Abstract
OBJECTIVE
To determine whether immunocomplexes (ICs) containing advanced glycation end product (AGE)–LDL (AGE-LDL) and oxidized LDL (oxLDL) contribute to the development of retinopathy over a 16-year period in subjects with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Levels of AGE-LDL and oxLDL in ICs were measured in 517 patients of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort. Retinopathy was assessed by stereoscopic fundus photography. Cox proportional hazards models were used to assess the effect of AGE-LDL-ICs and oxLDL-ICs on retinopathy progression.
RESULTS
In unadjusted models, higher baseline levels of AGE-LDL-ICs and oxLDL-ICs significantly predicted progression of diabetic retinopathy outcomes. After adjustment by study-design variables (treatment group, retinopathy cohort, duration of type 1 diabetes, and baseline albumin excretion rate [AER], hemoglobin A1c (HbA1c), and Early Treatment Diabetic Retinopathy Study [ETDRS] score), one SD increase in IC levels was associated with 47% (hazard ratio [HR] 1.47 [95% CI 1.19–1.81]; AGE-LDL-IC) and 45% (1.45 [1.17–1.80]; oxLDL-IC) increased risk of developing proliferative diabetic retinopathy (PDR) and 37% (1.37 [1.12–1.66]; to both ICs) increased risk of progressing to severe nonproliferative retinopathy. Analyses were stratified by retinopathy cohort because results differed between primary and secondary cohorts. For AGE-LDL-ICs, HR for progression to PDR was 2.38 (95% CI 1.30–4.34) in the primary cohort and attenuated in the secondary cohort (1.29 [1.03–1.62]). Similar results were observed for oxLDL-ICs.
CONCLUSIONS
Increased levels of AGE-LDL and oxLDL in ICs are associated with increased risk for progression to advanced retinopathy in patients with type 1 diabetes, indicating that the antibody response to modified LDL plays a significant role in retinopathy progression.
Diabetic retinopathy is a major cause of vision loss in working-age adults (1) and affects the majority of people with type 1 diabetes at some stage of their lives (2). Established risk factors are long duration of diabetes, poor glycemic control, hypertension, dyslipidemia, smoking, and diabetic renal disease (3). In spite of this knowledge, diabetic retinopathy still occurs at an unacceptably high rate (4). Identification of novel markers and mechanisms for its onset and progression will facilitate new preventive and therapeutic strategies.
In people both with and without diabetes, conventional “quantitative” measures of dyslipidemia (e.g., high LDL cholesterol or low HDL cholesterol) are associated with increased risk for atherosclerosis (5). Among subjects with diabetes, conventional lipid profiles are also associated with microvascular complications, including diabetic retinopathy (4). However the associations of plasma lipoprotein levels with diabetic retinopathy are less pronounced than for atherosclerosis, likely because in the retina specialized barrier functions must break down before lipoprotein-mediated effects become operative (6).
In recent years, the importance of lipoprotein modification in the propagation of vascular damage has been recognized (7). Modifications including glycation, oxidation, and formation of advanced glycation end products (AGEs) are observed predominantly in extravasated lipoproteins and are enhanced in diabetes. These lipoprotein modifications are sufficient to elicit the synthesis of auto-antibodies that, in turn, lead to the formation of immunocomplexes (ICs) (8).
Previous studies have focused on the effects of modified lipoproteins on atherosclerosis, but we have demonstrated that similar principles apply in diabetic retinopathy (6). Normally, the inner and outer blood retinal barriers prevent any extravasation of plasma lipoproteins, a special property of the retina; however, in diabetes, damage to these barriers enables extravasation and subsequent lipoprotein modification. High levels of modified forms of LDL in circulating ICs have been implicated as risk factors for atherosclerosis (9), but few studies have examined their associations with diabetic retinopathy. In this study, we have examined progression of retinopathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort of patients with type 1 diabetes, and we report a significant relationship between circulating levels of ICs containing AGE-LDL and oxidized LDL (oxLDL) and progression of diabetic retinopathy over a 16-year period.
