Abstract
OBJECTIVE
Insulin resistance is a risk factor for cardiovascular and noncardiovascular diseases. Impaired kidney function is linked with insulin resistance and may affect relationships of insulin resistance with health outcomes.
RESEARCH DESIGN AND METHODS
We performed a cohort study of 3,138 Cardiovascular Health Study participants (age ≥65 years) without diabetes. Insulin sensitivity index (ISI) was calculated from fasting and 2-h postload insulin and glucose concentrations. Associations of ISI and fasting insulin concentration with all-cause mortality were tested using Cox proportional hazards models, adjusting for demographic variables, prevalent cardiovascular disease, lifestyle variables, waist circumference, and LDL cholesterol. Subsequent models were additionally adjusted for or stratified by glomerular filtration rate estimated using serum cystatin C (eGFR).
RESULTS
A total of 1,810 participants died during the 14.7-year median follow-up. Compared with the highest quartile of ISI, the lowest quartile (most insulin resistant) was associated with 21% (95% CI 6–41) and 11% (−3 to 29) higher risks of death without and with adjustment for eGFR, respectively. Compared with the lowest quartile of fasting insulin concentration, the highest quartile was associated with 22% (4–43) and 4% (−12 to 22) higher risks of death without and with adjustment for eGFR, respectively. Similar attenuation by eGFR was observed when blood pressure, triglycerides, HDL cholesterol, and C-reactive protein were included in models.
CONCLUSIONS
Insulin resistance measured as ISI or fasting insulin concentration is associated with increased risk of death among older adults, adjusting for conventional confounding characteristics. Impaired kidney function may mediate or confound this relationship.
Insulin resistance is an established risk factor for cardiovascular and noncardiovascular diseases. Insulin resistance is associated with increased risk of cardiovascular disease events in diverse community-based populations, whether it is measured directly (1,2), estimated using fasting insulin concentration (e.g., homeostasis model assessment [HOMA]) (3–9), or calculated using dynamic testing (e.g., oral glucose tolerance test [OGTT]) (9,10). Insulin resistance is also associated with increased risk of noncardiovascular diseases, including cancer (2,11). Insulin resistance promotes endothelial dysfunction, oxidative stress, and inflammation and is closely linked with other cardiovascular risk factors (obesity, hypertension, and dyslipidemia) as part of metabolic syndrome (12). Through these mechanisms, insulin resistance may be causally related to adverse clinical outcomes.
Kidney function may play an important role in the relationship of insulin resistance with adverse health outcomes. Impaired kidney function is known to be linked with insulin resistance (13). The causal nature of this relationship is not well defined: impaired kidney function may promote insulin resistance through retained uremic toxins, acidosis, and active vitamin D deficiency; insulin resistance may contribute to the development of impaired kidney function by damaging glomerular endothelial and epithelial cells; and/or shared risk factors (e.g., obesity or genetic predisposition) may underlie both insulin resistance and impaired kidney function (14–18). Lower glomerular filtration rate (GFR), even within the normal range (≥60 mL/min/1.73 m2), is strongly associated with increased risks of cardiovascular disease and death, particularly among older adults (19,20). It is therefore possible that impaired kidney function confounds or mediates known associations of insulin resistance with cardiovascular and noncardiovascular diseases.
We explored whether impaired kidney function confounds or mediates the relationship of insulin resistance with mortality. We chose all-cause mortality as our primary outcome to reflect the pleiotropic effects of insulin resistance. We studied this relationship in the Cardiovascular Health Study (CHS), a community-based population of older adults, because insulin resistance and impaired kidney function are each known strong risk factors for adverse health outcomes among older people (1,19,20). In addition, CHS obtained baseline data ascertaining insulin resistance in both fasting and dynamic states; measured baseline serum cystatin C, which may better discriminate differences in kidney function and its associated health risks in the normal range (20–23); and followed participants for >15 years. These data allow a comprehensive evaluation of the relationships of interest.
