Abstract
OBJECTIVE
To evaluate the relationship between diabetes care and types of comorbidity, classified by the degree to which their treatment is concordant with that for diabetes.
RESEARCH DESIGN AND METHODS
Retrospective cohort study (fiscal year [FY] 2001 to FY 2004) of 42,826 veterans with new-onset diabetes in FY 2003. Veterans were classified into five chronic comorbid illness groups (CCIGs): none, concordant only, discordant only, both concordant and discordant, and dominant. Five diabetes-related care measures were assessed in FY 2004 (guideline-consistent testing and treatment goals for HbA1c and LDL cholesterol and diabetes-related outpatient visits). Analyses included logistic regressions adjusting for age, race, sex, marital status, priority code, and interaction between CCIGs and visit frequency.
RESULTS
Only 20% of patients had no comorbidities. Mean number of visits per year ranged from 7.8 (no CCIG) to 17.5 (dominant CCIG). In unadjusted analyses, presence of any illness was associated with equivalent or better care. In the fully adjusted model, we found interaction between CCIG and visit frequency. When visits were <7 per year, the odds of meeting the goal of HbA1c <8% were similar in the concordant (odds ratio 0.96 [95% CI 0.83–1.11]) and lower in the discordant (0.90 [0.81–0.99]) groups compared with the no comorbidity group. Among patients with >24 visits per year, these odds were insignificant. Dominant CCIG was associated with substantially reduced care for glycemic control for all visit categories and for lipid management at all but the highest visit category.
CONCLUSIONS
Our study indicates that diabetes care varies by types of comorbidity. Concordant illnesses result in similar or better care, regardless of visit frequency. Discordant illnesses are associated with diminished care: an effect that decreases as visit frequency increases.
Comorbid illnesses among patients may complicate care by competing for time, attention, or other resources (1–5). This is particularly applicable for patients with chronic illnesses, such as diabetes. As a consequence, the quality of diabetes care might be compromised unless additional resources are made available to compensate.
Comorbid illnesses are common among patients with diabetes. In 2004, 88.6% of people with diabetes who responded to the Medical Expenditure Panel Survey reported having at least one additional chronic illness, while close to 15% reported having four or more, illustrating how common comorbidity is among the diabetic population (2). The prevalence of both diabetes and comorbid illness is likely to increase as the U.S. population ages.
Despite a high level of comorbidity among diabetic patients, the literature studying the effect of comorbidity on diabetes care predominantly focuses on a single coexisting condition, such as a mental illness (6–9). On the other hand, researchers accounting for all concurrent morbidity have applied aggregate morbidity counts or one-dimensional scores (10,11). Both approaches fail to reveal the true impact of multiple comorbid illnesses because not all illnesses are likely to have the same impact. Measuring patient complexity still poses a challenge to both clinicians and researchers, as described in a recent article (12).
Piette and Kerr (13) have proposed a novel theoretical framework as a way to categorize the effect of comorbidity on patients with diabetes and other chronic illnesses. The Piette and Kerr framework groups comorbid illnesses as concordant illnesses (illnesses that overlap with diabetes in their pathogenesis and management plans [e.g., cardiovascular diseases]), discordant illnesses (illnesses with unrelated pathogenesis or management plans [e.g., mental health illnesses and musculoskeletal disorders]), and dominant illnesses (illnesses whose severity eclipses all other illness management plans [e.g., end-stage kidney and liver diseases and metastatic cancer]). The framework hypothesizes that effects differ depending on the nature of comorbid illness (13–15). The presence of a discordant illness may draw resources away from diabetes management and result in compromised diabetes care, the presence of a concordant illness may result in similar or better diabetes care, and the presence of a dominant illness may result in substantially worse diabetes care. The primary purpose of this study was to evaluate the relationship between diabetes care and different types of comorbid illnesses, classified by the degree to which their treatment is concordant with that for diabetes as described by Piette and Kerr (13). We hypothesized that having concordant illnesses would be associated with similar or better diabetes care outcomes, having discordant illnesses would be associated with worse diabetes care outcomes, and the presence of dominant illnesses would lead to substantially worse diabetes care outcomes.