RESEARCH DESIGN AND METHODS
Subjects
This study was performed on a subgroup of 517 subjects from the DCCT/EDIC cohort who had AGE-LDL and oxLDL measured in ICs isolated from samples obtained at entry into the DCCT study as well as Early Treatment Diabetic Retinopathy Study (ETDRS) scores performed throughout the DCCT/EDIC study. The original DCCT cohort included 1,441 patients with type 1 diabetes, 13–39 years of age, who were generally in good health and had 1–15 years of diabetes duration at study entry (10). Subjects in the primary prevention cohort were retinopathy free (ETDRS = 1), had diabetes for 1–5 years, and had normal albumin excretion rates (AERs <40 mg/24 h). The subjects in the secondary intervention cohort had mild to moderate nonproliferative diabetic retinopathy (ETDRS = 2–9), diabetes for 1–15 years, and AER ≤200 mg/24 h. Subjects were randomized to intensive or conventional diabetes therapy within each cohort. The entire DCCT cohort was randomized and followed for an average of 6.5 years. In 1993, the interventional phase of the study was stopped, and in 1994, the observational phase of the study (EDIC phase) was initiated (11). EDIC was aimed at assessing the development of macrovascular disease in type 1 diabetes and the progression of microvascular disease. During the EDIC phase, all patients were under the care of their personal health care provider and encouraged to practice intensive insulin therapy. At DCCT baseline (1983–1989), none of the patients had hypertension (defined as ≥140 [systolic] and/or ≥90 mmHg (diastolic) or dyslipidemia [defined as total cholesterol >200 and/or LDL >160 mg/dL]).
Of the 1,441 DCCT participants, 905 had blood collected for a substudy on biomarkers of vascular disease. From these 905 subjects, 517 patients were selected for measurement of oxLDL and AGE-LDL in ICs. In the selection of these 517 patients, those with abnormal albuminuria, increased ETDRS score (≥10), and elevated carotid atherosclerosis (≥25% stenosis at a lesion) were oversampled (i.e., all available cases were sampled), resulting in 157 of the 517 patients having one of these three end points and 361 of the patients having none of these end points.
Serum samples were obtained after an overnight fast at entry into the DCCT study (between 1983 and 1989) and assayed at the time for hemoglobin A1c (HbA1c), creatinine, and lipids, and aliquots were stored at −70°C. The Institutional Review Board of all participating DCCT/EDIC centers approved the DCCT/EDIC study, and all participants provided written informed consent.
Assessment of diabetic retinopathy
During DCCT, diabetic retinopathy was assessed on each eye every 6 months in all patients. During EDIC, retinopathy was assessed in approximately one-quarter of the cohort during each follow-up year, and the entire cohort was assessed at EDIC years 4 and 10. Severity of retinopathy was determined using stereoscopic seven-field fundus photographs and graded according to the ETDRS protocol (12) using methods standardized by the DCCT/EDIC group (10). This study used the abbreviated final version of the ETDRS scale of diabetic retinopathy severity (12), which provides a composite score on a scale of 1–23 for both eyes on each subject. Retinopathy severity levels were defined as follows: ETDRS score 1–3 = none to minimal retinopathy; ETDRS score 4–9 = mild to moderate nonproliferative retinopathy; and ETDRS score 10–23 = severe preproliferative and proliferative retinopathy (13). Four predefined, primary retinal outcomes of interest were established for this study: 1) time to develop proliferative diabetic retinopathy (PDR), 2) time to develop severe nonproliferative diabetic retinopathy (NPDR) (ETDRS score ≥10), 3) time to clinically significant macular edema (CSME), and 4) time to significant progression of diabetic retinopathy beyond what was measured at DCCT baseline (defined as a three-step or greater increase in ETDRS from that obtained at DCCT baseline). Patients having any scatter laser photocoagulation performed during the study were included in the retinopathy groups, and patients having focal laser photocoagulation were considered to have progressed to CSME.
Measurement of AGE-LDL and oxLDL in isolated ICs
Circulating ICs were precipitated from serum samples with 3.5% (weight/volume) polyethylene glycol 8000 and then fractionated by protein G affinity chromatography, thus separating the predominant IgG antibody from the modified LDL (14). The concentrations of oxLDL and AGE-LDL in the fractionated ICs were assayed with a capture assay developed in our laboratory using specific oxLDL and AGE-LDL antibodies, respectively (15). The assays were calibrated with modified forms of LDL prepared in our laboratory. The calibration of our oxLDL control has been described elsewhere (15). The AGE calibrator was a highly reactive AGE-LDL preparation of known protein concentration. The effect of long-term freezing at −70°C was assessed and found to have no effect in the measurements performed. The levels of oxLDL and AGE-LDL in circulating ICs (oxLDL-ICs and AGE-LDL-ICs, respectively) were expressed as a function of the amount of apolipoprotein-B (apoB) contained in the ICs, and the final values were given as the concentrations per milliliter of serum.