RESEARCH DESIGN AND METHODS
Study population
The CHS is a prospective, community-based cohort designed to study risk factors for the development and progression of cardiovascular disease in people aged ≥65 years (24). Participants were recruited from four U.S. communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Eligible participants were sampled using Medicare eligibility lists, were not institutionalized, and were expected to remain in the area for at least 3 years. People who were wheelchair bound in the home or receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded.
The original CHS cohort of 5,201 participants was enrolled in 1989–1990. For the current study, we excluded from this group 1,176 participants with prevalent diabetes (use of insulin or oral hypoglycemic agents, fasting blood glucose ≥126 mg/dL, or 2-h OGTT glucose ≥200 mg/dL) (25), 424 participants with missing measurements of insulin and/or glucose concentrations (fasting and/or 2-h OGTT), and 463 participants with missing covariate data (serum cystatin C, waist circumference, blood pressures, lipid concentrations, and C-reactive protein). Another 687 predominantly African American participants enrolled in 1992–1993 were not included because 2-h OGTT insulin was not measured in this group. The final analytic cohort included 3,138 participants.
Insulin resistance
Insulin resistance was evaluated primarily as the insulin sensitivity index (ISI) derived from an OGTT (26). Insulin and glucose were measured in the fasting state and 2 h after oral consumption of 75 g of glucose. Insulin was measured by competitive radioimmunoassay (Diagnostic Products Corp., Malvern, PA), mean coefficient of variation 10.7%. Glucose was measured using the Kodak Ektachem 700 analyzer (Eastman Kodak, Rochester, NY), mean coefficient of variation 0.93%. ISI was calculated using the formula ISI = 10,000/sqrt(G0 × I0 × G120 × I120). ISI calculated using this method correlates well with insulin sensitivity measured using the hyperinsulinemic euglycemic clamp (r = 0.772, P < 0.01) (26). We evaluated fasting insulin concentration (in quartiles), impaired fasting glucose (100–125 mg/dL), and impaired glucose tolerance (2-h glucose concentration 140–199 mg/dL) as parallel secondary exposures (25). Fasting insulin correlated strongly with HOMA of insulin resistance (HOMA-IR; r = 0.979) (27), which was therefore not evaluated as a separate exposure.
Mortality
Mortality was ascertained through 30 June 2008, by semiannual telephone contact. Follow-up for vital status was 100% complete through this date. Cardiovascular death was examined as a secondary end point defined as death due to atherosclerotic coronary heart disease, heart failure, peripheral vascular disease, or cerebrovascular disease, with cases adjudicated using hospital discharge summaries, diagnostic test records, and consultation reports (28).
Other clinical characteristics
Covariates were ascertained at the baseline CHS study visit in 1989–1990. Age, sex, race (Caucasian or African American), and current smoking were defined by self-report. Prevalent cardiovascular disease was categorized as clinical (self-reported history of myocardial infarction, angina pectoris, or coronary revascularization), subclinical (ankle/arm index <0.9, major electrocardiogram changes, common and internal carotid artery intima-media thickness in the upper 20%, or common carotid stenosis >25% in the absence of clinical cardiovascular disease), or none (29). Medication inventories were completed by CHS staff using participants’ prescription and nonprescription medication bottles (24). Total physical activity was quantified in kilocalories per week using validated questionnaires assessing a broad range of common activities (30). BMI was calculated as weight (kg) divided by height (m2). Waist circumference was measured at the level of the umbilicus. Serum cystatin C was measured using a BNII nephelometer (N Latex Cystatin C; Dade Behring, Deerfield, IL) and used to estimate GFR (eGFR) using the equation: eGFR = 76.7 × [cystatin C]−1.19 (31). Total cholesterol, HDL cholesterol, and triglyceride concentrations were measured using conventional enzymatic methods, with LDL cholesterol calculated using the Friedewald formula (32). C-reactive protein was measured with an enzyme-linked immunosorbent assay developed in the CHS laboratory (33).
