Abstract
OBJECTIVE
Physical fitness is inversely related to mortality in the general population and in subjects with type 2 diabetes. Here, we present data concerning the relationship between changes in physical fitness and modifiable cardiovascular risk factors in subjects with type 2 diabetes from the Italian Diabetes and Exercise Study.
RESEARCH DESIGN AND METHODS
Sedentary patients with type 2 diabetes (n = 606) were enrolled in 22 outpatient diabetes clinics and randomized to twice-a-week supervised aerobic and resistance training plus exercise counseling versus counseling alone for 12 months. Baseline to end-of-study changes in cardiorespiratory fitness, strength, and flexibility, as assessed by Vo2max estimation, a 5–8 maximal repetition test, and a hip/trunk flexibility test, respectively, were calculated in the whole cohort, and multiple regression analyses were applied to assess the relationship with cardiovascular risk factors.
RESULTS
Changes in Vo2max, upper and lower body strength, and flexibility were significantly associated with the variation in the volume of physical activity, HbA1c, BMI, waist circumference, high-sensitivity C-reactive protein (hs-CRP), coronary heart disease (CHD) risk score, and inversely, HDL cholesterol. Changes in fitness predicted improvements in HbA1c, waist circumference, HDL cholesterol, hs-CRP, and CHD risk score, independent of study arm, BMI, and in case of strength, also waist circumference.
CONCLUSIONS
Physical activity/exercise-induced increases in fitness, particularly muscular, predict improvements in cardiovascular risk factors in subjects with type 2 diabetes independently of weight loss, thus indicating the need for targeting fitness in these individuals, particularly in subjects who struggle to lose weight.
Large studies have shown that physical activity (PA) provides significant health benefits by reducing cardiovascular disease (CVD) and all-cause mortality in the general population (1,2) and also in subjects with type 2 diabetes (3,4). A recent meta-analysis has shown that aerobic and resistance exercise are both effective in reducing HbA1c in diabetic individuals (5). Two randomized controlled trials, the Diabetes Aerobic and Resistance Exercise study (6) and the Health benefits of Aerobic and Resistance Training in individuals with type 2 Diabetes study (7), have demonstrated that combined aerobic and resistance training is more effective than either one alone.
In the recent Italian Diabetes and Exercise Study (IDES) (8), a strategy combining a prescribed and supervised mixed (aerobic and resistance) training program with structured exercise counseling was more effective than counseling alone in improving HbA1c and other modifiable CVD risk factors, decreasing the number and/or dosage of medications, and reducing coronary heart disease (CHD) 10-year risk scores in a large cohort of sedentary subjects with type 2 diabetes. In addition, physical and mental health–related quality-of-life improved significantly with supervised exercise, but not with counseling alone (9). The incremental health benefits of supervised exercise beyond those of counseling alone were associated with a higher volume of PA, both total and unsupervised, and more marked improvements in physical fitness (8).
Because physical fitness is mainly determined by the level of PA/exercise, it increases with training, namely, aerobic training enhancing cardiorespiratory fitness and resistance training augmenting muscular fitness (10,11). Both components of physical fitness have been shown to be inversely related to all-cause mortality in the general population (12,13), whereas, for subjects with type 2 diabetes, an association with all-cause and CVD mortality has been shown so far for cardiorespiratory fitness only (14–17). In these studies (12–17), the relation of physical fitness with mortality was independent of BMI. However, the detrimental effect of overweight/obesity on mortality risk disappeared or was significantly reduced after adjustment for a number of confounding variables, including fitness and amount of exercise (18).
A recent systematic review showed that although the risk of death was higher in unfit/nonfat individuals than in fit/fat subjects, having a high BMI, even in the presence of high PA, posed a greater risk for the incidence of type 2 diabetes and the prevalence of CVD risk factors compared with normal BMI in the presence of low PA (19). Thus, it is unclear whether the benefits of PA and exercise on the unfavorable profile of CVD risk factors in subjects with type 2 diabetes and/or the metabolic syndrome, which appears to be mainly driven by increased adiposity (19), are predicted by improvements in the fitness components, independently of body weight loss. To address this issue, we analyzed the relationship between changes in physical fitness and improvements in modifiable CVD risk factors in subjects with type 2 diabetes participating in the IDES.