RESEARCH DESIGN AND METHODS
Data source
This study used data from the Diabetes Epidemiology Cohort (DEpiC), an administrative research database created by merging matched data files from the Veterans Health Administration (VHA) and Centers for Medicare and Medicaid Services (CMS). The DEpiC database identifies all VHA users with diabetes using a validated approach of having two or more diabetes-related ICD-9-CM codes (250.xx, 357.2, 362.0, and 366.41) from both inpatient and outpatient visits or any prescription for antiglycemic medication using a 24-month window (16).
Study cohort
A retrospective cohort study design was used to study patients with incident diabetes in the DEpiC database. The study observation period extended from fiscal year (FY) 2001 to FY 2004. The incident diabetes cohort was composed of patients with new-onset diabetes in FY 2003. We chose to study patients with incident diabetes over those with prevalent diabetes because the former tend to be more homogenous with respect to diabetes duration and management needs/demands.
We identified patients with incident diabetes in the baseline year (FY 2003) by excluding those with diabetes-related codes and/or medications in a 2-year look-back period (FY 2001 to FY 2002). Comorbidities were identified using a minimum of two codes during the look-back and baseline years. Data on number of laboratory tests performed were obtained from both VHA and CMS files, which allowed for enumeration of tests’ frequency and consistency. However, the VHA Decision Support System files were the only source for laboratory test results. Study outcomes were assessed in the follow-up year (FY 2004).
From the DEpiC database, we identified 51,043 patients who were VHA system users throughout the study period (FY 2001 to FY 2004) and had new-onset diabetes in the baseline year (FY 2003). Patients enrolled in Medicare HMO plans (n = 6,581) (whose clinical data are not reported to CMS) were excluded. Patients with less than three visits in the baseline year (n = 1,636) were also excluded to reduce potential underassessment of comorbid illnesses. After the above exclusions, there were 42,826 patients with incident diabetes in the analysis cohort. Data were available on visits and testing for HbA1c and LDL cholesterol (LDL-C) for all 42,826 patients. However, results for HbA1c and LDL-C tests were available only for those patients who underwent laboratory testing in the VHA system. This reduced the cohort size to 39,516 and 39,332 when analyzing the intermediate measures HbA1c <8% and LDL-C <130 mg/dL, respectively.
Study variables
Outcome variables.
Our study assessed five diabetes-related care measures (three process measures and two intermediate [or treatment goal] measures) that were based on the Diabetes Quality Improvement Project (DQIP) measures (17). The process measures included a test for HbA1c at least once every 6 months, a diabetes-related visit at least once every 6 months, and a test for LDL-C at least once a year. We used the last test result in FY 2004 from a subset of patients who underwent laboratory testing in the VHA system to assess two intermediate measures (or treatment goals): HbA1c <8% and LDL-C <130 mg/dL.
Independent variable.
Selection of relevant chronic comorbid illnesses and their subsequent grouping into chronic comorbid illness groups (CCIGs) was done using a nominative group process informed by VHA–Department of Defense diabetes guidelines and opinions of field experts from multiple VHA centers (both internal and external to our study team). We categorized patients using a comprehensive list of 53 chronic illnesses into the five CCIGs: none, concordant only, discordant only, both concordant and discordant, and dominant. Patients with no illness other than diabetes belonged to the none CCIG group. Presence of a dominant illness was given priority over other illnesses for CCIG classification. See the Supplementary Data for a listing of chronic comorbid illnesses used for CCIG categorization. We built on our team’s prior work for compilation of the ICD-9-CM code list (18). The variable CCIG was our main independent variable.
Covariates.
