Abstract
OBJECTIVE
To provide a comprehensive assessment of multiorgan insulin sensitivity in lean and obese subjects with normal glucose tolerance.
RESEARCH DESIGN AND METHODS
The hyperinsulinemic-euglycemic clamp procedure with stable isotopically labeled tracer infusions was performed in 40 obese (BMI 36.2 ± 0.6 kg/m2, mean ± SEM) and 26 lean (22.5 ± 0.3 kg/m2) subjects with normal glucose tolerance. Insulin was infused at different rates to achieve low, medium, and high physiological plasma concentrations.
RESULTS
In obese subjects, palmitate and glucose Ra in plasma decreased with increasing plasma insulin concentrations. The decrease in endogenous glucose Ra was greater during low-, medium-, and high-dose insulin infusions (69 ± 2, 74 ± 2, and 90 ± 2%) than the suppression of palmitate Ra (52 ± 4, 68 ± 1, and 79 ± 1%). Insulin-mediated increase in glucose disposal ranged from 24 ± 5% at low to 253 ± 19% at high physiological insulin concentrations. The suppression of palmitate Ra and glucose Ra were greater in lean than obese subjects during low-dose insulin infusion but were the same in both groups during high-dose insulin infusion, whereas stimulation of glucose Rd was greater in lean than obese subjects across the entire physiological range of plasma insulin.
CONCLUSIONS
Endogenous glucose production and adipose tissue lipolytic rate are both very sensitive to small increases in circulating insulin, whereas stimulation of muscle glucose uptake is minimal until high physiological plasma insulin concentrations are reached. Hyperinsulinemia within the normal physiological range can compensate for both liver and adipose tissue insulin resistance, but not skeletal muscle insulin resistance, in obese people who have normal glucose tolerance.
Obesity is associated with a constellation of metabolic alterations that are risk factors for coronary heart disease, including diabetes, dyslipidemia, and nonalcoholic fatty liver disease (1). It is likely that insulin resistance in specific organ systems, namely adipose tissue, liver, and skeletal muscle, is involved in the pathogenesis of these metabolic abnormalities (2,3). The effect of insulin resistance on daily glucose and free fatty acid (FFA) metabolism in obese people who do not have diabetes is unclear, however, because it is possible that hyperinsulinemia associated with obesity can overcome the defect in insulin action and normalize metabolic function. In fact, data from large studies have demonstrated that basal plasma glucose and FFA concentrations in obese people are not different than those in lean subjects (4). Accordingly, it is possible that many obese people have multiorgan insulin resistance and are at increased risk for development of metabolic diseases even when basal glucose and FFA concentrations are normal.
The assessment of insulin action is complex because insulin has multiple metabolic functions that differ across organ systems and require different doses of insulin to achieve maximal effects (5). A multistage hyperinsulinemic-euglycemic clamp procedure (HECP), conducted in conjunction with isotopically labeled tracer infusions to measure substrate kinetics, can be used to determine simultaneously insulin action in the liver (suppression of glucose Ra into plasma), muscle (stimulation of glucose Rd from plasma), and adipose tissue (suppression of adipose tissue triglyceride lipolysis; i.e., glycerol and palmitate Ra into plasma).
The primary purpose of the current study was to further understand the potential insulin-related metabolic dysfunction associated with obesity by providing a comprehensive assessment of multiorgan insulin sensitivity across a physiological range of plasma insulin concentrations in lean and obese subjects through the use of a multistage HECP in conjunction with stable isotopically labeled glucose, palmitate, and glycerol tracer infusions. Only subjects who had normal fasting plasma glucose concentrations and who did not have impaired glucose tolerance or diabetes were included in this study to eliminate the potential confounding influences of basal hyperglycemia and diabetes therapy on the assessment of insulin action. Most obese people do not have impaired glucose tolerance or diabetes, so our group represents the majority of the obese population (4). We hypothesized that hepatic glucose production and adipose tissue lipolytic rate are much more sensitive to insulin than is muscle glucose uptake. Basal hyperinsulinemia and physiological increases in plasma insulin concentration in obese subjects with normal glucose tolerance should therefore be able to overcome insulin resistance in the liver and adipose tissue but not skeletal muscle, resulting in normal rates of endogenous glucose production (EGP) and lipolysis but not glucose disposal.