Statistical analysis
Spearman correlation was used to quantify cross-sectional correlations of skewed variables. Unadjusted mortality rates by quartiles of ISI and fasting insulin were calculated using the person-years approach. We constructed a cubic spline model to describe the continuous association of ISI with mortality risk, adjusting for age, sex, race, and study site. We tested associations of ISI and fasting insulin with mortality using Cox proportional hazards models. Analysis of Schoenfeld residuals and rescaled residuals plotted versus time demonstrated that the proportional hazards assumption was not violated. Serial models were 1) adjusted for age, sex, race, and study site; 2) additionally adjusted for potential confounders of the insulin resistance–mortality relationship, including prevalent cardiovascular disease, smoking, lipid-lowering medication use, LDL cholesterol, physical activity, and waist circumference; and 3) additionally adjusted for variables that may confound or mediate the insulin resistance–mortality relationship, including systolic and diastolic blood pressures, antihypertensive medication use, HDL cholesterol, triglycerides, and C-reactive protein. eGFR was subsequently added to models 2 and 3.
RESULTS
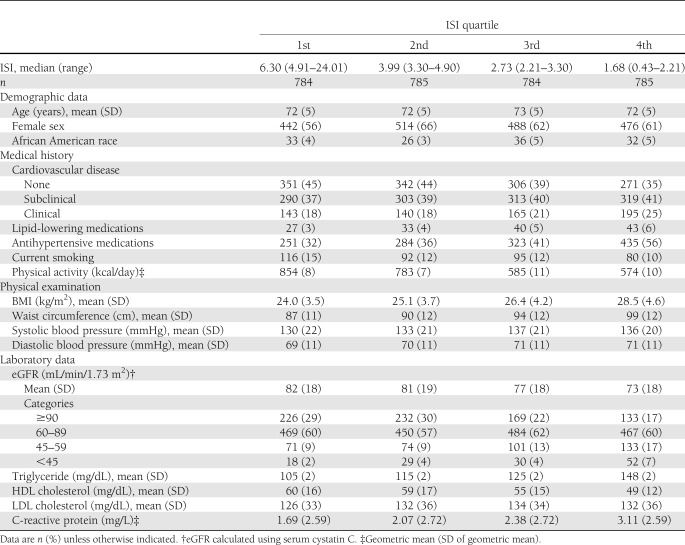
Mean (SD) age was 72 (5) years, and 1,920 participants were women (61%). Distributions of ISI and fasting insulin concentration were skewed, with median (interquartile range) values of 3.30 (2.21–4.91) units and 12 (9–16) IU/mL, respectively. ISI correlated most strongly with 2-h OGTT insulin concentration (r = −0.927), followed by concentrations of fasting insulin (r = −0.734), 2-h OGTT glucose (r = −0.634), and fasting glucose (−0.412). Lower ISI (i.e., greater insulin resistance) was associated with prevalent cardiovascular disease, more prevalent use of lipid-lowering medications, less prevalent smoking, lower physical activity, larger BMI and waist circumference, higher blood pressures, unfavorable lipid concentrations, and higher C-reactive protein concentration (Table 1).
Table 1.
Baseline characteristics of 3,138 participants in the CHS by quartiles of ISI
eGFR was correlated with ISI (r = 0.182, P < 0.001) and fasting insulin concentration (r = −0.236, P < 0.001). These associations were monotonic across the full range of eGFR (Supplementary Fig. 1). Compared with participants with eGFR ≥60 mL/min/1.73 m2, participants with eGFR <60 mL/min/1.73 m2 had lower ISI (median [interquartile range] 2.79 [1.77–4.28] vs. 3.41 [2.31–5.02]) and higher fasting insulin concentration (14 [11–19] vs. 12 [9–15] IU/mL) but no substantial difference in fasting glucose (mean [SD] 99.9 [9.1] vs. 98.7 [9.0] mg/dL).