We included health care use and additional sociodemographic variables available in the database as covariates. Face-to-face (F2F) visit frequency was used to measure overall and diabetes-related visits. F2F visits refer to in-person visits to a medical professional with decision-making capacity in either the Medicare or VHA outpatient services that were identified using the current procedural terminology codes for visits as outlined in the health care effectiveness data and information set measures (the Supplementary Data lists current procedural terminology codes used to define F2F visits). The visits were classified as being diabetes related if they were assigned a diabetes-specific ICD-9-CM code within the given visit. Total F2F visits were categorized as <7, 7–12, 13–24, and >24 visits per year.
Sociodemographic variables included age categories of <55, 55–64, 65–74, and >75 years; sex; race/ethnicity divided into white, African American, Hispanic, and other; marital status of married or not married; and VHA priority code of low income, severely disabled, moderately disabled, and copay. The VHA priority code is derived from VHA enrollment group assignment based on assessment of an individual’s income and service-connected disability.
Statistical analyses
First, we cross-tabulated study covariates with CCIGs and diabetes-related care measures to describe their bivariate associations and to identify potential confounders. Second, we tabulated the levels at which patients met care guidelines across the CCIGs in the overall cohort and within each F2F visit frequency stratum. Third, logistic regression modeling was used to test for association between CCIGs and diabetes care, sequentially without (model 1) and with (model 2) sociodemographic variables (age, sex, race, marital status, and VHA priority code). Model 3 added visit frequency to model 2. In model 4, we tested for interaction between CCIGs and F2F visit frequency to determine the effect of visit frequency on the strength of association between CCIGs and diabetes care. We assigned each veteran to a parent facility where he or she had the most outpatient encounters. We then used this information to adjust for the effects of clustering by VHA facility. Patients belonging to the none CCIG group were used as the reference category in all our logistic regression models. We report odds ratios (ORs) and their 95% CIs. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC). Models accounting for clustering by facility were built using PROC GENMOD, and the CONTRAST option was used to generate ORs for the interaction terms.
RESULTS
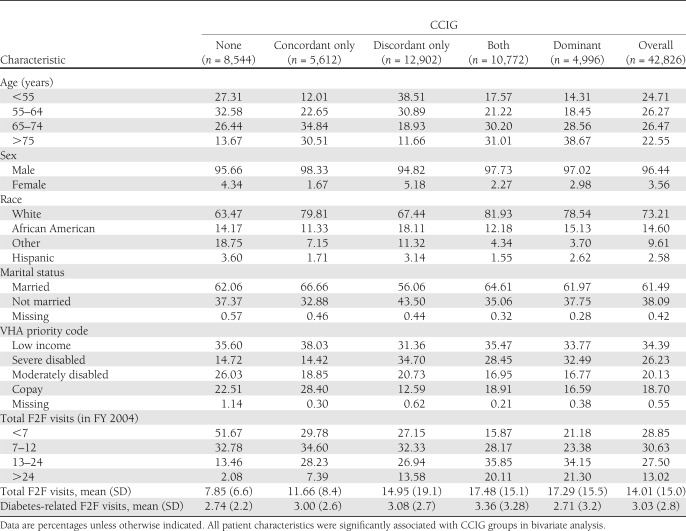
Only 20% of the 42,826 patients were free of chronic comorbid illnesses. Patients with concordant illnesses constituted ∼13% of the study cohort, 30.13% had discordant illnesses, and 25.15% had both concordant and discordant illnesses. Approximately 12% of patients were diagnosed with a dominant illness (Table 1).
Table 1.