RESEARCH DESIGN AND METHODS
A total of 26 lean (BMI 18.5–24.9 kg/m2) and 40 obese (30.3–44.2 kg/m2) sedentary (<1 h of exercise per week) subjects participated in this study. Lean and obese subjects underwent a two-stage HECP in conjunction with stable isotopically labeled tracer infusions as part of previous studies (6,7) and for this study. All subjects completed a comprehensive medical evaluation, which included a history and physical examination, blood tests, and a 2-h oral glucose tolerance test. No subject had impaired fasting glucose or diabetes or took medications that can affect glucose or lipid metabolism. All subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine in St. Louis, Missouri.
Body composition analysis
Body fat mass and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Delphi-W densitometer; Hologic, Waltham, MA).
HECP
Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine in the evening before the HECP. Between 1800 and 2000 h, subjects consumed a standard meal, containing ∼12–15 kcal/kg FFM comprising 55% carbohydrate, 30% fat, and 15% protein. Subjects then fasted until completion of the HECP the next day.
At 0500 h the next morning, one catheter was inserted into a forearm vein to infuse stable isotopically labeled tracers (Cambridge Isotope Laboratories, Andover, MA), dextrose, and insulin, and a second catheter was inserted into a radial artery in the contralateral hand to obtain blood samples. When radial artery cannulation was not possible, a catheter was inserted into a hand vein, which was heated to 55°C with a thermostatically controlled box, to obtain arterialized blood samples. A primed, continuous infusion of [6,6-2H2]glucose (priming dose 22.5 μmol · kg−1, infusion rate 0.25 μmol · kg−1 · min−1) was started at 0600 h, followed at 0800 h by a continuous infusion of [2,2-2H2]palmitate (0.035 μmol · kg−1 · min−1) or [U-13C]palmitate (0.006 μmol · kg−1 FFM−1 · min−1), and in obese subjects only, a primed, continuous infusion of [1,1,2,3,3-2H5]glycerol (priming dose 1.2 μmol · kg−1, infusion rate 0.08 μmol · kg−1 · min−1) was administered. After 3.5 h of [6,6-2H2]glucose infusion (basal period), a two-stage HECP was started and continued for ∼6.5 h. Either [2,2-2H2]palmitate or [U-13C]palmitate tracer infusion could be used to evaluate palmitate kinetics, because both tracers result in the same palmitate Ra values (B.W.P., S.K., unpublished observation).
In all 26 lean subjects and in 14 obese subjects, insulin was infused at a rate of 7 mU · m−2 body surface area (BSA) · min−1 (initiated with a priming dose of 28 mU · m−2 BSA · min−1 for 5 min and then 14 mU · m−2 BSA · min−1 for 5 min) for ∼180 min during stage 1. In the remaining 26 obese subjects, insulin was infused at a rate of 20 mU · m−2 BSA · min−1 (initiated with a priming dose of 80 mU · m−2 BSA · min−1 for 5 min and then 40 mU · m−2 BSA · min−1 for 5 min) during stage 1. In all 66 subjects, insulin was infused at a rate of 50 mU · m−2 BSA · min−1 (initiated with a priming dose of 200 mU · m−2 BSA · min−1 for 5 min and then 100 mU · m−2 BSA · min−1 for 5 min) and continued for ∼210 min during stage 2. These three different insulin infusion rates allowed assessments of adipose tissue and liver insulin sensitivities (low-dose and medium-dose insulin infusion rates during stage 1 to suppress adipose tissue lipolysis and hepatic glucose production submaximally) and skeletal muscle insulin sensitivity (high-dose insulin infusion to stimulate muscle glucose uptake during stage 2) (3). Euglycemia (5.5 mmol/L) was maintained by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose at variable rates. The glucose, glycerol, and palmitate tracer infusion rates were decreased by 50% during stage 1 of the clamp procedure and by additional increments of 50% (glucose) and 75% (glycerol and palmitate) during stage 2 to account for the expected declines in hepatic glucose production and lipolytic rates.