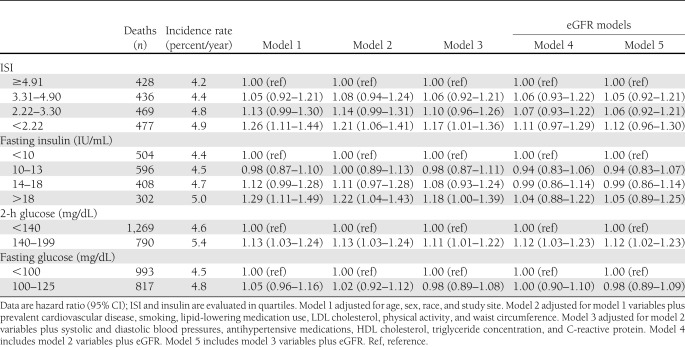
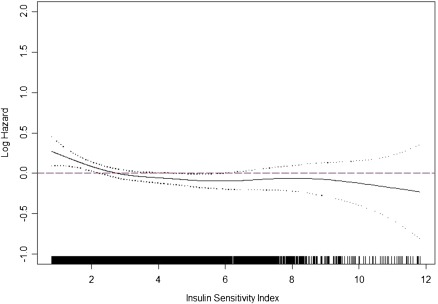
During the 14.7-year median follow-up, 1,810 participants died (58% of the study population), including 634 from adjudicated cardiovascular causes (35% of deaths). Lower ISI was associated with higher unadjusted mortality rates (Table 2 and Supplementary Fig. 2). Adjusting for age, sex, race, and study site, there was a monotonic relationship of ISI with mortality risk below the 75th percentile of ISI (4.91 units) (Table 2 and Fig. 1). The magnitude of this association was modestly attenuated adjusting for potential confounders (Table 2, model 2) or potential confounders and/or mediators (Table 2, model 3). Adjusted models did not differ using BMI in place of waist circumference. However, addition of eGFR to either model substantially attenuated associations of ISI with mortality (Table 2, models 4 and 5). Similar relationships were observed for fasting insulin, with more dramatic attenuation by eGFR. Associations of ISI with cardiovascular mortality displayed similar patterns as with all-cause mortality but were less robust, possibly as a result of smaller numbers of events or the competing risk of noncardiovascular mortality, while no strong, consistent, or statistically significant associations of ISI with noncardiovascular mortality were observed (Supplementary Table 1).
Table 2.
Associations of ISI, fasting insulin concentration, glucose concentration 2 h after glucose load, and fasting glucose concentration with all-cause mortality among 3,138 CHS participants
Figure 1.
Association of ISI with all-cause mortality as modeled by cubic spline, adjusted for age, sex, race, and study site, among 3,138 participants in the CHS. Ticks on the x-axis represent the distribution of ISI observations. (A high-quality color representation of this figure is available in the online issue.)
eGFR did not modify associations of ISI or fasting insulin concentration with mortality (interaction P values 0.304 and 0.835, respectively). Adjustment for ISI or fasting insulin concentration did not attenuate associations of lower eGFR with increased mortality risk (data not shown).
Significant associations of impaired glucose tolerance with mortality were not substantially attenuated by adjustment for eGFR (Table 2). Impaired fasting glucose was not associated with increased mortality risk in unadjusted or adjusted analyses (Table 2).
CONCLUSIONS
Insulin resistance was associated with increased risk of all-cause mortality among community-based older adults, adjusting for conventional confounding clinical characteristics. Associations were of similar magnitude, evaluating insulin resistance as ISI derived from dynamic testing or as fasting insulin concentration. However, all associations were substantially attenuated and rendered statistically insignificant with additional adjustment for eGFR, an effect that was most pronounced evaluating fasting insulin concentration as the exposure. In contrast, associations of impaired glucose tolerance with mortality were not attenuated by adjustment for eGFR.