Characteristics of veterans with incident diabetes in FY 2003
All covariates were significantly associated with type of comorbidity (P < 0.001). Diabetic patients with either no comorbidities or those with discordant illnesses were more likely to be younger, female, and nonwhite (Table 1). The concordant group had the highest levels of married (66.7%), poverty (38.4%), and copay (28.4%). The discordant group had the lowest levels in all these categories (56.1, 31.4, and 12.6%). A service-connected disability, as measured by the VHA priority code, was more prevalent among patients with discordant and dominant illnesses. F2F visits increased as comorbidities increased. The annual F2F visits ranged from mean (SD) 7.85 (6.6) for the CCIG with no illnesses to 17.48 (15.05) for the CCIGs with both concordant and discordant illnesses and 17.29 (15.5) for the dominant CCIG. (Table 1)
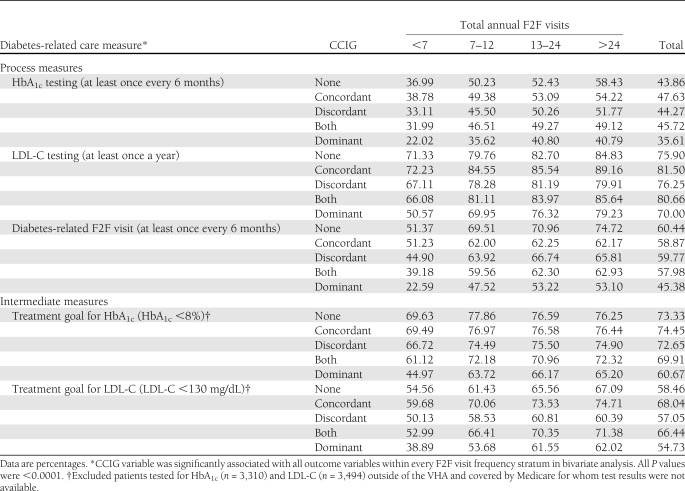
All study covariates showed statistically significant bivariate associations with study outcomes and were entered into the multivariable logistic regression models. Table 2 displays the unadjusted proportions of patients who met diabetes-related care guidelines and treatment goals by CCIGs and visit frequency. Approximately 44% were tested for HbA1c once every 6 months in FY 2004. Three out of four (71%) patients met the HbA1c goal of <8%. The LDL-C measures were met at a higher rate (LDL-C testing 77.2% and LDL-C <130 mg/dL 60.7%). A total of 58% of the study cohort had a diabetes-related visit once every 6 months as recommended. For all the diabetes care measures, the proportion of patients meeting them increased as F2F visits increased. Comparing across CCIGs, the highest proportions were almost always observed in either the none or the concordant CCIG and the lowest in the dominant CCIG.
Table 2.
Veterans with incident diabetes in FY 2003 who met recommended diabetes-related care measures in FY 2004 by CCIGs and visit frequency
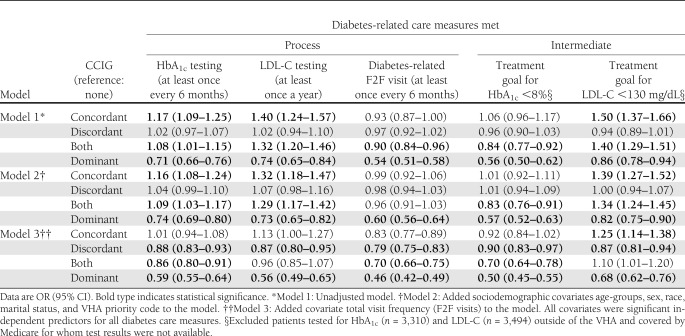
Table 3 presents results from three sequential main effects models built to assess the association between CCIGs and the five study outcomes (unadjusted, adjusted for sociodemographic covariates, and then further adjusted for F2F visit frequency). Results from the unadjusted models (model 1) showed that comorbidity type was associated with odds of meeting diabetes guidelines and goals. Increased odds were seen among the concordant and the both concordant and discordant CCIGs. Discordant and dominant groups were associated with similar and lower odds, respectively, for meeting diabetes guidelines and goals compared with the no comorbidity group. For example, patients with concordant (OR 1.17 [95% CI 1.09–1.25]) illness had 17% higher odds for getting tested for HbA1c as per guideline compared with those with no comorbidity, and patients with both concordant and discordant comorbidities had 8% higher odds. The dominant group had 29% lower odds of meeting the guideline. This trend was seen for two other outcomes—LDL-C testing and LDL-C treatment goal. Model 2 additionally controlled for sociodemographic variables, and the results were similar to model 1. The initial models showed a pattern of improved or similar diabetes care among patients with either concordant, discordant, or both concordant and discordant illnesses, contrary to the study hypotheses.