Analyses of samples
Plasma glucose concentration was measured with an automated glucose analyzer (Yellow Spring Instruments Co., Yellow Springs, OH). Plasma insulin and C-peptide concentrations were measured with a chemiluminescent immunoassay (Immulite 1000; Diagnostic Products Corporation, Los Angeles, CA). Plasma FFA concentrations were determined by gas chromatography, and plasma glucose, palmitate, and glycerol tracer-to-tracee ratios in plasma were determined by using electron impact ionization gas chromatography–mass spectrometry, as previously described (6,7). Triglyceride and cholesterol concentrations in plasma were measured enzymatically by using a Hitachi 917 autoanalyzer (Hitachi, Tokyo, Japan); LDL cholesterol was calculated by using the Friedewald equation (8).
Calculations.
Isotopic steady-state conditions were achieved during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure. Steele’s equation for steady-state conditions was therefore used to calculate substrate kinetics (9). Glucose Rd from plasma was equal to endogenous glucose Ra plus the rate of exogenously infused dextrose and glucose tracer. Palmitate and glycerol kinetics were expressed in micromoles per kilogram of fat mass per minute to provide an index of adipose tissue lipolytic activity in relation to the amount of endogenous fat stores and in micromoles per kilogram of FFM per minute to provide an index of FFA availability for lean tissues that use fatty acids for fuel. Portal vein and hepatic sinusoidal insulin concentrations in obese subjects were estimated from the measured arterial insulin and C-peptide concentrations according to the method of Staehr et al. (10).
Statistical analyses
Statistical analyses were performed with SPSS for Windows (version 17.0; SPSS, Chicago, IL). Results are reported as means ± SEM (normally distributed data sets) or medians and interquartile ranges (skewed data sets). Accordingly, the Student t test for independent samples or the Mann-Whitney U test were applied as appropriate to compare differences in subject characteristics and multiorgan insulin sensitivity between lean (n = 26) and obese (n = 14) subjects who received the same insulin infusion rates during the HECP (i.e., low- and high-dose infusions). The paired Student t test or the Wilcoxon signed rank test were used to evaluate the effect of insulin infusion on substrate kinetics within lean (n = 26) and obese (n = 14 for low-dose infusion [7 mU · m−2 BSA · min−1], n = 26 for medium-dose infusion [20 mU · m−2 BSA · min−1], and n = 40 for high-dose infusion [50 mU · m−2 BSA · min−1]) groups. P ≤ 0.05 was considered statistically significant.
RESULTS
Subject characteristics
The characteristics of the study subjects are shown in Table 1. Although mean basal plasma glucose concentrations were not significantly different between lean and obese subjects, mean plasma insulin concentration was threefold higher in the obese group than in the lean group. Mean plasma triglyceride concentration was greater in obese than lean subjects, and HDL cholesterol concentration was lower; however, LDL cholesterol concentration did not differ between lean and obese subjects. Total plasma FFA concentration did not differ between lean and obese subjects.
Table 1.
Characteristics of the study subjects
Insulin and C-peptide concentrations in obese subjects
Three distinct systemic plasma insulin (SPI) concentrations, which spanned the physiological range, were achieved during the HECP in obese subjects. Insulin infusion of 7, 20, and 50 mU · m−2 BSA · min−1 resulted in significantly different (P < 0.01) SPI concentrations of 151 ± 13, 295 ± 19, and 595 ± 8 pmol/L, respectively. Plasma C-peptide concentration at baseline was 0.79 ± 0.05 nmol/L. Neither the low-dose infusion (7 mU · m−2 BSA · min−1) nor the medium-dose infusion (20 mU · m−2 BSA · min−1) resulted in significant changes in C-peptide concentrations from baseline (0.89 ± 0.07 and 0.76 ± 0.08 nmol/L at the low- and medium-dose infusions, respectively). Plasma C-peptide concentration, however, decreased from baseline during high-dose insulin infusion to 0.58 ± 0.04 nmol/L (P < 0.01). Portal and hepatic sinusoidal insulin concentrations were estimated in obese subjects and were greater than SPI concentration during basal conditions and at all insulin infusion rates. During the basal stage, portal and hepatic sinusoidal insulin concentrations were 215 ± 18 and 190 ± 16 pmol/L, respectively, values that increased with each progressive increase in insulin infusion rate (P ≤ 0.01) to 288 ± 29, 416 ± 33, and 809 ± 43 pmol/L, respectively in the portal vein and 261 ± 26, 392 ± 31, and 766 ± 39 pmol/L, respectively in the hepatic sinusoids. The portal venous-to-peripheral insulin concentration gradient was assumed to be 2.4 pmol/L in the postabsorptive state (10) and decreased to 1.85 ± 0.5, 1.38 ± 0.15, and 1.35 ± 0.2 pmol/L during low-, medium-, and high-dose insulin infusions, respectively (P ≤ 0.01 vs. basal for all insulin infusion values).
