TG (very low-density lipoprotein) levels as a result of abnormal fatty acid metabolism and excess hepatic TG synthesis and/or low clearance of TGs from the circulation (24). In turn, increases in LDL-C and decreases in HDL-C levels ensue (24).
The current study has certain limitations in that it lacks direct assessments of postchallenge glucose, in vivo insulin action and secretion, glycosylated hemoglobin, and body fat mass and distribution. Instead, we used well-established simple surrogate measures of glucose homeostasis that are applicable to population studies. Parental diabetes and fasting status in the study were self-reported. Previous studies, including our own, have found ∼90% of the self-reported diabetic information to be valid (25). Although diabetes cases were excluded at participant entry, type 1 and type 2 diabetes could not be distinguished in insulin users at follow-up because of the lack of measurements of glutamic acid decarboxylase antibodies and fasting C-peptide levels, which are used for clinical diagnosis of diabetes types. Even with CDC quality controls, variability in the accuracy of measured variables over time is of concern. However, any potential measurement errors (drifts) due to different methodologies, if any, over time would have resulted in the underestimation of the observed relationships. Finally, there was uncertainty about the precise time of the onset of diabetes.
In summary, these findings indicate that adverse levels of traditional cardiometabolic risk factors, parental (especially maternal) diabetes, black race, adiposity, and measures of glucose homeostatis characterize the early natural history of the development of type 2 diabetes. In relatively young to middle age adults, maternal history of type 2 diabetes is an important predictor of diabetes onset, especially at the younger age. The feasible composition of elementary office and insulin measurements with the knowledge of parental history of diabetes should add in the clinical assessment to begin early prevention in children and adolescents to preclude the onset of type 2 diabetes.
Acknowledgments
This work was supported by grants HD-061437 and HD-062783 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and AG-16592 from the National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
Q.M.N. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. J.-H.X. researched and helped create laboratory data. W.C. researched data and reviewed and edited the manuscript. S.R.S. researched data, contributed to discussion, and reviewed and edited the manuscript. G.S.B. researched data, helped with the concept, and reviewed and edited the manuscript. G.S.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The Bogalusa Heart Study is a joint effort of many investigators and staff members, whose contributions are gratefully acknowledged. The authors would especially like to thank the study participants. The authors also appreciate Dr. Janet Rice (Tulane Department of Biostatistics and Bioinformatics) for her assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1818/-/DC1.
References
- 1.Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997-2003. Am J Prev Med 2006;30:371–377 [HAY] [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 3.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care 2008;31:2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Influence of childhood parental history of type 2 diabetes on the pre-diabetic and diabetic status in adulthood: the Bogalusa Heart Study. Eur J Epidemiol 2009;24:537–539 [DOI] [PubMed] [Google Scholar]
- 5.Lyssenko V, Almgren P, Anevski D, et al. Botnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan SR, Frontini MG, Berenson GS, Bogalusa Heart Study Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa Heart Study. Metabolism 2003;52:443–450; discussion 451–453 [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 8.Franks PW, Hanson RL, Knowler WC, et al. Childhood predictors of young-onset type 2 diabetes. Diabetes 2007;56:2964–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000;49:2201–2207 [DOI] [PubMed] [Google Scholar]
- 10.Kirtland KA, Li YF, Geiss LS, Thompson TJ. State-specific incidence of diabetes among adults-participating states, 1995-1997 and 2005-2007. MMWR Weekly, 31 October 2008:57:1169–1173 [PubMed]
- 11.Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in U.S. adults age 45 to 64 years. Ann Intern Med 2009;150:741–751 [DOI] [PubMed] [Google Scholar]
- 12.Pickoff AS, Berenson GS, Schlant RC. Introduction to the symposium celebrating the Bogalusa Heart Study. Am J Med Sci 1995;310(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenson GS, McMahan CA, Voors AW, et al. Cardiovascular Risk Factors in Children: The Early Natural History of Atherosclerosis and Essential Hypertension. New York, Oxford University Press, 1980 [Google Scholar]
- 15.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In Handbook of Electrophoresis. Lewis LA, Ed. Boca Raton, FL, CRC Press, 1983, p. 185–204 [Google Scholar]
- 16.Morrison JA, Glueck CJ, Horn PS, Schreiber GB, Wang P. Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18-19 y: a 10-y prospective study of black and white girls. Am J Clin Nutr 2008;88:778–788 [DOI] [PubMed] [Google Scholar]
- 17.Morrison JA, Glueck CJ, Horn PS, Wang P. Childhood predictors of adult type 2 diabetes at 9- and 26-year follow-ups. Arch Pediatr Adolesc Med 2010;164:53–60 [DOI] [PubMed] [Google Scholar]
- 18.Thomas F, Balkau B, Vauzelle-Kervroedan F, Papoz L, CODIAB-INSERM-ZENECA Study Group Maternal effect and familial aggregation in NIDDM. The CODIAB Study. Diabetes 1994;43:63–67 [DOI] [PubMed] [Google Scholar]
- 19.Ehm MG, Karnoub MC, Sakul H, et al. American Diabetes Association GENNID Study Group. Genetics of NIDDM Genomewide search for type 2 diabetes susceptibility genes in four American populations. Am J Hum Genet 2000;66:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berenson GS, Bao W, Srinivasan SR. Abnormal characteristics in young offspring of parents with non-insulin-dependent diabetes mellitus. The Bogalusa Heart Study. Am J Epidemiol 1996;144:962–967 [DOI] [PubMed] [Google Scholar]
- 21.Luo ZC, Fraser WD, Julien P, et al. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses 2006;66:38–44 [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan SR, Myers L, Berenson GS. Temporal association between obesity and hyperinsulinemia in children, adolescents, and young adults: the Bogalusa Heart Study. Metabolism 1999;48:928–934 [DOI] [PubMed] [Google Scholar]
- 23.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003;52:1779–1785 [DOI] [PubMed] [Google Scholar]
- 24.Nikkilä EA. Regulation of hepatic production of plasma triglycerides by glucose and insulin. In Regulation of Hepatic Metabolism. Lundquist F, Tygstrup N, Eds. Copenhagen, Denmark, Munksgaard, 1974, p. 360–387 [Google Scholar]
- 25.Kahn LB, Marshall JA, Baxter J, Shetterly SM, Hamman RF. Accuracy of reported family history of diabetes mellitus. Results from San Luis Valley Diabetes Study. Diabetes Care 1990;13:796–798 [DOI] [PubMed] [Google Scholar]