RESEARCH DESIGN AND METHODS
The design and methods of this multicenter, randomized controlled trial have been detailed elsewhere (8,20) and will be briefly reported here. Locally appointed ethics committees approved the research protocol, and participants gave written informed consent.
Setting and participants
The IDES involved 22 outpatient diabetes clinics throughout Italy between October 1, 2005, and March 31, 2006. Each center was connected with a metabolic fitness center, a dedicated facility where patients trained under the supervision of an exercise professional. Sedentary patients with type 2 diabetes, according to the definition of the American Diabetes Association (21) and who fulfilled the International Diabetes Federation criteria for the metabolic syndrome (22), were eligible for this study. Subjects who had any condition limiting or contraindicating PA were excluded (8,20). Of the 691 eligible patients, 85 were excluded for various reasons and 606 were recruited and randomized to supervised training plus structured exercise counseling (EXE group; n = 303) versus a control group that received counseling alone as part of standard care (CON group; n = 303) for 12 months. Randomization was stratified by center and, within each center, by age (<60 vs. ≥60 years) and type of diabetes treatment (diet with or without oral agents vs. insulin) using permuted-block randomization software. The study flow chart has been reported elsewhere (8). Physicians and patients were not blinded to group assignment, whereas sample blinding at the central laboratory was achieved using bar codes.
Interventions
Subjects from both groups received structured, individualized counseling (23) aimed at achieving the currently recommended amount of PA (24) by encouraging any type of commuting, occupational, home, and leisure-time PA. Counseling was reinforced every 3 months.
The training program for the EXE group consisted of 150 min/week in two supervised sessions of progressive mixed (aerobic and resistance) training (8,20). Aerobic training was performed at 55–70% of predicted Vo2max using a treadmill, step, elliptical, arm, or cycle-ergometer. The exercise load for the equipment was calculated to achieve prescribed exercise intensity, expressed as percentage of Vo2max, by the use of standard equations (25). Resistance training was performed at 60–80% of predicted 1 repetition maximum (1-RM) and consisted of four resistance exercises, comprising a thrust movement on the transverse plane (chest press or equivalent), traction movement on the frontal plane (lateral pull down or equivalent), squat movement (leg press or equivalent), trunk flexion for the abdominals, and three stretching positions. Intensity was adjusted according to improvements in predicted Vo2max and 1-RM, as recorded throughout the study. In addition, caloric expenditure was increased progressively by 0.1-kcal/kg body weight/session every month.
Standard care consisted of a treatment regimen aimed at achieving optimal glycemic, lipid, blood pressure (BP), and body weight targets, as established by current guidelines, and including diet prescription and glucose-, lipid-, and BP-lowering agents, as needed (8,20). For ethical reasons and to test the real-world applicability of the IDES results, the treatment regimen was adjusted according to results of biochemical tests performed locally at 3-month intervals, and changes in type, number, and dosage of drugs were recorded.
Main outcome measures
The primary outcome was HbA1c reduction. Secondary outcomes included improvements in other modifiable CVD risk factors, change in the number and/or dosage of glucose-, lipid-, and BP-lowering drugs, UK Prospective Diabetes Study (UKPDS) global CHD 10-year risk scores, health-related quality-of-life, and the relationship between changes in physical fitness and modifiable CVD risk factors. Results of this trial have been reported elsewhere (8,9), except those concerning the relation of physical fitness with modifiable CVD risk factors.
