Abstract
OBJECTIVE
To test the hypothesis that replacement of sucrose with isomaltulose in sweet foods and beverages improves metabolic control in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
One hundred ten patients with type 2 diabetes were randomized to receive sweet foods containing either 50 g/day isomaltulose or sucrose for 12 weeks as part of their habitual diet under free-living conditions. HbA1c at 12 weeks was the primary outcome parameter.
RESULTS
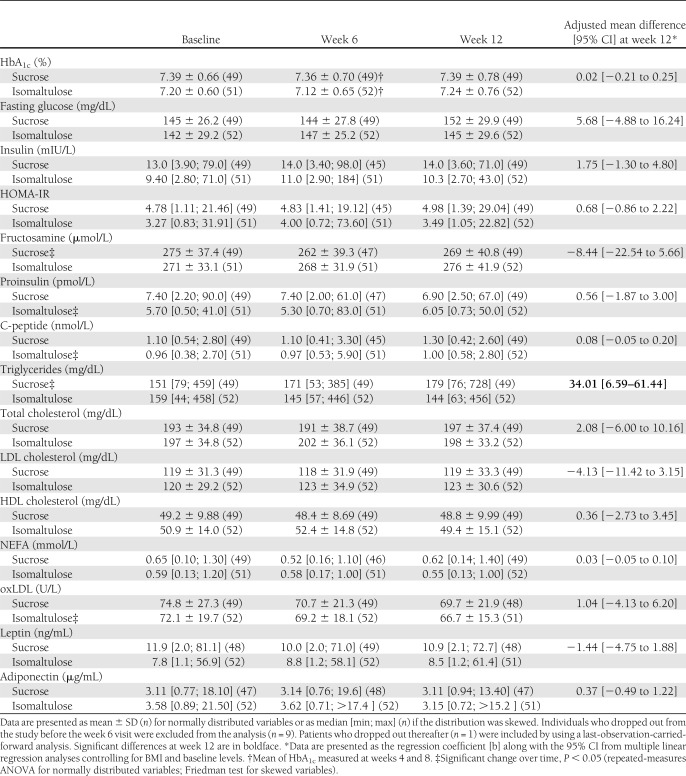
In the final analysis comprising 101 patients, isomaltulose did not significantly affect HbA1c at 12 weeks (sucrose: 7.39 ± 0.78%; isomaltulose: 7.24 ± 0.76%; regression coefficient [b]: 0.02 [95% CI: −0.21 to 0.25], P = 0.844). Triglycerides at 12 weeks were significantly lower in the isomaltulose versus the sucrose group (b: 34.01 [6.59–61.44], P = 0.016). Other secondary parameters did not significantly differ between groups.
CONCLUSIONS
Isomaltulose did not influence glycemic control assessed as HbA1c in type 2 diabetes under free-living conditions but was associated with lower triglyceride levels.
In patients with type 2 diabetes, a low glycemic diet is recommended to reduce postprandial hyperglycemia and, thereby, improve glycemic control (1). Isomaltulose (Palatinose), a disaccharide composed of α-1,6–linked glucose and fructose, was recently introduced as an alternative sugar with delayed digestion and absorption (2) resulting in a low glycemic index (GI) of 32 (3).
The aim of this study was to examine whether replacing a daily intake of 50 g sucrose by isomaltulose in sweet foods and beverages over a period of 12 weeks would result in improved glycemic control assessed as HbA1c and metabolic parameters in individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The study followed a randomized, controlled, double-blind design with two parallel groups. One hundred ten patients with type 2 diabetes (age > 18 years, BMI 25–40 kg/m2, HbA1c 6.5–9.0) treated by diet alone or with oral antidiabetic agents were recruited through advertisements between March 2007 and December 2008 in two study centers (Munich and Wuerzburg, Germany) and randomly assigned to either isomaltulose (n = 57) or sucrose (n = 53) intervention. The study protocol was approved by the ethical committees of the Technical University of Munich and the University of Wuerzburg, Germany.
The participants received sweet foods and beverages (biscuits, toffees, milk drinks, soft drinks) containing either 50 g of isomaltulose or sucrose per day in a double-blinded fashion for a period of 12 weeks and were asked to maintain their habitual diet but to refrain from additional sweetened foods other than the test products.