Other procedures
At baseline, all DCCT participants completed a physical examination, medical history, electrocardiogram, and laboratory testing including serum creatinine and HbA1c (11,16). Lipid profiles and 4-h urine collections for measurement of AER and creatinine clearance were also obtained. Covariates for the current analyses were obtained from DCCT baseline history, physical examination, and laboratory data (fasting lipids, renal function, and HbA1c). The methodology used to perform the routine measurements used as conventional risk factors in this study were previously described (11,16).
Statistical analysis
The concentrations of AGE-LDL and oxLDL in ICs purified from serum samples collected at DCCT baseline were used to determine whether increases in these ICs could predict elevated risk to develop or induce accelerated progression of retinopathy. Values of AGE-LDL-ICs and oxLDL-ICs were log transformed due to their non-normal distribution. Baseline demographic and clinical variables were stratified by retinopathy cohort (primary prevention, retinopathy free vs. secondary intervention, and mild-moderate baseline retinopathy) and levels of AGE-LDL-ICs at DCCT baseline (i.e., median split [±6.42 µg AGE-LDL in apoB-IC/mL serum]). A two-sample Wilcoxon rank sum test was used to compare groups for ordinal/continuous variables, whereas a Pearson χ2 test was used to compare categorical characteristics. Outcome event rates and their 95% CIs are presented as events per 100 patient-years (17).
Inverse probability-weighted Cox proportional hazards regression models (with 95% CIs) were used to assess the effect of the modified LDL in ICs and of clinical and demographic variables on the risk of progression of retinal disease in the presence of uneven sampling (i.e., oversampling of individuals with possible complications, including albuminuria, retinopathy, and carotid atherosclerosis) (18). Study design–adjusted models accounted for DCCT treatment assignment, presence of baseline retinopathy, duration of diabetes, and baseline measures of AER, HbA1c, and ETDRS score. Recent evidence has shown a strong and consistent protective effect of hydroxymethylglutaryl-CoA reductase inhibitors (statins) and ACE inhibitors on the risk of cardiovascular complications of diabetes and has also shown promise in reducing the risk of diabetic retinopathy progression and the development of macular edema (19,20). In light of this, final models were additionally adjusted for effects of ACE/angiotensin receptor blocker (ARB) and lipid-lowering therapy (the use of these drugs increased as the DCCT/EDIC study progressed; as such, their use at any time leading up to an event or censor time [t] is entered into the model as time-varying covariate). The final models were also adjusted for the weighted mean study HbA1c, sex, smoking, and DCCT baseline measures of HDL cholesterol, LDL cholesterol, triglycerides, systolic blood pressure, and age. Modifying effects of DCCT treatment group, sex, and baseline retinopathy status on the effect of the concentrations of AGE-LDL-ICs and oxLDL-ICs on time to events were also examined. The baseline for the time-to-event analysis was set as the date of DCCT randomization.
All statistical analyses were performed using SAS System version 9.3 (SAS Institute, Cary, NC). A type I error rate was controlled for significance at 0.05 for all analyses.
RESULTS
Demographics and event rates
The characteristics of the DCCT/EDIC subgroup studied in this analysis (n = 517) were similar to those of the subgroup not included in the study (n = 924) (Supplementary Table 1). Baseline measures of height, weight, blood pressures (systolic and diastolic), and lipid profiles were similar. The DCCT baseline AER, HbA1c, and randomized treatment group assignment (standard or intensive therapy) were also similar. The only significant baseline difference between the members of the subgroup studied and the remaining cohort was a higher likelihood for those included of belonging to the secondary intervention cohort (55.0 vs. 46.7%; P = 0.003).