Table 3.
Results from logistic regression models assessing the effect of CCIGs on diabetes-related care measures
However, after adjusting for differences in F2F visit frequency, model 3 results supported the study hypotheses. For all study outcomes, patients in the concordant illness group had similar or increased likelihood of meeting recommended diabetes care measures compared with those with no illnesses. Those with discordant and dominant illnesses reported statistically significant lower likelihood of meeting recommended diabetes-related care measures compared to those with no illnesses. The magnitude of reduction in odds ranged between 10–21% for discordant CCIG and 32–54% for dominant CCIG. The complete set of results for model 3 is provided in Supplementary Table 1.
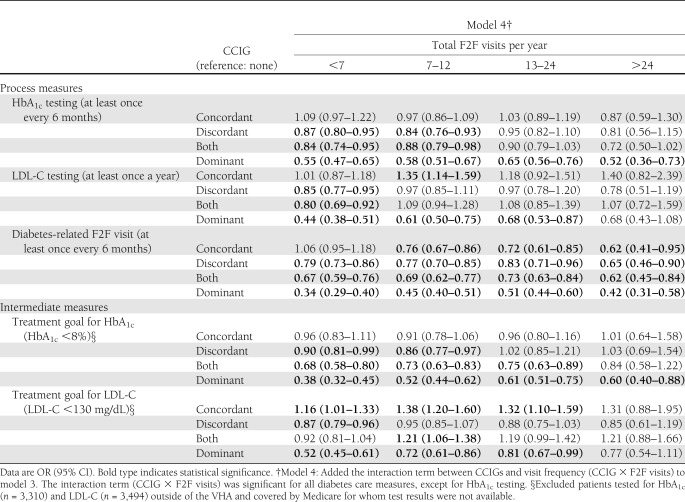
Table 4 presents results from model 4, which included all covariates from model 3 along with an additional interaction term between CCIGs and F2F visit frequency. The interaction term was significant for four out of five outcomes (HbA1c goal, LDL-C goal, LDL-C testing, and diabetes-related visits), indicating that the association between CCIGs and study outcomes was modified by visit frequency.
Table 4.
Results from logistic regression models assessing the effect of interaction between CCIGs and visit frequency on diabetes-related care measures
Presence of concordant illnesses was associated with similar odds for HbA1c-related measures regardless of visit frequency and increased odds for LDL-C–related measures only at lower visit frequency (<24 visits). Presence of discordant illnesses resulted in lower odds for HbA1c-related measures when annual visit frequency was ≤12 and for LDL-C–related measures when there were <7 annual visits. Presence of dominant illnesses was associated with significantly lower odds for HbA1c-related measures regardless of visit frequency and LDL-C–related measures when number of visits made in a year were ≤24. For all illness groups, the odds for having diabetes-related F2F visits as recommended were significantly lower than those with no illnesses, regardless of visit frequency.
Using results from LDL-C treatment goal measure (LDL-C level <130 mg/dL) as a specific illustration, among patients who had more than seven visits per year, having concordant illnesses significantly increased the odds (OR 1.16 [95% CI 1.01–1.33]) of meeting the goal compared with patients with no comorbidity. The odds were significantly lower for patients with discordant (0.87 [0.79–0.96]) and dominant (0.52 [0.45–0.61]) illnesses.
As visit frequency increased to 7–12 annual visits, those with concordant illnesses had significantly higher odds of meeting the LDL-C goal (OR 1.38 [95% CI 1.20–1.60]). Those with both concordant and discordant illnesses also had higher odds (1.21 [1.06–1.38]). Those with discordant illnesses had lower odds (0.95 [0.85–1.07]), but the findings were not significant. Those with dominant illnesses had lower odds (0.72 [0.61–0.86]). These results were similar among patients with 13–24 annual visits.