At baseline, the volume of PA was assessed retrospectively using the Minnesota Leisure Time Physical Activity questionnaire (26). The amount of PA was evaluated prospectively by asking patients to fill in a daily diary based on the range of activities considered in this questionnaire. Volume was calculated by multiplying the metabolic equivalent (MET) scores corresponding to each Minnesota code (8), by time in hours per week spent in each activity, and expressed as METs · h · week−1. For aerobic exercise, energy expenditure during supervised sessions was calculated automatically by the machines from workload (i.e., the combination of speed and slope for treadmill, steps per minute for step and power for ergometer), using standard equations (25). For resistance exercise, a conservative estimate of 3 METs · h was established, based on direct measurements in subjects with type 2 diabetes (27).
The following modifiable CVD risk factors were evaluated at baseline and end-of-study: HbA1c, fasting blood glucose and serum insulin, waist circumference, BMI, BP, triglycerides, total and HDL cholesterol, and high-sensitivity C-reactive protein (hs-CRP). Biochemical tests were performed at the central laboratory, at baseline, and end-of-study, and locally, throughout the study period, to adjust treatment regimen (8,20). The Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) index was calculated by the formula: HOMA-IR = [insulin (in pmol/L) × FPG (in mmol/L)]/156.3, whereas LDL cholesterol was estimated by the equation: LDL cholesterol (in mmol/L) = total cholesterol – [HDL cholesterol + (triglycerides/2.17)], as previously reported (20). Global CHD 10-year risk scores were calculated using the UKPDS risk engine (28).
Parameters of physical fitness (i.e., cardiorespiratory fitness, strength, and flexibility) were evaluated at baseline, end-of-study, and in the EXE group, also during the study period, to adjust training loads (8,20). Assessment of cardiorespiratory fitness consisted of a submaximal Vo2max evaluation, (i.e., at 80% of the predicted maximal heart rate [= 220 – age]). It was preferred to a maximal test, because 1) the latter cannot be performed without a cardiologist, according to Italian law; and 2) heart rate varies linearly with Vo2 to the point of maximum exertion, thus allowing extrapolation of the actual Vo2max value.
The test was preceded by two consecutive run-in sessions to become familiar with testing devices and protocols. All patients performed the test at the treadmill, which was preferred to the cycloergometer to avoid early muscle exhaustion in untrained subjects, using a protocol modified form the Balke and Ware procedure (29). Indirect calorimetry was used to measure Vo2 by the use of a gas exchange analyzer (FitMate, Cosmed, Rome, Italy), with concurrent assessment of heart rate. For patients taking medications that affect heart rate, such as β-adrenergic blockers, the Borg Rate of Perceived Exertion scale was used (version 1–10). Patients were stopped at the perceived value of 5–6 (hard), corresponding to a heart rate of 70–89% (30).
For strength assessment, a test was performed with the following modalities: thrust movement on the transverse plane (chest press or equivalent), traction movement on the frontal plane (lateral pull down or equivalent), and squat movement (leg press or equivalent). Although the 1-RM test is the most reliable test for evaluating the maximal dynamic strength of a muscle or group of muscles, because of the very low fitness profile of patients enrolled in this study, a 5–8 maximal repetition test was preferred, for safety (to avoid maximal loads to the joint structures) and validity (untrained subjects are not always able to properly reach their 1-RM) reasons. Then, 1-RM was predicted from the weight loaded and the number of repetitions executed after a proper warm up using the Brzycki equation (31). Results were expressed as upper body (average of chest press and lateral pull down) and lower body (leg press) strength.
A standard bending test was executed to assess hip and truck flexibility (8,20). Patients stood on a step with legs fully extended and were asked to bend the torso forward to try to touch the ground with their fingertips. The test was performed three times, and the distance between the finger and the ground was measured by the exercise specialist at the third attempt.
Statistical analysis
Baseline to end-of-study changes (expressed as median and interquartile range [IQR]) in total PA, CVD risk factors, and total CHD risk score were calculated by quintiles of changes in physical fitness parameters (i.e., Vo2max and upper and lower body strength and flexibility) and analyzed using Kruskal-Wallis one-way ANOVA. A test for linear trend was also applied. A value of P < 0.05 was considered statistically significant. To encompass the entire range of fitness values, the whole cohort of 606 patients was considered.