At study entry, after 6 and 12 weeks, venous blood was taken in the morning after a 12-h overnight fast to determine clinical routine and metabolic parameters.
HbA1c, fasting glucose, serum fructosamine, insulin, C-peptide, proinsulin, nonesterified fatty acids (NEFA), total cholesterol, triglycerides, LDL-cholesterol, HDL-cholesterol, and standard clinical parameters were analyzed by a certified laboratory. Homeostasis model assessment–insulin resistance (HOMA-IR) was calculated as previously described (4).
Commercial ELISA kits were used to analyze oxidized (ox)LDL (Mercodia, Uppsala, Sweden), leptin, and adiponectin (R&D Systems, Abingdon, U.K.).
The primary end point of the study was HbA1c at week 12. With a sample size of 55 patients per group, the study had a power of 80% to show a statistically significant difference of at least 0.3% in HbA1c at week 12 between the groups with a type I error level of 0.05 assuming a common standard deviation of 0.5.
RESULTS
One hundred patients completed the study (isomaltulose, n = 52; sucrose, n = 48). Ten patients dropped out for various reasons but not because of side effects (Supplementary Fig. 1). One patient who dropped out after the week 6 visit was included in the analysis by use of a last-observation-carried-forward analysis. Baseline patient characteristics were comparable (Supplementary Table 1), with the exception of higher BMI at baseline in the patients receiving sucrose (32.3 ± 4.5 vs. 29.9 ± 4.2 kg/m2, P = 0.007).
Over the course of the study, there was no significant change in HbA1c within both groups (Table 1). Mean HbA1c at 12 weeks was 7.39 ± 0.78% in the sucrose group and 7.24 ± 0.76% in the isomaltulose group, respectively, with no significant difference between the groups (regression coefficient [b]: 0.02 [95% CI −0.21 to 0.25], P = 0.844) in the final analysis controlling for BMI and baseline levels. There were no significant differences between the groups for insulin, fasting glucose, fructosamine, proinsulin, C-peptide, and HOMA-IR (Table 1). Within the sucrose group, a significant linear increase in triglycerides was observed over the course of the study (P = 0.023), whereas in the isomaltulose group, there was a tendency toward reduced levels, resulting in a significant difference between the groups at week 12 (b: 34.01 [6.59–61.44], P = 0.016).
Table 1.
HbA1c and secondary target parameters over the course of the 12-week intervention period in the sucrose vs. isomaltulose group
In addition, there were significant changes over time within the sucrose group for fructosamine (P = 0.019) and within the isomaltulose group for proinsulin (P = 0.039), C-peptide (P = 0.038), and oxLDL (P = 0.013). All other parameters remained unchanged.
CONCLUSIONS
Short-term studies have consistently shown a reduced glycemic and insulin response after isomaltulose compared with sucrose/glucose ingestion in healthy patients as well as in individuals with type 2 diabetes (5–8). Our study is the first to investigate the effects of a 12-week dietary intervention with 50 g/day isomaltulose compared with sucrose in sweet foods and beverages in patients with type 2 diabetes under free-living conditions. Both dietary interventions were well-tolerated by the participants, independent of the antidiabetic medication. HbA1c, the primary outcome parameter, remained virtually unchanged in both groups after 12 weeks of intervention. Likewise, no profound effects on most other secondary metabolic parameters and cardiovascular risk factors were observed. However, triglyceride levels were significantly lower in the isomaltulose group, which is in accordance with findings from an animal study (9) and might indicate a potential metabolic benefit if sustained over the longer term.
Thus, the results of our study suggest that replacement of 50 g/day sucrose with isomaltulose is not enough to induce a pronounced and clinically relevant effect on HbA1c in individuals with type 2 diabetes in addition to their standard antidiabetic treatment and under free-living conditions. This may be explained by the fact that isomaltulose and sucrose, respectively, constituted only approximately 10% of total caloric intake resulting in an approximate reduction of the overall GI by ∼6 units according to a simple calculation (3), and it is obvious that this proportion within a mixed diet is too small to evoke distinct effects on metabolic control. This finding is in line with a recent 1-year trial in type 2 diabetic patients that shows that an only modest reduction of the dietary GI does not affect HbA1c as a long-term marker for glycemic control (10), although meta-analyses have provided evidence for low GI diets as a useful strategy in the management of diabetes (11–13).