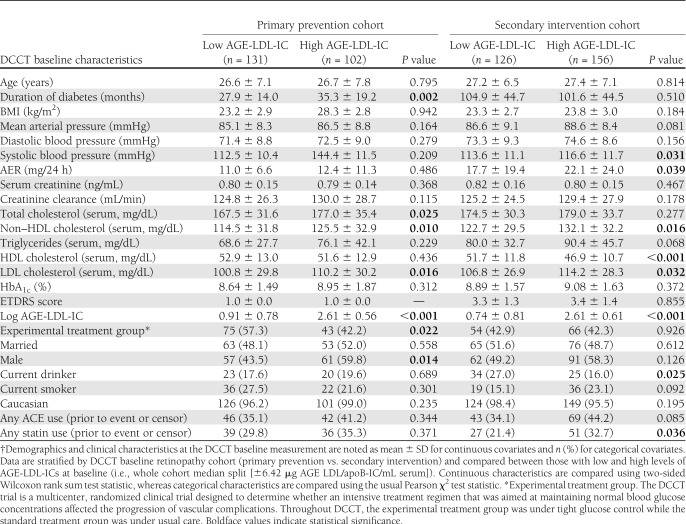
In both the primary prevention (retinopathy free at baseline) and secondary intervention (mild-moderate retinopathy at baseline) cohorts, LDL cholesterol levels were higher in patients in the upper half of the AGE-LDL-IC distribution versus the lower (Table 1). In the primary prevention cohort, patients with levels of AGE-LDL-ICs in the upper half had slightly longer diabetes duration at DCCT entry and slightly higher total cholesterol levels and were more likely to be male than those with levels in the lower half. In contrast, in the secondary cohort, there was a significant negative association between baseline IC level and HDL cholesterol levels. Additionally, in the secondary cohort, being in the upper versus lower half of the AGE-LDL-IC distribution at baseline was associated with increased use of statins or ACE inhibitors during EDIC.
Table 1.
Demographics and clinical characteristics at DCCT baseline measurement†
Of the 517 patients studied, 121 (23.4%) progressed to PDR, 137 (26.5%) progressed to severe NPDR, 113 (21.9%) developed CSME, and 323 (62.5%) experienced a three-step progression beyond their baseline ETDRS score (Supplementary Table 2). The progression to PDR occurred at a lower rate in the primary versus secondary cohort (0.7 [95% CI 0.4–1.0] vs. 2.4 [1.9–2.9] events per 100 person-years, respectively), as did the rate of progression to severe NPDR (0.8 [0.5–1.1] vs. 2.8 [2.3–3.3]) and to CSME (0.8 [0.6–1.2] vs. 2.1 [1.7–2.6]). However, the rate of progression of the first three-step change was similar in the primary and secondary retinopathy cohorts (5.9 [5.0–6.9] and 6.2 [5.3–7.2] events per 100 person-years).
In both the primary and secondary cohorts, the rates for progression to PDR (0.2 [0.1–0.5] vs. 1.3 [0.8–1.9]), progression to severe NPDR (0.3 [0.1–0.7] vs. 1.4 [0.9–2.1]), development of CSME (0.5 [0.3–0.9] vs. 1.3 [0.8–2.0]), and time to first three-step change (4.7 [3.8–6.0] vs. 7.6 [6.1–9.6]) were lower in patients with low AGE-LDL-IC levels than in those with high levels (Supplementary Fig. 1).
Levels of AGE-LDL in isolated ICs
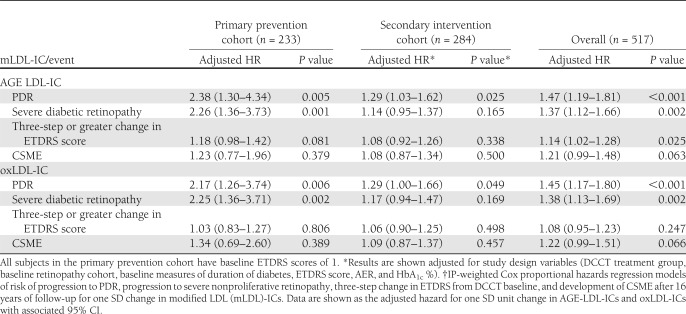
In unadjusted models of the whole subgroup, higher baseline levels of AGE-LDL-ICs were significantly associated with development of all the diabetic retinopathy outcomes assessed in the study. The significant association between AGE-LDL-ICs and retinopathy outcomes was maintained after adjustment for study design variables (Table 2). A change of one SD in AGE-LDL-ICs after log transformation (one SD = 1.138) was associated with a 47% increased risk of developing PDR (hazard ratio [HR] 1.47 [95% CI 1.19–1.81]), 45% increased risk of progressing to severe NPDR (1.45 [1.17–1.80]), 14% increased risk of a three-step or greater change in ETDRS score from DCCT baseline (1.14 [1.02–1.28]), and 21% increased risk of developing CSME (1.21 [0.99–1.48]).
Table 2.
