Abstract
OBJECTIVE
To assess efficacy and safety of lixisenatide monotherapy in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Randomized, double-blind, 12-week study of 361 patients not on glucose-lowering therapy (HbA1c 7–10%) allocated to one of four once-daily subcutaneous dose increase regimens: lixisenatide 2-step (10 μg for 1 week, 15 μg for 1 week, and then 20 μg; n = 120), lixisenatide 1-step (10 μg for 2 weeks and then 20 μg; n = 119), placebo 2-step (n = 61), or placebo 1-step (n = 61) (placebo groups were combined for analyses). Primary end point was HbA1c change from baseline to week 12.
RESULTS
Once-daily lixisenatide significantly improved HbA1c (mean baseline 8.0%) in both groups (least squares mean change vs. placebo: −0.54% for 2-step, −0.66% for 1-step; P < 0.0001). Significantly more lixisenatide patients achieved HbA1c <7.0% (52.2% 2-step, 46.5% 1-step) and ≤6.5% (31.9% 2-step, 25.4% 1-step) versus placebo (26.8% and 12.5%, respectively; P < 0.01). Lixisenatide led to marked significant improvements of 2-h postprandial glucose levels and blood glucose excursions measured during a standardized breakfast test. A significant decrease in fasting plasma glucose was observed in both lixisenatide groups versus placebo. Mean decreases in body weight (∼2 kg) were observed in all groups. The most common adverse events were gastrointestinal—nausea was the most frequent (lixisenatide 23% overall, placebo 4.1%). Symptomatic hypoglycemia occurred in 1.7% of lixisenatide and 1.6% of placebo patients, with no severe episodes. Safety/tolerability was similar for the two dose regimens.
CONCLUSIONS
Once-daily lixisenatide monotherapy significantly improved glycemic control with a pronounced postprandial effect (75% reduction in glucose excursion) and was safe and well tolerated in type 2 diabetes.
In recent years, the use of glucagon-like peptide 1 (GLP-1) receptor agonists has become established as an important therapeutic option in the management of patients with type 2 diabetes (1,2). As a class, GLP-1 receptor agonists possess a number of favorable clinical characteristics in addition to their glucose-lowering effects, including a low propensity to cause hypoglycemia (as a result of their glucose-dependent action) and promotion of weight loss (3,4). Preclinical studies using in vitro and animal models also suggest that GLP-1 receptor agonists have the potential to preserve pancreatic islet β-cells, which may help to provide more stable metabolic control long term (5). As of today, three representatives of the GLP-1 receptor agonist class have been marketed: exenatide (twice-daily and once-weekly formulations) and liraglutide once daily.
Lixisenatide (AVE0010) is a new selective once-daily GLP-1 receptor agonist in development for the treatment of type 2 diabetes mellitus (6–8). It is a 44-amino acid peptide that is amidated at the C-terminal end and shares some structural elements with exendin-4 (the main difference being the addition of 6 lysine residues at the C terminus) (9). Lixisenatide is highly selective for the GLP-1 receptor and exerts about fourfold higher affinity for the GLP-1 receptor than native human GLP-1 (9). The preclinical pharmacological profile of lixisenatide suggests that stimulation of insulin secretion by lixisenatide is strictly glucose dependent. In animal models, lixisenatide enhances insulin biosynthesis and stimulation of β-cell proliferation and delays gastric emptying and reduces food intake (9). In human islets, lixisenatide prevents lipotoxic islet insulin depletion and preserves insulin production, storage, and pancreatic β-cell function (9). Lixisenatide undergoes renal metabolism, but mild or moderate renal impairment does not appear to influence its pharmacokinetics or tolerability (10).
In a 13-week, randomized, double-blind, placebo-controlled, parallel-group, phase II dose-ranging study, lixisenatide administered at doses of 5, 10, 20, or 30 μg once daily or twice daily in patients with type 2 diabetes inadequately controlled with metformin significantly improved HbA1c compared with placebo (8). Dose-dependent improvements were also observed for fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) associated with the morning meal and average self-monitored seven-point blood glucose levels (7). The dose of 20 μg administered once daily was found to provide the optimal balance of efficacy and tolerability (8).