In conclusion, substitution of 50 g/day sucrose by isomaltulose in sweet food and beverages over 12 weeks did not significantly affect HbA1c and most other metabolic and cardiovascular risk parameters, despite significantly lower triglyceride levels in the isomaltulose versus the sucrose group.
Although the principle of isomaltulose action is unquestionable, a more marked modification of the dietary GI may be required to achieve a clinically significant improvement in glycemic control in type 2 diabetic patients.
Acknowledgments
The study was funded by Suedzucker AG, Mannheim/Ochsenfurt, Germany. The study results and data contained in the publication have been developed by and/or for the Suedzucker group. The Suedzucker group reserves the exclusive right to use the results and data for possible health claim requests.
S.B., A.G., R.M., P.W., U.A.-G., and H.H. received a defined grant paid to their institutions from Suedzucker AG, Mannheim/Ochsenfurt, Germany, to conduct this study.
I.H. and S.T. are employed by Suedzucker AG, Mannheim/Ochsenfurt, Germany. No other potential conflicts of interest relevant to this article were reported.
S.B. and I.H. interpreted data and wrote the manuscript. I.H., S.T., A.G., U.A.-G., W.S., and H.H. conceived and designed the study. I.H. and U.A.-G. implemented the study. R.M., W.S., and H.H. supervised the study. P.W. performed statistical analysis. S.T., W.S., and H.H. were responsible for critical revision of the manuscript and its important intellectual content. H.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank K. Backhaus, Y. Breusing, D. Dorbath, E. Kelber, H. Lichtlein, A. Volk (University Hospital Wuerzburg), E. Titova (Juliusspital Wuerzburg), E. Jobst and E. Hammerl (Else Kröner-Fresenius Center for Nutritional Medicine, Technische Universität München) for excellent assistance; Dr. C. Groeger (Labor Dr. Limbach and colleagues, Heidelberg) for coordination of blood analysis; and M. Arenz and T. Doerr (Suedzucker AG Mannheim/Ochsenfurt) for development and preparation of test products.
Footnotes
German clinical trial reg. no. DRKS00003486, https://drks-neu.uniklinik-freiburg.de/drks_web/setLocale_EN.do.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1485/-/DC1
S.B. and I.H. contributed equally to this work.
References
- 1.Bantle JP, Wylie-Rosett J, Albright AL, et al. American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 2.Lina BAR, Jonker D, Kozianowski G. Isomaltulose (Palatinose): a review of biological and toxicological studies. Food Chem Toxicol 2002;40:1375–1381 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 5.Kawai K, Okuda Y, Yamashita K. Changes in blood glucose and insulin after an oral palatinose administration in normal subjects. Endocrinol Jpn 1985;32:933–936 [DOI] [PubMed] [Google Scholar]
- 6.Kawai K, Yoshikawa H, Murayama Y, Okuda Y, Yamashita K. Usefulness of palatinose as a caloric sweetener for diabetic patients. Horm Metab Res 1989;21:338–340 [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Mizuno A, Sakuma M, et al. Effects of a palatinose-based liquid diet (Inslow) on glycemic control and the second-meal effect in healthy men. Metabolism 2007;56:115–121 [DOI] [PubMed] [Google Scholar]
- 8.Holub I, Gostner A, Theis S, et al. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br J Nutr 2010;103:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai H, Mizuno A, Matsuo K, et al. Effect of a novel palatinose-based liquid balanced formula (MHN-01) on glucose and lipid metabolism in male Sprague-Dawley rats after short- and long-term ingestion. Metabolism 2004;53:977–983 [DOI] [PubMed] [Google Scholar]
- 10.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114–125 [DOI] [PubMed] [Google Scholar]
- 11.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87:258S–268S [DOI] [PubMed] [Google Scholar]
- 12.Opperman AM, Venter CS, Oosthuizen W, Thompson RL, Vorster HH. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–381 [DOI] [PubMed] [Google Scholar]
- 13.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–2267 [DOI] [PubMed] [Google Scholar]