Study design–adjusted* HRs associated with one SD increase in modified LDL-IC†
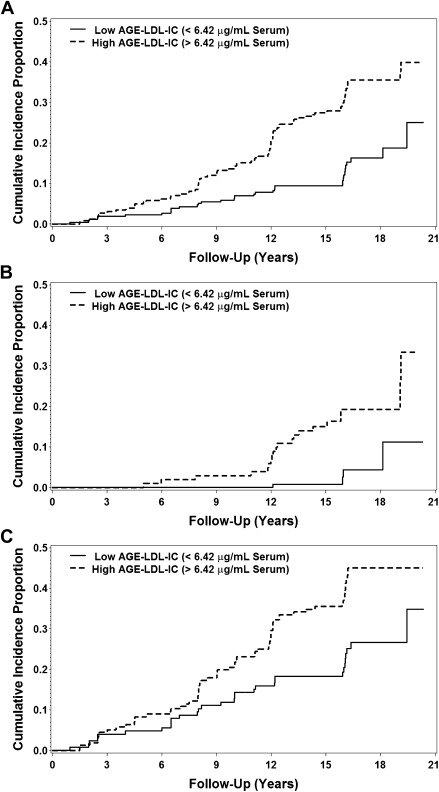
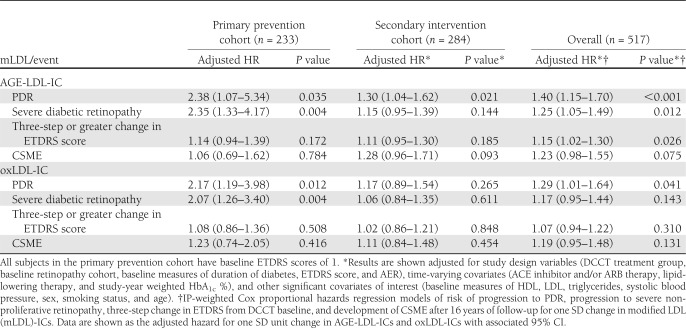
There was also a significant interaction between the subject’s baseline retinopathy status (primary prevention vs. secondary intervention) and levels of AGE-LDL-ICs for both development of PDR (t514 = 2.6; P = 0.011) and progression to severe NPDR (t514 = 3.2; P = 0.001). Figure 1 illustrates the interaction between a subject’s baseline retinopathy status and levels of AGE-LDL-ICs with cumulative incidence of PDR. Due to this interaction, the analysis was further stratified by the retinopathy status at baseline. In the primary prevention, higher baseline levels of AGE-LDL-ICs were significantly associated with development of diabetic retinopathy outcomes but not with the CSME. In the model adjusted by the study design variables (Table 2), one SD increase in log AGE-LDL-ICs was associated with a more than twofold increase in risk of developing both PDR (HR 2.38 [95% CI 1.30–4.34]) and progression to severe NPDR (2.26 [1.36–3.73]), and it was also moderately associated with an increased risk of a three-step or greater change in ETDRS score (1.18 [0.98–1.42]). When the final model was adjusted for other covariates of interest, such as the effects of ACE/ARB treatment and statin use (time adjusted), as well as the mean study HbA1c, the risk of each end point associated with one SD increase in log AGE-LDL-ICs remained significant (Table 3). In the secondary intervention cohort, after adjustment for the study design variables, higher baseline levels of AGE-LDL-ICs were significantly associated with the development of PDR (1.29 [1.03–1.62]) but they were not associated with any of the other outcomes (Table 2). After adjustment for additional covariates, including medications, the effects of AGE-LDL-ICs on progression of PDR, in the secondary cohort, maintained significance (1.30 [1.04–1.62]) (Table 3).
Figure 1.
Cumulative incidence of progression to PDR by baseline high/low AGE-LDL-IC levels (median split: ±6.42 AGE-LDL-IC μg apoB/mL serum) (A) as well as stratified for the primary prevention cohort (B) and secondary intervention cohort (C).
Table 3.
Study design plus other covariate-adjusted* HRs associated with one SD increase in modified LDL-ICs†
Levels of oxLDL in isolated ICs
Unadjusted analysis of the subgroup showed that one SD increase in oxLDL-ICs was associated with a significant increase in the risk of developing each diabetic retinopathy outcome. After adjustment for study design variables (Table 2), relationships between one SD change in oxLDL-ICs (one SD = 0.907) and progression to PDR and severe NPDR remained significant (HR 1.45 [95% CI 1.17–1.80] and 1.38 [1.13–1.69], respectively).