Without yet considering the role of kidney function, this study adds two new facets to the literature evaluating the health impact of insulin resistance. First, we evaluated all-cause mortality as our primary study outcome. Others have focused on cardiovascular disease events and cardiovascular disease mortality (1–10). In our study, results for all-cause mortality were stronger than results for cardiovascular disease mortality, possibly because the larger number of events led to increased statistical precision or because all-cause mortality accounted for the competing risk of noncardiovascular death. These results emphasize the potential effects of insulin resistance on overall health.
Second, we focused on insulin resistance evaluated as ISI during dynamic testing. Because postprandial glucose is largely disposed of in peripheral tissues, such as muscle, ISI may capture peripheral insulin resistance more completely than estimates of insulin resistance based on fasting insulin concentration, which in comparison more strongly reflect hepatic insulin resistance. It is interesting that while ISI and fasting insulin concentration reflect different aspects of insulin resistance, they were similarly associated with mortality in conventionally adjusted analyses. Similar results were observed in the Framingham Offspring Study, in which 1) ISI was calculated using a different formula that additionally incorporates body weight and 2) HOMA-IR scores were each associated with increased risk of incident cardiovascular disease (9). The current study is the first to our knowledge assessing ISI as a risk factor for mortality, and it broadens the measures of insulin resistance evaluated in relation to health outcomes.
The most novel finding in this study is the marked attenuation of the insulin resistance–mortality association by adjustment for kidney function. Specifically, even after adjustment for conventional confounders and mediators of the insulin resistance–mortality relationship, further adjustment for cystatin C–based eGFR markedly attenuated observed magnitudes of association, and the degree of attenuation by eGFR exceeded that for any other covariate.
It is possible that impaired kidney function mediates, in part, the relationship of insulin resistance with mortality. Systemic insulin resistance may directly contribute to kidney damage by creating an unfavorable renal microvascular environment, and the hyperinsulinemia that compensates for systemic insulin resistance may promote the intrarenal renin-angiotensin-aldosterone system, mesangial matrix production, and tubulointerstitial fibrosis (15,17,34). In turn, impaired kidney function, even within the normal range, may promote cardiovascular disease and mortality by leading to oxidative stress, anemia, disturbed mineral metabolism, and other unique metabolic abnormalities (35).
Alternatively, impaired kidney function may represent a sensitive marker of the adverse biologic pathways activated by insulin resistance without lying in the direct causal pathway. Adjustment for eGFR attenuated the association of insulin resistance with mortality even after adjustment for blood pressure, dyslipidemia, and inflammation, suggesting a role for other biologic pathways. Diseased endothelial and epithelial kidney cells demonstrate resistance to insulin actions on cell proliferation and differentiation (16,18). Resistance to nonglycemic actions of insulin at the level of the kidney may represent parallel processes occurring systemically.
Finally, impaired kidney function may confound the relationship of insulin resistance with mortality. Impaired kidney function independently promotes insulin resistance, at least at very low levels of GFR (14). Potential mechanisms include retained uremic toxins, acidosis, and active vitamin D deficiency. In addition, the kidney clears ∼50% of peripheral insulin, such that higher insulin levels in the setting of lower GFR may reflect impaired kidney function instead of or in addition to insulin resistance (36). If impaired kidney function contributes substantially to both insulin resistance (true or apparent) and mortality, the independent contribution of insulin resistance to adverse outcomes may be overestimated in studies that do not accurately account for kidney function.
In contrast to ISI and fasting insulin concentration, associations of impaired glucose concentration with mortality risk were not attenuated by adjustment for eGFR. This suggests that the adverse effects of glucose intolerance are mediated through nonrenal mechanisms or that the relationship of glucose intolerance with adverse health outcomes is less sensitive to confounding by impaired kidney function.
Strengths of this study include the broad, community-based population of older adults, the large numbers of participants and events, assessment of insulin resistance in both the fasting and dynamic states, evaluation of a hard outcome with complete follow-up, and availability of detailed covariate data, including a relatively sensitive marker of impaired kidney function. Study limitations include the lack of direct measurements of insulin resistance and kidney function, lack of urine albumin measurements to ascertain whether accounting for kidney dysfunction more broadly might even further attenuate associations of insulin resistance with mortality, exclusion of a large number of African American participants because of missing OGTT data as a result of the CHS design, lack of detailed data on socioeconomic status, and inability of epidemiologic analysis to specifically delineate causal pathways to differentiate confounding and mediation.