To assess whether improvements in fitness predicted changes from baseline in HbA1c, other CVD risk factors, and CHD risk score, independently of body weight loss, multiple regression analyses were applied, with baseline to end-of-study changes in each modifiable CVD risk factor as a dependent variable. Covariates were the baseline value of each CVD risk factor, the change in each fitness parameter (and, for upper and lower body strength and flexibility, also in Vo2max), change in BMI, body weight, or waist circumference to assess dependence on body weight loss, and study arm, to account for the independent effect of intervention. Additional regression analyses were performed with PA/exercise volume as the covariate forced in the model or, to account for change in medication throughout the 12-month period, with treatment at baseline and treatment initiation during the study included in the model as dichotomous (yes vs. no) variables.
RESULTS
Subjects from the IDES cohort had a mean age of 58.8 years (SD 8.5), a median diabetes duration of 6 years (IQR 3–10), and a male-to-female ratio of 58/42. Baseline values for Vo2max, upper and lower body strength, and flexibility were 25.9 ± 6.2 mL/kg/min, 40.0 ± 16.6 kg, 107.3 ± 68.5 kg, and 11.7 ± 9.8 cm, respectively. The two study groups did not differ for any of these parameters (8).
As previously reported (8), the median attendance of the supervised exercise sessions in the EXE group was 80.3% (IQR, 75–99%). Moreover, Vo2max (mean difference 2.8 mL/kg/min [95% CI 2.1–3.5], P < 0.001) and flexibility (−4.6 cm [−5.7 to −3.6]) improved more markedly in EXE than in CON subjects, whereas upper (11.0 kg [9.5–12.5]) and lower (30.8 kg [25.1–35.6]) body strength increased significantly only in EXE participants (8) (Supplementary Fig. 1).
Changes in Vo2max, and upper and lower body strength (Tables 1, 2, and 3) were significantly and linearly associated with variation in PA volume, HbA1c, BMI, waist circumference, hs-CRP, total CHD risk score, and, inversely, with change in HDL cholesterol. In addition, flexibility was significantly associated with these parameters (P < 0.0001 for trend), except for HbA1c (P = 0.003) and hs-CRP (P = 0.041; data not shown). Less significant associations were found for Vo2max with insulin and HOMA-IR, for upper body strength with LDL cholesterol, and for lower body strength with insulin, HOMA-IR, and total and LDL cholesterol. Associations with insulin and HOMA-IR remained after excluding the 73 individuals on insulin treatment, which significantly influences these parameters.
Table 1.
Baseline to end-of-study changes (median and IQR) in PA volume, CVD risk factors, and total CHD risk score by quintiles of Vo2max
Table 2.
Baseline to end-of-study changes (median and IQR) in PA volume, CVD risk factors, and total CHD risk score by quintiles of upper body strength
Table 3.
Baseline to end-of-study changes (median and IQR) in PA volume, CVD risk factors, and total CHD risk score by quintiles of lower body strength
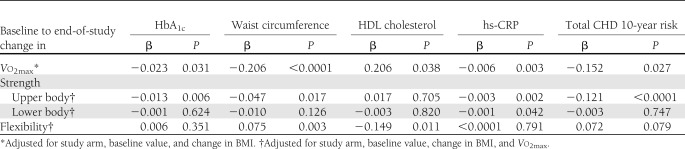
Regression analyses (Table 4) showed that changes in Vo2max predicted a reduction in HbA1c, waist circumference, hs-CRP, and total CHD UKPDS risk score, and an increase in HDL cholesterol, independently of study arm and change in BMI. In addition, changes in upper body strength predicted improvements in HbA1c, waist circumference, hs-CRP, and total CHD risk score, whereas an increase in lower body strength predicted only amelioration of hs-CRP, independently of study arm, and changes in BMI and Vo2max. When changes in BMI and Vo2max were excluded from the model, lower body strength was also significantly associated with waist circumference reduction (P = 0.039). Finally, improved flexibility predicted changes in waist circumference and HDL cholesterol, again independently of study arm, and changes in BMI and Vo2max.