Similar to what was observed for AGE-LDL-ICs, the effect of oxLDL-ICs on the progression of both PDR and severe NPDR was different in the primary prevention and secondary intervention retinopathy cohorts (PDR t516 = 2.0, P = 0.048; severe NPDR t516 = 2.5, P = 0.013). In the primary cohort, the increase in oxLDL-ICs was associated with an increased risk of developing PDR progression ( 2.17 [1.26–3.74]) or severe NPDR (2.25 [1.36–3.71]) but not with a three-step change or with CSME (Table 2). When additionally adjusted for other covariates of interest, such as time-varying effects of ACE/ARB and statin use, as well as the mean study HbA1c, the significant risk increases associated with one SD increase in oxLDL-ICs in the primary prevention cohort remained stable and statistically significant (Table 3). In the secondary intervention cohort, higher baseline levels of oxLDL-ICs were significantly associated with progression to PDR (1.29 [1.00–1.66]) (Table 2) but not with any of the other retinopathy progression outcomes or CSME in the models adjusted by study design variables only or for study design and other covariates of interest (Table 3).
Secondary covariates
Additional risk factors associated with progression to PDR included DCCT treatment group (conventional vs. intensive, HR 2.89 [95% CI 1.80–4.63]) and baseline retinopathy cohort (secondary intervention cohort vs. primary prevention, 2.32 [1.28–4.22]). Additionally, there was an increased risk of retinal complications in patients with greater diabetes duration (1 year, 1.10 [1.03–1.17]) and increased HbA1c at baseline (1% increase, 1.41 [1.24–1.59]). As previously reported (21), an increase in the weighted mean study HbA1c was also significantly associated with an increased risk of progression to PDR (1% increase, 2.17 [1.82–2.57]).
CONCLUSIONS
The effect of hyperlipidemia, as well as the importance of oxidative stress and modified lipoproteins, mainly AGE-LDL, in the development of retinopathy is well established (2–4,6,13,22,23). The possible involvement of ICs in the pathogenesis of diabetic retinopathy has also been suggested by several groups (24,25). The data presented in this study show that higher baseline AGE-LDL and oxLDL levels in ICs were significantly associated with all the outcomes related to progression of retinopathy. After adjustment by study design variables and conventional risk factors, the significant relationship between high levels of AGE-LDL-ICs and oxLDL-ICs and progression to PDR as well as progression to severe NPDR was maintained in the primary prevention cohort.
At baseline, the subgroup used in our study was quite similar to the whole cohort. However there was a greater prevalence of patients belonging to the secondary prevention cohort in the subgroup studied than in the subgroup not included in the study. Some associations between conventional risk factors and oxLDL-ICs and AGE-LDL-ICs were observed at baseline, including higher levels of total cholesterol, triglycerides, and LDL cholesterol in patients with high levels of oxLDL and AGE-LDL in ICs, and an inverse correlation between the concentrations of AGE-LDL and oxLDL in ICs and HDL cholesterol, which suggests that low HDL cholesterol may be associated with increased oxidative stress and therefore contributes to high levels of modified lipoproteins and IC formation. Men also had higher levels of modified LDL in ICs at baseline. It is noteworthy that although the levels of total cholesterol, triglycerides, and LDL are increased in patients with high levels of ICs, the levels are still within “normal” limits, and therefore their usefulness to identify patients at high risk to develop retinopathy is quite limited.
To increase the statistical power, available participants with possible complications (albuminuria, retinopathy, or carotid atherosclerosis) were oversampled. To overcome this selection bias, inverse sampling probability–weighted analysis techniques were used in all parameter estimation models. Additionally, all analyses were controlled or stratified by baseline markers of diabetes severity (i.e., DCCT retinopathy status, diabetes duration, and HbA1c) and albuminuria. Additionally, we determined that DCCT treatment group was not acting as an effect modifier of associations of interest, thus indicating that the predictive ability of ICs was similar across different DCCT treatment groups. However, residual confounding may still be present in the analysis.
The deposition of AGE-modified proteins starts very early in the evolution of diabetes (26), and perhaps as a consequence of local oxidative stress, oxidized proteins are also generated and colocalize with AGE-modified proteins in the retina of patients with diabetic retinopathy (27). Among all the modified proteins that can emerge as a consequence of glycoxidation and lipid peroxidation, LDL seems to be particularly important. As increased permeability in retinal vessels develops in early retinopathy, oxLDL and AGE-LDL IgG antibodies can diffuse to the extravascular space, thus favoring the formation of proinflammatory ICs in the vessel walls in the retina; indeed we have shown that oxLDL-ICs are present in diabetic retina but not in normal retina (28). Also, recent in vitro work has shown that oxLDL-ICs lead to pericyte loss, one of the initial steps in the development of retinopathy. Interestingly, through engagement of Fcγ receptor I, oxLDL-ICs are able to induce oxidative stress, mitochondrial dysfunction, and apoptosis in retinal pericytes (29).