Substrate kinetics in obese subjects
Palmitate and glycerol kinetics.
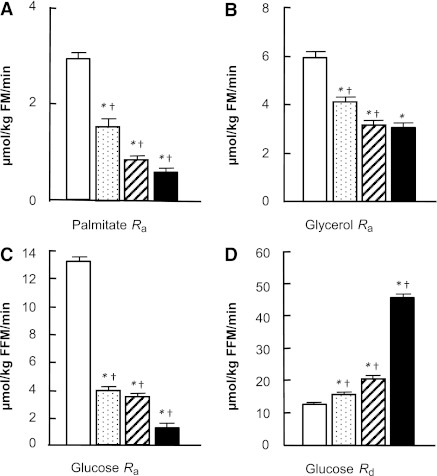
Palmitate Ra and glycerol Ra decreased progressively with increasing plasma insulin concentrations (Fig. 1A and B); however, the relative suppression of glycerol Ra was much less than the relative suppression of palmitate Ra at all insulin infusion rates (Fig. 2). The ratio of palmitate Ra to glycerol Ra thus decreased progressively as plasma insulin concentration increased from a basal value of 0.53 ± 0.02 to 0.22 ± 0.01 μmol during the high-dose insulin infusion (P ≤ 0.01).
Figure 1.
Palmitate Ra (A), glycerol Ra (B), glucose Ra (C), and glucose Rd (D) at baseline (white bars) and during low-dose (speckled bars), medium-dose (hatched bars), and high-dose (black bars) insulin infusions in obese subjects. Values are means ± SEM. *P < 0.01 significantly different from baseline. †P < 0.01 significantly different from lower insulin dose. FM, fat mass.
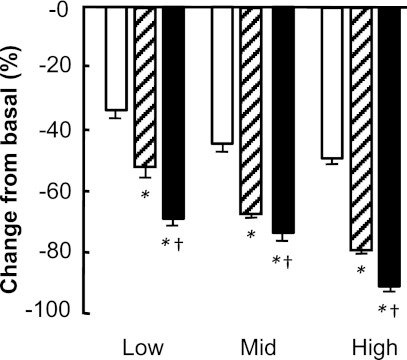
Figure 2.
Suppressions of glycerol Ra (white bars), palmitate Ra (hatched bars), and glucose Ra (black bars) during low-dose, medium-dose, and high-dose insulin infusions in obese subjects. Values are means ± SEM. *P < 0.01 significantly different from glycerol suppression. †P < 0.01 significantly different from palmitate suppression.
Glucose kinetics.
Endogenous glucose Ra decreased progressively with increasing plasma insulin concentrations (Fig. 1C). The relative suppression of glucose Ra increased from 69 ± 2% during low-dose insulin infusion to 90 ± 2% during high-dose insulin infusion (P < 0.01) (Fig. 2). The relative suppression of glucose Ra was significantly greater than the relative suppression of palmitate Ra and glycerol Ra at all insulin infusion rates (P < 0.01) (Fig. 2).
The change in glucose Rd during insulin infusion increased progressively with increasing insulin infusion rates (Fig. 1D). The relative stimulation of glucose Rd (24 ± 5%) was lower than the relative suppression of glucose Ra (69 ± 2%) during low-dose insulin infusion (P < 0.01) (Fig. 1). Glucose Rd increased more than threefold relative to baseline during high-dose insulin infusion (Fig. 1D).