In conclusion, the association of insulin resistance with mortality among community-based older adults appeared to be largely mediated or confounded by impaired kidney function. Future studies assessing associations of insulin resistance with health outcomes should take kidney function into account. Additional studies are needed to define the joint roles of insulin resistance and impaired kidney function in disease.
Acknowledgments
The research reported in this article was supported by Contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, and N01-HC-45133 and Grant U01-HL-080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support came from National Institutes of Health Grants R01-AG-027002 and R01-DK-087726. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
No potential conflicts of interest relevant to this article were reported.
I.H.d.B. researched data and wrote the manuscript. R.K. researched data, contributed to discussion, and reviewed and edited the manuscript. M.B.C., L.F.F., J.H.I., B.K., K.J.M., C.A.P., and D.S.S. contributed to discussion and reviewed and edited the manuscript. I.H.d.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1657/-/DC1.
References
- 1.Zethelius B, Lithell H, Hales CN, Berne C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 2005;48:862–867 [DOI] [PubMed] [Google Scholar]
- 2.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 2001;86:3574–3578 [DOI] [PubMed] [Google Scholar]
- 3.Pyöräla K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care 1979;2:131–141 [DOI] [PubMed] [Google Scholar]
- 4.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334:952–957 [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002;25:1177–1184 [DOI] [PubMed] [Google Scholar]
- 6.Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med 2002;19:470–475 [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol 2007;49:2112–2119 [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck Study. Diabetes Care 2007;30:318–324 [DOI] [PubMed] [Google Scholar]
- 9.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005;54:3252–3257 [DOI] [PubMed] [Google Scholar]
- 10.Welborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care 1979;2:154–160 [DOI] [PubMed] [Google Scholar]
- 11.Parekh N, Lin Y, Hayes RB, Albu JB, Lu-Yao GL. Longitudinal associations of blood markers of insulin and glucose metabolism and cancer mortality in the third National Health and Nutrition Examination Survey. Cancer Causes Control 2010;21:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Muntner P, Hamm LL, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 2003;14:469–477 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest 1981;67:563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrass CK, Spicer D, Raugi GJ. Insulin induces a change in extracellular matrix glycoproteins synthesized by rat mesangial cells in culture. Kidney Int 1994;46:613–620 [DOI] [PubMed] [Google Scholar]
- 16.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 2005;54:3095–3102 [DOI] [PubMed] [Google Scholar]
- 17.Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 2007;56:476–485 [DOI] [PubMed] [Google Scholar]
- 18.Mima A, Ohshiro Y, Kitada M, et al. Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int 2011;79:883–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 2007;167:2490–2496 [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 2006;145:237–246 [DOI] [PubMed] [Google Scholar]
- 21.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 1995;47:312–318 [DOI] [PubMed] [Google Scholar]
- 22.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis 2000;36:29–34 [DOI] [PubMed] [Google Scholar]
- 23.O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem 2003;40:648–655 [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care 2010;33:e93. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 28.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–285 [DOI] [PubMed] [Google Scholar]
- 29.Kuller LH, Velentgas P, Barzilay J, Beauchamp NJ, O’Leary DH, Savage PJ. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol 2000;20:823–829 [DOI] [PubMed] [Google Scholar]
- 30.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 2009;169:2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 33.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 1997;43:52–58 [PubMed] [Google Scholar]
- 34.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol 2006;26:232–244 [DOI] [PubMed] [Google Scholar]
- 35.Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis 2003;41(Suppl.):11–17 [DOI] [PubMed] [Google Scholar]
- 36.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 1998;19:608–624 [DOI] [PubMed] [Google Scholar]