Although the incidence of PDR, severe retinopathy, and maculopathy is higher in the secondary intervention cohort, increased levels of both AGE-LDL-ICs and oxLDL-ICs are stronger predictors of PDR and severe NPDR in the primary prevention cohort than in the secondary intervention cohort. In contrast, the predictive value of AGE-LDL-ICs and oxLDL-ICs for the first three-step changes in ETDRS was similar in the primary and secondary cohorts. These findings provide evidence and support the hypothesis that oxLDL and AGE-LDL–containing ICs are causally related with the development of retinopathy.
Much of the retinal damage that characterized diabetic retinopathy results from endothelial cell damage, retina vascular leakage, and lack of perfusion. These processes are known to begin well before any clinical evidence of retinopathy is detected (30–32). Increased vascular leakage, lack of perfusion, and endothelial cell dysfunction are associated with increased retinal leukostasis, mediated by increased expression of intracellular adhesion molecule 1 and CD18 (31,33). Leukocyte adhesion to the diabetic vascular endothelium can promote endothelial apoptosis through a Fas/FasL mechanism (31). Interestingly some studies have shown that alterations in the leukocyte-endothelial interaction may be responsible for pericyte loss (30), one of the earliest changes in the diabetic retina.
AGE-LDL and oxLDL-containing ICs are likely to play a role in enhancing retinal leukostasis. ICs prepared with human oxLDL and the human antibodies are known to strongly activate macrophages, leading to the release of a variety of products, including cytokines and matrix metalloproteinases (34–36). Since the interaction between oxLDL-ICs and macrophages is associated with Fcγ receptor ligation (35,37), similar effects are to be expected with ICs prepared with human AGE-LDL and the corresponding antibodies. Interleukin-1β, tumor necrosis factor, and interleukin-6 are among the cytokines released (35,36). These cytokines are known to activate endothelial cells, leading to increased expression of intracellular adhesion molecule 1 and endothelial leukocyte adhesion molecule 1 (E-selectin) (38).
Other possible mechanisms by which oxLDL and AGE-LDL–containing ICs may contribute to the changes observed in diabetic retinopathy include stimulation of growth factors, like vascular endothelial growth factor, and production of matrix proteins, thus leading to thickening of the retinal vascular basement membrane, a well-known characteristic of diabetic retinopathy. Macrophages, when exposed to oxLDL-ICs, have been shown to express increased levels of anti-apoptotic and proliferation-inducing genes (39). Recently we have shown that oxLDL-ICs are able to stimulate the production of collagen IV by mesangial cells (40) as well as other matrix proteins, integrins, and growth factors (data not published).
Further studies are needed to clearly detail the pathogenic mechanisms by which ICs containing AGE-LDL and oxLDL contribute to the development of diabetic retinopathy, but the current study provides strong clinical evidence that a link may likely exist between their formation and/or deposition in the retina and the accelerated progression to retinopathy in patients with type 1 diabetes.
Acknowledgments
This work was supported by a Program Project funded by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (P01-HL-55782), R01 grants funded by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01-DK-081352 and R01-DK-088778), and by a Juvenile Diabetes Research Foundation grant (2006-49). This work was also supported by the Research Service of the Ralph H. Johnson VA Medical Center. The DCCT/EDIC was sponsored through research contracts from the Division of Diabetes, Endocrinology, and Metabolic Diseases (NIDDK) of the NIH. Additional support was provided by the National Center for Research Resources through the General Clinical Research Centers program and by Genentech Inc., through a Cooperative Research and Development Agreement with the NIDDK. No other potential conflicts of interest relevant to this article were reported.
M.F.L.-V. and G.V. acquired and analyzed samples and wrote, reviewed, and edited the manuscript. N.L.B. and K.J.H. analyzed data and wrote, reviewed, and edited the manuscript. T.J.L. and A.J.J. wrote, reviewed, and edited the manuscript. M.F.L.-V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors would like to thank Dr. John M. Lachin (George Washington University, Washington, DC) for his critical feedback and invaluable assistance with analysis methodologies and Charlyne Chassereau (Medical University of South Carolina) and Joan Colglazier (Medical University of South Carolina) for their excellent technical assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2040/-/DC1.
A slide set summarizing this article is available online.
*A complete list of EDIC participants can be found in the Supplementary Data online.