Comparison of insulin sensitivity in lean and obese subjects
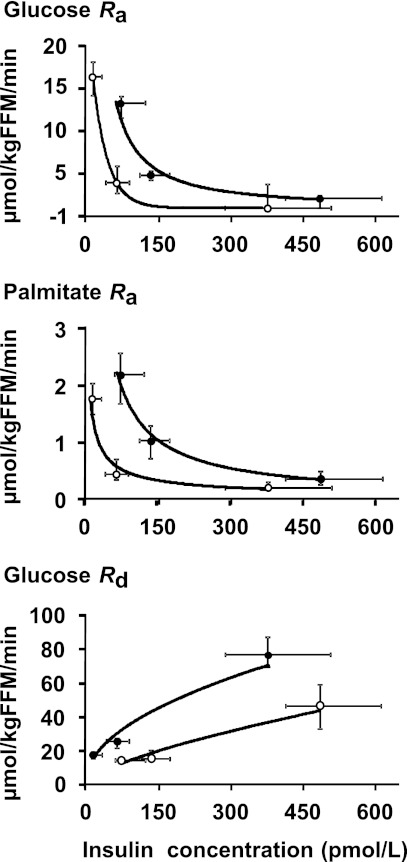
Multiorgan insulin sensitivity was compared in lean (n = 26) and obese (n = 14) subjects who received the same insulin infusion rates during the HECP (i.e., 7 mU · m−2 BSA · min−1 and 50 mU · m−2 BSA · min−1, respectively). In the lean group, insulin infused at 7 (low dose) and 50 (high dose) mU · m−2 BSA · min−1 resulted in significantly different (P ≤ 0.01) SPI concentrations of 70 ± 7 and 399 ± 25 pmol/L, respectively. During low-dose insulin infusion, the SPI concentration in lean subjects was ∼50% lower than the corresponding SPI concentration in obese subjects (P < 0.01). During high-dose insulin infusion, it was ∼20% lower than in obese subjects (P < 0.05). Basal glucose Ra and Rd, expressed as per kilogram of FFM, were greater in lean (16.3 ± 0.5 and 16.6 ± 0.5 μmol · kg FFM−1 · min−1, respectively) than obese (12.7 ± 0.5 and 13.1 ± 0.5 μmol · kg FFM−1 · min−1, respectively) subjects (P < 0.001), whereas palmitate Ra, expressed as per kilogram of FFM, was significantly higher in obese (2.1 ± 0.2 μmol · kg FFM−1 · min−1) than lean subjects (1.7 ± 0.1 μmol · kg FFM−1 · min−1; P < 0.05). The suppressions of palmitate Ra and glucose Ra were greater in lean than obese subjects when plasma insulin concentrations were in the low physiological range but were the same in both groups when insulin concentrations were in the high physiological range (Fig. 3). In contrast, the stimulation of glucose Rd during insulin infusion was greater in lean than obese subjects across the entire physiological range of plasma insulin concentrations (Fig. 3).
Figure 3.
Correlation between systemic plasma insulin concentration and substrate kinetics in lean (○; n = 26) and obese (●; n = 14) subjects. A: Glucose Ra (R2 = 1 and R2 = 0.968, respectively). B: Palmitate Ra (R2 = 0.9486 and R2 = 0.988, respectively). C: Glucose Rd (R2 = 0.957 and R2 = 0.932, respectively). Values are medians and interquartile ranges.
CONCLUSIONS
Insulin resistance in liver (suppression of glucose production), muscle (stimulation of glucose uptake), and adipose tissue (suppression of lipolysis) is involved in the pathogenesis of many of the metabolic complications associated with obesity, including diabetes, dyslipidemia, nonalcoholic liver disease, and the metabolic syndrome. We evaluated insulin sensitivity during basal postabsorptive conditions and across a physiological range of plasma insulin concentrations designed to simulate those observed during postprandial conditions in lean and obese subjects who had normal oral glucose tolerance. Our data demonstrate that EGP and adipose tissue lipolytic rate are much more sensitive to insulin infusion than muscle glucose uptake; glucose production and lipolytic rate were nearly completely suppressed in both lean and obese subjects at plasma insulin concentrations that only minimally stimulated muscle glucose uptake. Although obese subjects demonstrated multiorgan insulin resistance relative to lean subjects, higher basal postabsorptive and higher “simulated” postprandial plasma insulin concentrations in the obese group were able to overcome the impairment in liver and adipose tissue insulin sensitivity so that glucose and FFA Ra into plasma during basal conditions and high-dose insulin infusion were similar between the groups. In contrast, insulin-stimulated muscle glucose uptake was ∼40% higher in lean than obese subjects during high-dose insulin infusion, despite higher insulin concentrations in the obese group. These results suggest that hyperinsulinemia can compensate for both liver and adipose tissue insulin resistance, but not skeletal muscle insulin resistance, in obese people who have normal glucose tolerance. Impaired insulin-stimulated skeletal muscle glucose uptake, rather than suppression of hepatic glucose production or lipolysis of adipose tissue triglycerides, should therefore be considered the major manifestation of insulin resistance in obese people.