Accordingly, in this Phase III study, we assessed the safety and efficacy of 20 μg lixisenatide once daily in a 12-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group monotherapy trial in patients with type 2 diabetes not currently receiving glucose-lowering therapy.
RESEARCH DESIGN AND METHODS
Study participants
The study population comprised male and female patients aged 20–85 years with type 2 diabetes mellitus not currently receiving glucose-lowering therapy and with glycated hemoglobin (HbA1c) ≥7.0% and ≤10.0%. The main exclusion criteria were as follows: treatment with a glucose-lowering pharmacological agent within the previous 3 months; FPG at screening >250 mg/dL (13.9 mmol/L); amylase and/or lipase >3 times the upper limit of the normal (ULN) laboratory range; clinically relevant history of gastrointestinal disease with prolonged nausea and vomiting during the previous 6 months, chronic pancreatitis, or stomach/gastric surgery; history of myocardial infarction, stroke, or heart failure requiring hospitalization within the previous 6 months; hepatic disease, end-stage renal disease, and/or dialysis at screening.
The study was approved by the institutional review boards or ethics committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave written informed consent to participate in the study.
Study design
This 12-week, multinational, randomized, parallel-group, placebo-controlled study was conducted at 61 centers in 12 countries (Belgium, India, Israel, Japan, South Korea, Mexico, Poland, Romania, Russian Federation, Tunisia, Ukraine, and United States) between 2008 and 2009. The study drug was double-blind regarding active treatment or placebo and open-label regarding the treatment volume and dose increase regimen.
After a 2-week screening phase, eligible patients entered into a 1-week, single-blind, placebo run-in period. Eligible patients were then randomized to receive one of four treatment regimens in a 1:1:1:1 ratio for 12 weeks: 1) lixisenatide 2-step dose increase (10 μg for 1 week, 15 μg for 1 week, and then 20 μg), 2) lixisenatide 1-step dose increase (10 μg for 2 weeks and then 20 μg), 3) placebo 2-step dose increase, and 4) placebo 1-step dose increase. This was followed by a 3-day, posttreatment follow-up period. All treatments were administered once daily within 1 h before breakfast. Routine fasting self-measured plasma glucose and central laboratory alerts on FPG were set up to ensure that glycemic parameters remained under predefined thresholds values (defined depending on study period as: FPG >270 mg/dL [15.0 mmol/L] from baseline visit [day 1] to visit 8, week 8; FPG >240 mg/dL [13.3 mmol/L] from visit 8, week 8 to visit 9, week 12). Rescue medication was introduced in subjects with FPG above those thresholds values, if no reasonable explanation existed for insufficient glucose control, or if appropriate action failed to decrease FPG. Metformin was recommended as first-line rescue medication.
Randomization of subjects, allocation of medication, and management of drug supplies was performed using an Interactive Voice Response System. Patients were stratified by screening values of HbA1c (<8%, ≥8%) and BMI (<30 kg/m2, ≥30 kg/m2). All patients received diet and lifestyle counseling according to the American Diabetes Association guidelines (11).
Study assessments
The primary efficacy end point was change in HbA1c from baseline to study end for the intent-to-treat population. All clinical laboratory assessments, including HbA1c, were measured at a National Glycohemoglobin Standardization Program Level 1 certified central laboratory (Covance), using a high performance liquid chromatography method.