Finally, among patients making >24 annual visits, there were no statistically significant differences among the five CCIGs in the odds for attaining the LDL-C treatment goal.
CONCLUSIONS
In the initial analysis, our study found that an increasing burden of comorbidity was associated with increased visit frequency and higher levels of receiving recommended diabetes care regardless of type of CCIG. However, after adjustment for visit frequency, the results supported the study hypotheses that having concordant illnesses was associated with similar or better diabetes care, having discordant illnesses was associated with decreased diabetes care, and the presence of dominant illnesses resulted in markedly decreased diabetes care. This difference was more pronounced among patients who made less frequent visits.
There are some studies that report a similar relationship between comorbidity type and receipt of guideline-concordant care, for example, a study by Sales et al. (19) among postacute myocardial infarction patients and a Lagu et al. (20) study among hypertensive patients. Krein et al. (21) showed that chronic pain affected hypertension care in diabetes. Our findings support the underlying premise of the competing demands framework proposed by Piette and Kerr (13) among veterans with new-onset diabetes. Health care resources are finite, and diabetic patients burdened with additional discordant or dominant illness may not be able to receive all the care they need to address both their diabetes and nondiabetes needs (13,22).
However, the phenomenon of competing demands was not consistent. As visit frequency increased, differences in diabetes care became less pronounced. Health care systems’ ability to compensate in this way will depend on availability of resources, including subspecialty care and care coordination. Physicians’ capacity will depend on how well they manage visit time to address multiple illnesses. Finally, patients’ ability to compensate may depend on access to health care, availability of a caregiver, and how they prioritize their self-care (13–15,22–24). Such compensatory mechanisms are a likely explanation for the association between increased comorbidity burden and a seemingly paradoxical improvement in quality of care that has been reported in several studies (11,25–28).
Few other studies report a similar interaction between type of comorbidity and visit frequency when examining quality of care. Kodl et al. (29) reported that among veterans, when visit frequency was not accounted for, presence of a mental health diagnosis was associated with either increased or similar likelihood of colorectal cancer screening. However, after adjusting for visit frequency, presence of a mental health diagnosis increases risk of not receiving colorectal cancer screening. Along similar lines, Fenton et al. (30) demonstrated substandard preventive care for diabetes among HMO-enrolled patients who made either infrequent outpatient visits (less than eight per year) or more frequent but low-priority visits.
We identified two studies of patients with diabetes that were based on the competing demands framework proposed by Piette and Kerr (13) and whose results fail to support the framework’s hypotheses. Woodard et al. (31) studied the effect of concordant and discordant illnesses on quality care among all veterans with diabetes. They concluded that complexity of comorbidity was associated with superior care, regardless of comorbidity type. Their results remained unchanged after accounting for visit frequency. The difference between their study and ours is that their sample included patients with prevalent as well as incident diabetes, used different comorbid illnesses to determine CCIGs, and used the relative risk score from the diagnostic cost groups (DxCG) as an illness burden indicator. DxCG is correlated with both comorbidity type and visit frequency, and its inclusion might modify the effect of the other variables. Bayliss et al. (32) used a population of prevalent diabetes patients to study the pre- and posteffect of three discordant incident conditions (cancer, depression, and exacerbation of chronic obstructive pulmonary disease) on intermediary outcomes (HbA1c, LDL-C, and blood pressure) and reported no short-term or long-term effects on study outcomes.
Our implementation of the Piette and Kerr (13) framework can refine the assessment of comorbidities when evaluating diabetes care. In studies examining pay for performance, for example, comorbidities were measured in aggregate for risk-adjustment purposes, whereas our findings indicate that different types of comorbidities have different effects. It can also be used to evaluate the adequacy of the compensatory response across health care systems: adequate compensation should attenuate the adverse effect of discordant comorbidities. It might also help in identifying system factors that favor adequate compensation, such as better care coordination. Additional applications might include evaluating whether diabetes care quality measures need tailoring for certain illness groups.