Table 4.
Multiple regression analyses of association of baseline to end-of-study changes in physical fitness parameters with variation in selected CVD risk factors and total CHD risk score
In these analyses, BMI was significantly associated with changes in HbA1c, and waist circumference, but not HDL cholesterol, hs-CRP, or total UKPDS CHD risk score, whereas study arm predicted changes in all of these variables with most combinations of fitness components and measures of adiposity as covariates (data not shown). Results were similar when BMI values were substituted for body weight as a covariate in the regression models (data not shown). In contrast, when waist circumference was included in the model as a measure of adiposity in place of BMI or body weight, an independent association of Vo2max and flexibility with CVD risk factors was no longer observed, except for a reduction in hs-CRP (P = 0.017) and an increase in HDL cholesterol (P = 0.018), respectively, whereas strength, particularly upper body, continued to predict HbA1c (P = 0.016), hs-CRP (P = 0.005), and total CHD risk score (P < 0.0001). Finally, all fitness parameters no longer predicted changes in CVD risk factors when PA/exercise volume was forced in the model, whereas associations remained significant when change in medication was included as a covariate.
CONCLUSIONS
This report shows that PA/exercise-induced improvements in physical fitness, particularly in cardiorespiratory fitness and upper body strength, significantly predict changes in several modifiable CVD risk factors and in the total UKPDS CHD 10-year risk score, independently of study arm, change in BMI or body weight, and, in case of strength, also of Vo2max and waist circumference.
A previous meta-analysis of small-sized studies showed that structured aerobic exercise training improves cardiorespiratory fitness in subjects with type 2 diabetes, along with a reduction of HbA1c (32). Moreover, cardiorespiratory and/or muscular fitness correlated with changes in HbA1c in sedentary diabetic individuals performing supervised aerobic and/or resistance training (33). Our data indicate for the first time that in these subjects, PA/exercise-induced amelioration of physical fitness is linked to improvements in a wide range of modifiable CVD risk factors in addition to HbA1c, thus resulting in a significantly reduced estimated total CHD 10-year risk. These factors included waist circumference, insulin, HOMA-IR, HDL cholesterol, and hs-CRP, which are the metabolic and inflammatory abnormalities related to central fat distribution in subjects with type 2 diabetes and the metabolic syndrome (34). Interestingly, waist circumference and HOMA-IR were also independent predictors of HbA1c reduction in the IDES participants (8). Taken together, these observations are consistent with the inverse association of cardiorespiratory fitness with the prevalence of type 2 diabetes (35) and the metabolic syndrome (36) as well as of low levels of PA with traditional and nontraditional CVD risk factors (37) in the general population.
A very important finding of our study is that the relationship of changes in physical fitness with improvements in modifiable CVD risk factors was independent of body weight loss, although fitness correlated significantly with BMI and waist circumference. This observation, which is consistent with the lack of interaction between fitness and fatness (38), supports the concept that fitness ameliorates the unfavorable CVD risk factor profile driven by increased adiposity through mechanisms independent, at least partly, of weight loss. These mechanisms seem to involve enhanced muscle fuel supply and storage, which increase insulin sensitivity (33), as well as production of myokines, which act locally in a paracrine fashion and also as hormones in other organs of the body, including fat, to improve metabolism and reduce inflammation (39). A role for muscle independent of central fat distribution is strongly supported by the observation that muscular fitness, at variance with cardiorespiratory fitness, predicted changes in modifiable CVD risk factors even after adjustment for waist circumference, a measure of visceral adiposity.