The secondary efficacy measures included the percentage of patients achieving an HbA1c <7.0% or ≤6.5%, change in FPG, and change in body weight. The secondary efficacy measures of 2-h PPG and 2-h glucose excursion (defined as 2-h PPG minus plasma glucose 30 min before the breakfast meal test before study drug administration) were assessed for a subgroup of patients at selected sites during a standardized meal test. For logistic reasons, the 2-h PPG after standardized meal challenge test was only assessed in a subgroup of all the patients in selected sites (∼50% of the randomized patients). In a selected country, all sites and all patients should undergo the meal test. The countries for the meal test were selected according to the following criteria: the anticipated number of patients to be enrolled (based on feasibility); balanced ethnicity at the end, as far as possible; and easy implementation of the meal test substudy (i.e., fast approval, easy import license for the meal test drink (Ensure Plus).
The meal test consisted of a 600 kcal liquid meal (400 mL of Ensure Plus; Abbott Nutrition, Columbus, OH) composed of 53.8% carbohydrate, 16.7% protein, and 29.5% fat and was to be consumed within a 10-min period, 30 min after drug administration at week −1 and week 12. Blood was sampled at 3 time points: 1) 30 min before the meal test (before study drug administration), 2) just before consumption at the start of the meal test (0 min), and 3) 120 min after the start of the meal test. Antilixisenatide antibody levels were measured. Antilixisenatide antibody status and concentration were measured by Biacore. It is a commercial system that is validated and complies with the current regulatory standards, based on Surface Plasmon Resonance using Biacore technology.
Safety and tolerability were assessed by physical examination, blood pressure, heart rate, 12-lead electrocardiogram, standard laboratory measurements, antilixisenatide antibodies, and adverse events reporting (including, in particular, symptomatic and severe symptomatic hypoglycemia, local intolerability at injection site, allergic or allergic-like reactions, suspected pancreatitis, and major cardiovascular events). Symptomatic hypoglycemia was defined as symptoms consistent with hypoglycemia, with accompanying blood glucose <3.3 mmol/L (60 mg/dL) and/or prompt recovery with carbohydrate. Severe symptomatic hypoglycemia was defined as symptomatic hypoglycemia in which the patient required the assistance of another person and which was associated either with a plasma glucose level <36 mg/dL (2.0 mmol/L) or, if no plasma glucose measurement was available, with prompt recovery with carbohydrate.
The safety population comprised all randomized patients exposed to at least one dose of investigational drug.
Statistical analyses
The two placebo groups were combined in all analyses. Sample sizes of 120 patients in each lixisenatide treatment group and the combined placebo group were calculated to provide a statistical power of 90% to detect a 0.5% difference in HbA1c between active treatment and placebo assuming a standard deviation of 1.2%. Statistical significance was assumed at the 5% level. All statistical computations were performed using SAS, version 8.2 or higher.
Analyses of the primary efficacy variable (changes in HbA1c from baseline to end point) were performed using an ANCOVA model, with treatment group, screening strata for HbA1c and BMI, and country as fixed factors and baseline HbA1c as a covariate. The same method was used for analysis of secondary efficacy parameters. The Last Observation Carry Forward procedure was used to handle missing assessments, early discontinuation, or introduction of rescue therapy during the double-blind treatment period. Least squares (LS) means were calculated from the model to estimate treatment effect size. Comparisons between each lixisenatide group and placebo were based on treatment differences using the LS means. A step-down testing procedure was applied. The lixisenatide 2-step dose increase arm was first compared with the combined placebo group (primary objective); if the test was statistically significant, then the lixisenatide 1-step dose increase arm was compared with the combined placebo group (secondary objective). The percentage of patients achieving HbA1c <7.0% or ≤6.5% and the percentage of patients requiring rescue therapy were analyzed using a Cochran-Mantel-Haenszel test stratified according to screening HbA1c and screening BMI.
Unless otherwise indicated, all efficacy data were analyzed in the intent-to-treat population, comprising all randomized patients who received at least one dose of double-blind investigational drug (lixisenatide or placebo) and had both a baseline assessment and at least one postbaseline assessment of any primary or secondary efficacy variable. Only two patients were excluded from the efficacy analyses because of lack of postbaseline efficacy data. Results are presented as mean ± SEM, unless otherwise specified.