Our study has several strengths. First, we used a large population-based study cohort to evaluate the Piette and Kerr (13) framework. Second, we used a comprehensive list of 53 comorbid illnesses. Third, we evaluated five CCIG groups, including those with dominant illnesses. Fourth, the VHA population is known to have higher prevalence of comorbidity, which enabled us to successfully contrast the patterns of study outcomes across the various CCIGs, which might not be possible in populations with low prevalence of comorbidity. Fifth, use of a longitudinal study design preserved temporality between the exposure and outcome.
Our study has several limitations. First, the study results are not generalizable to the U.S. population or other populations because the VHA population is predominantly male and has a high prevalence of comorbidity. Second, we did not have access to laboratory results from Medicare. Data from private insurance was also unavailable. Third, the inclusion criteria in the baseline year (FY 2003) biased the study to those with at least three or more F2F visits. Fourth, our study cohort was drawn from an administrative database that does not include any patient-reported data on resources available for self-care of diabetes management; health care access barriers; knowledge, attitudes, beliefs, and perceptions on diabetes care; quality of patient-physician interaction; and other factors that are known to have an impact on our study outcomes. Fifth, when classifying comorbid illnesses, we looked for presence or absence of comorbid illnesses only; we did not account for their severity. Sixth, we classified all patients into broad CCIGs but did not assess the relative burden of illnesses within each CCIG.
Further research is required to extend this study’s findings. One such area is the impact of type of visits (primary or specialty) on diabetes care. In addition, this study is limited to understanding the impact of the framework on diabetes care for those with new-onset diabetes. We feel that further analysis will be required to determine whether these findings will apply to those with prevalent diabetes as well.
Comorbidity type affected diabetes care. Discordant illnesses were associated with decreased diabetes care, possibly as a result of competition for time, attention, or other limited resources. Concordant illnesses, on the other hand, were associated with either similar or better care, probably because their management is congruent with that for diabetes. Dominant illnesses were associated with significant decrease in diabetes care that may be appropriate given their poor prognoses. In addition, the effect of competing demands was greater at the lower end of the visit frequency spectrum. This suggests the need for better care coordination within health care systems to improve diabetes care among patients with comorbidities. The Piette and Kerr (13) framework, based on the competing demands model, can be used as a tool to compare diabetes care across health care systems and providers, to identify patient groups who might be receiving over- and undertreatment and design specific interventions to improve their care, and to design appropriate performance measures based on evidence-based benefits while accounting for individuals’ comorbidity type and life expectancy.
Acknowledgments
This study was conducted under VHA Health Services Research Grant Medications and Diabetes Morbidity in the VA DEpiC (principal investigators D.R.M. and L.M.P.), approved by the East Orange Veterans Affairs institutional review board.
No potential conflicts of interest relevant to this article were reported.
S.R.P. researched data, contributed to discussion, and wrote the manuscript. M.R. researched data, contributed to discussion, and reviewed and edited the manuscript. B.G.F., C.-L.T., D.R.M., C.L.C., and L.M.P. contributed to discussion and reviewed and edited the manuscript. E.A.K. reviewed and edited the manuscript. L.M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1569/-/DC1.