These findings suggest that lifestyle interventions aimed at reducing CVD risk in subjects with type 2 diabetes should target improvement of fitness, in addition to weight loss, and that strategies including diet plus PA/exercise would be more effective than diet alone at the same level of achieved BMI. In this view, physical fitness may represent a surrogate outcome in these subjects, especially in individuals who struggle to lose weight. However, although PA is the main determinant of physical fitness, as shown in our study by the strong association between these two parameters and by the disappearance of association between fitness and modifiable CVD risk factors after adjustment for PA/exercise volume, genetic factors also play a role in individuals with the low-fitness phenotype. It is unclear whether specific PA programs may reverse low-fitness in these subjects and whether those who fail to improve fitness with exercise still receive health benefits from training (40).
Finally, improvements of cardiorespiratory and muscular fitness appeared to exert at least partly independent effects on modifiable CVD risk factors, according to the observation that relation of strength, particularly upper body, with these parameters remained significant when Vo2max and also waist circumference were forced in the model as covariates. Moreover, because the EXE subjects followed a supervised mixed (aerobic and resistance) training program, whereas the CON participants performed predominantly aerobic PA (8), this is also in keeping with the association of study arm with improvements in modifiable CVD risk factors, although relation of fitness with these parameters occurred independently of it. This supports the finding that, given the same volume of PA, the combination of aerobic and resistance training is more effective than either one alone in reducing CVD risk in subjects with type 2 diabetes (6,7) and suggests that this is dependent of the concurrent improvement in cardiorespiratory and muscular fitness.
Strengths of this study are that the IDES was multicenter, thus less dependent on local factors, and of a larger size and longer duration than other exercise intervention trials in patients with type 2 diabetes (6,7). A potential limitation of this trial is the unblinded design, although it is impossible to keep assignment to supervised training hidden to both patients and physicians. However, sample analysis was blinded. In addition, in our study, although patients from both groups received specific dietary prescriptions and adherence to diet was verified at intermediate visits, diet was not considered in the data analysis.
In conclusion, this analysis of data from the IDES shows that PA/exercise-induced increases in physical fitness predict improvements in CVD risk factors and estimated CHD 10-year risk in subjects with type 2 diabetes independently of body weight loss and, for muscular fitness, also of waist circumference reduction. These findings indicate the need for targeting fitness, particularly muscular, in these individuals, especially in subjects who struggle to lose weight, by implementing specific training programs including resistance exercise.
Acknowledgments
This work was supported by LifeScan SrL, Novo Nordisk Ltd, Bristol-Myers Squibb Italy, Technogym SpA, and Cosmed SrL. No other potential conflicts of interest relevant to this article were reported.
The sponsors had no role in the design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, and approval of the manuscript.
S.B., S.Z., and A.N. researched data and reviewed and edited the manuscript. P.C., L.S., G.M., A.B., and C.I. researched data and contributed to the discussion. G.P. researched data and wrote the manuscript. G.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the IDES Investigators for participating in this study (see the complete list in the Supplementary Data online).
Footnotes
Clinical trial reg. no. ISRCTN04252749, www.isrctn.org.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1859/-/DC1.
A complete list of the IDES Investigators can be found in the Supplementary Data online.