RESULTS
Demographic and baseline characteristics
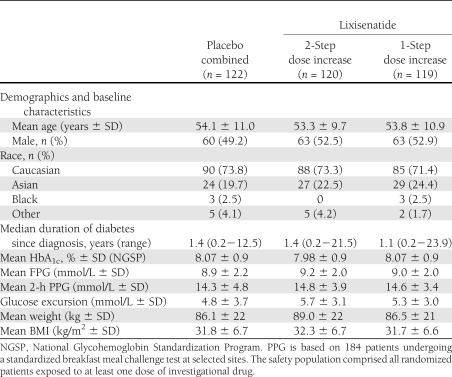
A total of 361 patients were randomized and treated from 795 patients screened. The main reason for screening failure was an HbA1c value out of the defined protocol range at the screening visit. The demographic and baseline characteristics of the combined placebo group and the two lixisenatide treatment groups were well matched, and there were no clinically relevant differences between groups (see Table 1 and Supplementary Fig. 1).
Table 1.
Patient disposition, demographics, and baseline characteristics (safety population)
The majority of patients (n = 331 [91.7%]) completed the 12-week double-blind treatment period. Overall, 30 patients discontinued treatment prematurely, including 10 (8.3%) in the lixisenatide 2-step dose increase arm, 11 (9.2%) in the lixisenatide 1-step dose increase arm, and 9 (7.4%) in the combined placebo group. A total of eight lixisenatide-treated patients (3.3%) discontinued as a result of an adverse event and none as a result of lack of efficacy (see Supplementary Fig. 1). Out of the 361 patients in the safety population, 335 (92.8%) were exposed to 57 days or more of study drug (mean 81.3 days for placebo, 81.2 days for lixisenatide 2-step, and 81.8 days for lixisenatide 1-step), 349 (96.7%) reached the target dose of 20 μg at the end of dose increase period (98.4% placebo, 95.0% lixisenatide 2-step, 96.6% lixisenatide 1-step), and 335 (92.8%) were receiving 20 μg at the end of double-blind treatment (99.2% placebo, 90.8% lixisenatide 2-step, 88.2% lixisenatide 1-step). Only six patients required rescue medication during the study (3 [2.5%] in the placebo group, 2 [1.7%] in the lixisenatide 2-step group, and 1 [0.8%] in the lixisenatide 1-step group).
Efficacy.
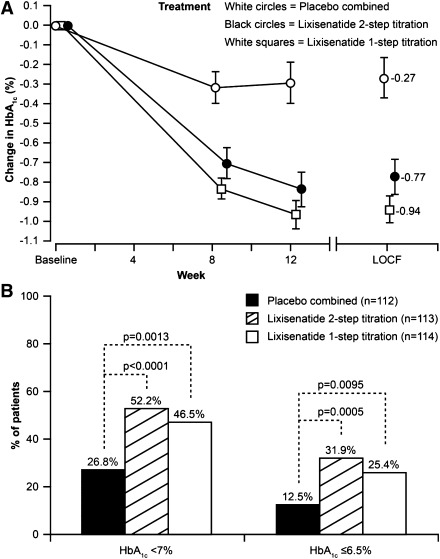
Lixisenatide once daily significantly decreased HbA1c from baseline to week 12 (Fig. 1A). The LS mean changes at end point in HbA1c were −0.19, −0.73, and −0.85% from a baseline of 8.07, 7.97, and 8.06% for the combined placebo, 2-step, and 1-step dose increase groups, respectively. LS mean differences versus placebo were −0.54% for the 2-step and −0.66% for the 1-step dose increase arm (both P values <0.0001). Out of the lixisenatide-treated patients, 56% in the 2-step dose increase group and 60% in the 1-step dose increase group developed antilixisenatide antibodies after 12 weeks of lixisenatide treatment. HbA1c reduction was similar in antilixisenatide antibody-positive and -negative patients.