References
- 1.Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA 2010;303:1303–1304 [DOI] [PubMed] [Google Scholar]
- 2.Clarke JL, Meiris DC. Building bridges: integrative solutions for managing complex comorbid conditions. Am J Med Qual 2007;22(Suppl.):5S–16S [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004;351:2870–2874 [DOI] [PubMed] [Google Scholar]
- 4.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005;294:716–724 [DOI] [PubMed] [Google Scholar]
- 5.Pogach LM, Tiwari A, Maney M, Rajan M, Miller DR, Aron D. Should mitigating comorbidities be considered in assessing healthcare plan performance in achieving optimal glycemic control? Am J Manag Care 2007;13:133–140 [PubMed] [Google Scholar]
- 6.Desai MM, Rosenheck RA, Druss BG, Perlin JB. Mental disorders and quality of diabetes care in the Veterans Health Administration. Am J Psychiatry 2002;159:1584–1590 [DOI] [PubMed] [Google Scholar]
- 7.Dixon LB, Kreyenbuhl JA, Dickerson FB, et al. A comparison of type 2 diabetes outcomes among persons with and without severe mental illnesses [corrected in: Psychiatr Serv 2004;55:1005]. Psychiatr Serv 2004;55:892–900 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009;194:491–499 [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RW, Kreyenbuhl JA, Medoff DR, et al. Quality of diabetes care among adults with serious mental illness. Psychiatr Serv 2007;58:536–543 [DOI] [PubMed] [Google Scholar]
- 10.Piette JD, Wagner TH, Potter MB, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care 2004;42:102–109 [DOI] [PubMed] [Google Scholar]
- 11.Bae SJ, Rosenthal MB. Patients with multiple chronic conditions do not receive lower quality of preventive care. J Gen Intern Med 2008;23:1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant RW, Ashburner JM, Hong CC, Chang Y, Barry MJ, Atlas SJ. Defining patient complexity from the primary care physician’s perspective: a cohort study. Ann Intern Med 2011;155:797–804 [DOI] [PubMed] [Google Scholar]
- 13.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725–731 [DOI] [PubMed] [Google Scholar]
- 14.Jaén CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract 1994;38:166–171 [PubMed] [Google Scholar]
- 15.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract 2001;14:352–361 [PubMed] [Google Scholar]
- 16.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl. 2):B10–B21 [DOI] [PubMed] [Google Scholar]
- 17.Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care 2001;24:1815–1820 [DOI] [PubMed] [Google Scholar]
- 18.Meduru P, Helmer D, Rajan M, Tseng CL, Pogach L, Sambamoorthi U. Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J Gen Intern Med 2007;22(Suppl. 3):408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sales AE, Tipton EF, Levine DA, et al. Are co-morbidities associated with guideline adherence? The MI-Plus study of Medicare patients. J Gen Intern Med 2009;24:1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagu T, Weiner MG, Hollenbeak CS, et al. The impact of concordant and discordant conditions on the quality of care for hyperlipidemia. J Gen Intern Med 2008;23:1208–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krein SL, Hofer TP, Holleman R, Piette JD, Klamerus ML, Kerr EA. More than a pain in the neck: how discussing chronic pain affects hypertension medication intensification. J Gen Intern Med 2009;24:911–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med 2007;5:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen LN, Kim C, Ettner SL, et al. Competing demands for time and self-care behaviors, processes of care, and intermediate outcomes among people with diabetes: Translating Research Into Action for Diabetes (TRIAD). Diabetes Care 2011;34:1180–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med 2007;22:1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med 2007;356:2496–2504 [DOI] [PubMed] [Google Scholar]
- 26.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care 2007;45:480–488 [DOI] [PubMed] [Google Scholar]
- 27.Ritchie C. Health care quality and multimorbidity: the jury is still out. Med Care 2007;45:477–479 [DOI] [PubMed] [Google Scholar]
- 28.Werner RM, Chang VW. The relationship between measured performance and satisfaction with care among clinically complex patients. J Gen Intern Med 2008;23:1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodl MM, Powell AA, Noorbaloochi S, Grill JP, Bangerter AK, Partin MR. Mental health, frequency of healthcare visits, and colorectal cancer screening. Med Care 2010;48:934–939 [DOI] [PubMed] [Google Scholar]
- 30.Fenton JJ, Von Korff M, Lin EH, Ciechanowski P, Young BA. Quality of preventive care for diabetes: effects of visit frequency and competing demands. Ann Fam Med 2006;4:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodard LD, Urech T, Landrum CR, Wang D, Petersen LA. Impact of comorbidity type on measures of quality for diabetes care. Med Care 2011;49:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayliss EA, Blatchford PJ, Newcomer SR, Steiner JF, Fairclough DL. The effect of incident cancer, depression and pulmonary disease exacerbations on type 2 diabetes control. J Gen Intern Med 2011;26:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]