References
- 1.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986;314:605–613 [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med 2002;347:716–725 [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Eriksson J, Barengo NC, et al. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation 2004;110:666–673 [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med 2003;163:1440–1447 [DOI] [PubMed] [Google Scholar]
- 5.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 6.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 7.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balducci S, Zanuso S, Nicolucci A, et al. Italian Diabetes Exercise Study (IDES) Investigators Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in type 2 diabetic subjects: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med 2010;170:1794–1803 [DOI] [PubMed] [Google Scholar]
- 9.Nicolucci A, Balducci S, Cardelli P, Zanuso S, Pugliese G, Italian Diabetes Exercise Study (IDES) Investigators Improvement of quality of life with supervised exercise training in subjects with type 2 diabetes mellitus.. Arch Intern Med 2011;171:1951–1953 [DOI] [PubMed] [Google Scholar]
- 10.Cauza E, Hanusch-Enserer U, Strasser B, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 2005;86:1527–1533 [DOI] [PubMed] [Google Scholar]
- 11.Larose J, Sigal RJ, Boulé NG, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc 2010;42:1439–1447 [DOI] [PubMed] [Google Scholar]
- 12.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801 [DOI] [PubMed] [Google Scholar]
- 13.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 15.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation 2003;107:2435–2439 [DOI] [PubMed] [Google Scholar]
- 16.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27:83–88 [DOI] [PubMed] [Google Scholar]
- 17.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med 2005;165:2114–2120 [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BK. Body mass index-independent effect of fitness and physical activity for all-cause mortality. Scand J Med Sci Sports 2007;17:196–204 [DOI] [PubMed] [Google Scholar]
- 19.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev 2010;11:202–221 [DOI] [PubMed] [Google Scholar]
- 20.Balducci S, Zanuso S, Massarini M, et al. Italian Diabetes Exercise Study (IDES) Group The Italian Diabetes and Exercise Study (IDES): design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr Metab Cardiovasc Dis 2008;18:585–595 [DOI] [PubMed] [Google Scholar]
- 21.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 23.Di Loreto C, Fanelli C, Lucidi P, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care 2003;26:404–408 [DOI] [PubMed] [Google Scholar]
- 24.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1433–1438 [DOI] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA, Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 26.Folsom AR, Jacobs DR, Jr, Caspersen CJ, Gomez-Marin O, Knudsen J. Test-retest reliability of the Minnesota leisure time physical activity questionnaire. J Chronic Dis 1986;39:505–511 [DOI] [PubMed] [Google Scholar]
- 27.Zanuso S, Balducci S, Gutierrez AJ, Bergamin M, Pugliese G. Similar energy expenditure from resistance training at moderate and vigorous intensity in subjects with type 2 diabetes. Diabetes Res Clin Pract 2009;85:e40–e41 [DOI] [PubMed] [Google Scholar]
- 28.Stevens RJ, Kothari V, Adler AI, Stratton IM, United Kingdom Prospective Diabetes Study (UKPDS) Group The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–679 [PubMed] [Google Scholar]
- 29.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J 1959;10:675–688 [PubMed] [Google Scholar]
- 30.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381 [PubMed] [Google Scholar]
- 31.Brzycki M. Strength testing – predicting a one-rep max from reps to fatigue. J Phys Educ, Recreat Dance 1993;64:88–90 [Google Scholar]
- 32.Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003;46:1071–1081 [DOI] [PubMed] [Google Scholar]
- 33.Larose J, Sigal RJ, Khandwala F, Prud’homme D, Boulé NG, Kenny GP, Diabetes Aerobic and Resistance Exercise (DARE) trial investigators Associations between physical fitness HbA1(c) in type 2 diabetes mellitus. Diabetologia 2011;54:93–102 [DOI] [PubMed] [Google Scholar]
- 34.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 2007;27:996–1003 [DOI] [PubMed] [Google Scholar]
- 35.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 1999;130:89–96 [DOI] [PubMed] [Google Scholar]
- 36.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002;25:1612–1618 [DOI] [PubMed] [Google Scholar]
- 37.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006;295:1412–1419 [DOI] [PubMed] [Google Scholar]
- 38.Stevens J, Evenson KR, Thomas O, Cai J, Thomas R. Associations of fitness and fatness with mortality in Russian and American men in the lipids research clinics study. Int J Obes Relat Metab Disord 2004;28:1463–1470 [DOI] [PubMed] [Google Scholar]
- 39.Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol 2009;587:5559–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Church T. The low-fitness phenotype as a risk factor: more than just being sedentary? Obesity (Silver Spring) 2009;17(Suppl. 3):S39–S42 [DOI] [PubMed] [Google Scholar]