We studied a group of lean subjects to serve as a comparison group for our obese subjects. Although relative to the lean group the obese group demonstrated evidence of insulin-resistant liver and skeletal muscle glucose metabolism and adipose tissue fatty acid metabolism during the HECP, basal glucose concentration was not significantly different between the two groups. The normalization of basal glucose metabolism was presumably caused by a threefold greater plasma insulin concentration in the obese than lean subjects, which compensated for the defect in insulin action. Thus, even though our obese subjects had normal fasting plasma glucose and oral glucose tolerance test results, they had evidence of multiorgan insulin resistance when challenged by an insulin infusion. These results underscore the complexity of evaluating the metabolic health of obese people. Although approximately one third of obese people are considered to be “metabolically normal” (11,12), it is likely that most of these individuals are “insulin resistant” with respect to glucose and fatty acid metabolism and therefore at increased risk for diabetes and coronary heart disease (13–15).
Our data demonstrate that physiological hyperinsulinemia causes a greater suppression of EGP than glycerol Ra and palmitate Ra (Fig. 2). This observation is consistent with the results from the only previous study of which we are aware that evaluated the effect of insulin on EGP and adipose tissue lipolytic activity (assessed by glycerol kinetics) simultaneously in the same subjects (16); however, even though hyperinsulinemia caused greater maximal suppression of EGP than glycerol Ra, the half-maximal suppression of glycerol Ra occurred at a lower plasma insulin concentration than that required for half-maximal suppression of EGP. Evaluation of hepatic insulin sensitivity with respect to glucose metabolism by using the HECP is complex, however, because of the dual blood supply to the liver and the effects of insulin on other organ systems that can affect glucose production. Normally, insulin is secreted from pancreatic β-cells into the portal vein and metabolized by the liver before entering the systemic circulation. During basal conditions, portal vein insulin concentration is ∼2.4-fold higher than systemic insulin concentration (10). Infusing insulin through a peripheral vein during the HECP decreases the gradient between portal and systemic insulin concentrations, so that the relationship between insulin delivered to the liver and insulin delivered to other organs changes with increasing insulin infusion. Furthermore, the extrahepatic effects of systemic insulin can decrease hepatic glucose production by suppressing adipose tissue lipolysis and circulating FFA (17), stimulating hypothalamic pathways (18), and inhibiting pancreatic glucagon secretion (19). In addition, systemic insulin decreases EGP by the kidney (20), which can account for ∼25% of EGP during basal conditions (21).
Infusions of palmitate and glycerol tracers are often used to evaluate adipose tissue lipolytic rate, because hydrolysis of 1 mol adipose tissue triglyceride releases 3 mol FFA and 1 mol glycerol into the bloodstream (22). We found that the suppression of palmitate Ra was always greater than the suppression of glycerol Ra during all rates of insulin infusion, indicating that there must be sources of glycerol release into plasma that are not suppressed by insulin. Palmitate released into plasma is derived primarily from lipolysis of adipose tissue triglycerides (23); however, plasma glycerol is derived from lipolysis of adipose tissue triglycerides, lipoprotein lipase–mediated lipolysis of circulating VLDL triglyceride, with spillover of glycerol into the bloodstream (24), and glycerol released from lipolysis of intramyocellular triglycerides (25). An increase in circulating insulin inhibits adipose tissue lipolysis and glycerol released from adipose tissue but stimulates adipose tissue lipoprotein lipase activity (26) and does not affect glycerol release from skeletal muscle (27). In addition, it is possible that increasing plasma insulin concentration stimulates re-esterification of FFA within adipocytes, preventing their release into the circulation (28). Thus, palmitate Ra provides an index of adipose tissue lipolytic rate and FFA availability, whereas glycerol Ra provides an index of whole-body lipolytic activity.