The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. government.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, 2008 [Google Scholar]
- 2.Klein R. The epidemiology of diabetic retinopathy: findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int Ophthalmol Clin 1987;27:230–238 [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Dorman JS, Maser RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care 1990;13:741–747 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotto AM., Jr Evolving concepts of dyslipidemia, atherosclerosis, and cardiovascular disease: the Louis F. Bishop Lecture. J Am Coll Cardiol 2005;46:1219–1224 [DOI] [PubMed] [Google Scholar]
- 6.Wu M, Chen Y, Wilson K, et al. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci 2008;49:2679–2685 [DOI] [PubMed] [Google Scholar]
- 7.Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem 2010;51:229–251 [DOI] [PubMed] [Google Scholar]
- 8.Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol 2010;134:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes 2011;60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 13.Lyons TJ, Jenkins AJ, Zheng D, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci 2004;45:910–918 [DOI] [PubMed] [Google Scholar]
- 14.Lopes-Virella MF, McHenry MB, Lipsitz S, et al. DCCT/EDIC Research Group Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis 2007;190:359–369 [DOI] [PubMed] [Google Scholar]
- 15.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol 2005;12:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The DCCT Research Group Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem 1987;33:2267–2271 [PubMed] [Google Scholar]
- 17.Penman AD, Johnson WD. A SAS program for calculating cumulative incidence of events (with confidence limits) and number at risk at specified time intervals with partially censored data. Comput Methods Programs Biomed 2008;89:50–55 [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Regression of Time to Event Data. New York, NY, Wiley, 1992 [Google Scholar]
- 19.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci 2008;49:3231–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi N. Modulation of the renin-angiotensin system and retinopathy. Heart 2000;84(Suppl. 1):i29–i31:discussion i50 [DOI] [PMC free article] [PubMed]
- 21.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol 2008;126:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol 2001;85:746–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gürler B, Vural H, Yilmaz N, Oguz H, Satici A, Aksoy N. The role of oxidative stress in diabetic retinopathy. Eye (Lond) 2000;14:730–735 [DOI] [PubMed] [Google Scholar]
- 24.Nicoloff G, Blazhev A, Petrova C, Christova P. Circulating immune complexes among diabetic children. Clin Dev Immunol 2004;11:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed E, Nityanand S, Mustafa A, Brismar K, Lefvert AK. Anti-cardiolipin antibodies and circulating immune complexes in type 1 diabetes mellitus: increased prevalence and relation to vascular complications. Clin Exp Immunol 1999;115:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCance DR, Dyer DG, Dunn JA, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest 1993;91:2470–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest 1997;100:2995–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu M, Chen Y, Abdel-Rezack SA, et al. Ox-LDL immunocomplexes are implicated in diabetic retinopathy. Diabetes 2009;58(Suppl. 1):A102 [Google Scholar]
- 29.Fu D, Wu M, Chen Y, et al. Oxidized-LDL immunocomplexes are implicated in diabetic retinopathy. Diabetes 2011;60(Suppl. 1):A53 [Google Scholar]
- 30.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joussen AM, Poulaki V, Mitsiades N, et al. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J 2003;17:76–78 [DOI] [PubMed] [Google Scholar]
- 32.Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994;145:574–584 [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch FC, Miyamoto K, Allport JR, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci 2000;41:1153–1158 [PubMed] [Google Scholar]
- 34.Huang Y, Fleming AJ, Wu S, Virella G, Lopes-Virella MF. Fc-gamma receptor cross-linking by immune complexes induces matrix metalloproteinase-1 in U937 cells via mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol 2000;20:2533–2538 [DOI] [PubMed] [Google Scholar]
- 35.Virella G, Atchley DH, Koskinen S, Zheng D, Lopes-Virella MF, DCCT/EDIC Research Group Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol 2002;105:81–92 [DOI] [PubMed] [Google Scholar]
- 36.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res 2006;47:1975–1983 [DOI] [PubMed] [Google Scholar]
- 37.Lopes-Virella MF, Binzafar N, Rackley S, Takei A, La Via M, Virella G. The uptake of LDL-IC by human macrophages: predominant involvement of the Fc gamma RI receptor. Atherosclerosis 1997;135:161–170 [DOI] [PubMed] [Google Scholar]
- 38.Cotran RS, Pober JS. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J Am Soc Nephrol 1990;1:225–235 [DOI] [PubMed] [Google Scholar]
- 39.Hammad SM, Twal WO, Barth JL, et al. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis 2009;202:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelsamie SA, Li Y, Huang Y, et al. Oxidized LDL immune complexes stimulate collagen IV production in mesangial cells via Fc gamma receptors I and III. Clin Immunol 2011;139:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]