Although our groups were not ideally matched with respect to age, it is unlikely that the age differences confounded our results or conclusions. Both lean and obese subjects were middle-aged, and our obese subjects were slightly younger than our lean subjects. Therefore, the difference in age between groups could not have contributed to the insulin resistance observed in our obese subjects compared with our lean group, because insulin sensitivity declines with aging (29,30).
In summary, even obese people who have normal oral glucose tolerance exhibit multiorgan insulin resistance relative to lean subjects. A small increase in plasma insulin concentration suppresses glucose production and lipolytic rates, whereas stimulation of muscle glucose uptake is minimal until high physiological plasma concentrations of insulin are reached. This stepwise, integrated organ response to increasing insulin concentrations (and glucose availability) helps maintain euglycemia and ensure that adequate glucose is delivered to the brain while preventing unnecessary mobilization of endogenous energy stores when there is a small increase in glucose availability; however, it prevents potentially toxic hyperglycemia when glucose availability and plasma insulin concentrations are high. The hyperinsulinemia associated with obesity helps normalize EGP and lipolytic rate but is not adequate to compensate for the defect in insulin-mediated skeletal muscle glucose uptake.
Acknowledgments
This work was supported by the National Institutes of Health Grants DK-37948, DK-56341 (Nutrition and Obesity Research Center), UL1-RR-024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource), and by a grant from the Longer Life Foundation.
No potential conflicts of interest relevant to this article were reported.
C.C. performed experiments, researched data, analyzed data, and wrote the manuscript. E.F. performed experiments, contributed to the discussion, and reviewed and edited the manuscript. M.K. performed experiments and reviewed and edited the manuscript. B.M. contributed to the discussion and reviewed and edited the manuscript. B.W.P. analyzed study samples and data, contributed to the discussion, and reviewed and edited the manuscript. S.K. designed the study, reviewed the data, contributed to the discussion, and reviewed and edited the manuscript. S.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Freida Custodio, Jennifer Shew, and Dr. Adewole Okunade (Washington University School of Medicine, St. Louis, MO) for their technical assistance; the staff of the Clinical Research Unit (Washington University School of Medicine, St. Louis, MO) for their help in performing the studies; and the study subjects for their participation.
References
- 1.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology 2002;123:882–932 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 6.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008;134:1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010;59:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 9.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956;187:15–24 [DOI] [PubMed] [Google Scholar]
- 10.Staehr P, Hother-Nielsen O, Levin K, Holst JJ, Beck-Nielsen H. Assessment of hepatic insulin action in obese type 2 diabetic patients. Diabetes 2001;50:1363–1370 [DOI] [PubMed] [Google Scholar]
- 11.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 12.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002;25:1177–1184 [DOI] [PubMed] [Google Scholar]
- 15.Howard G, O’Leary DH, Zaccaro D, et al. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators Insulin sensitivity and atherosclerosis. Circulation 1996;93:1809–1817 [DOI] [PubMed] [Google Scholar]
- 16.Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 1986;35:1326–1331 [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN. New concepts in extracellular signaling for insulin action: the single gateway hypothesis. Recent Prog Horm Res 1997;52:359–385; discussion 385–387 [PubMed] [Google Scholar]
- 18.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 19.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 2005;280:33487–33496 [DOI] [PubMed] [Google Scholar]
- 20.Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol 1999;276:E78–E84 [DOI] [PubMed] [Google Scholar]
- 21.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010;27:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday TD. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism 1990;39:384–390 [DOI] [PubMed] [Google Scholar]
- 23.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 2003;52:1641–1648 [DOI] [PubMed] [Google Scholar]
- 24.Ruge T, Hodson L, Cheeseman J, et al. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 2009;94:1781–1788 [DOI] [PubMed] [Google Scholar]
- 25.Moberg E, Sjöberg S, Hagström-Toft E, Bolinder J. No apparent suppression by insulin of in vivo skeletal muscle lipolysis in nonobese women. Am J Physiol Endocrinol Metab 2002;283:E295–E301 [DOI] [PubMed] [Google Scholar]
- 26.Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest 1982;69:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiens B, Lithell H, Mikines KJ, Richter EA. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J Clin Invest 1989;84:1124–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol 1999;276:E233–E240 [DOI] [PubMed] [Google Scholar]
- 29.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab 1985;60:13–20 [DOI] [PubMed] [Google Scholar]