Figure 1.
Changes in glycated hemoglobin (HbA1c) levels after 12 weeks’ treatment with lixisenatide (according to dose increase regimen) or placebo. A: Mean change (±SEM) in HbA1c over time. B: Percentage of patients achieving HbA1c goals <7.0% and ≤6.5%. LOCF, Last Observation Carry Forward.
The goal of HbA1c <7.0% was achieved by significantly more patients in both the lixisenatide 2-step (52%) and 1-step (47%) groups compared with placebo (27%; both P values <0.01) (Fig. 1B). A significantly greater response rate was also achieved for the goal of HbA1c ≤6.5% (32 and 25 vs. 13%; both P values <0.01) (Fig. 1B).
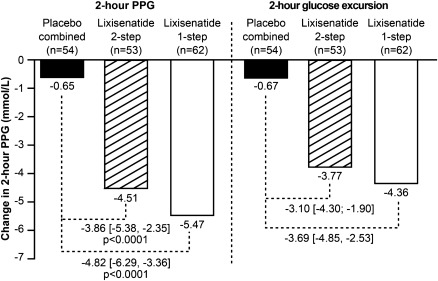
In the 169 patients who participated in the standardized meal test and had both baseline and end point evaluation, the treatment with lixisenatide significantly improved postprandial glycemic control as shown by the results of the 2-h postprandial plasma glucose assessment (both P values <0.0001) (Fig. 2). Treatment with lixisenatide significantly decreased the 2-h glucose excursion from baseline to week 12 compared with the combined placebo group (LS mean difference vs. placebo of −3.1 mmol/L; 95% CI [−4.30 to −1.90] for the lixisenatide 2-step and −3.7 mmol/L; 95% CI [−4.85 to −2.53] for the lixisenatide 1-step dose increase arm) (Fig. 2).
Figure 2.
Changes in postbreakfast glucose parameters from baseline after 12-weeks' treatment with lixisenatide (according to dose increase regimen) or placebo. LS mean change in mean (±SEM) 2-h PPG levels is shown. LS mean change in 2-h glucose excursion is also shown. Data are from patients undergoing a standardized breakfast meal test at selected sites. Glucose excursion = 2-h PPG, plasma glucose 30 min before the meal test before study drug administration. Mean ± SD baseline values for 2-h PPG: 13.99 ± 4.78 mmol/L (placebo), 14.67 ± 3.78 mmol/L (lixisenatide 2-step), 14.55 ± 3.36 mmol/L (lixisenatide 1-step). Mean ± SD baseline values for glucose excursion: 4.72 ± 3.65 mmol/L (placebo), 5.45 ± 3.02 mmol/L (lixisenatide 2-step), 5.25 ± 2.89 mmol/L (lixisenatide 1-step). To convert mmol/L to mg/dL, divide by 0.0555.
Treatment with lixisenatide also decreased FPG compared with the combined placebo group: LS mean difference versus placebo: −0.9 mmol/L for the 2-step (P value <0.001) and −1.1 mmol/L for the lixisenatide 1-step dose increase arm (P value <0.0001) (see Supplementary Fig. 2).
Body weight decreased by ∼2 kg in all groups, with no significant differences between lixisenatide and placebo.
Safety and tolerability
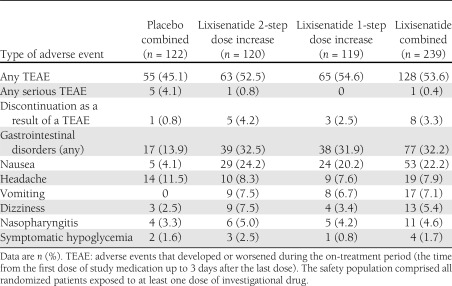
Approximately 50% of patients experienced a treatment-emergent adverse event (TEAE), with a slightly higher rate in the lixisenatide groups than the placebo group (Table 2). There was only one serious TEAE among lixisenatide-treated patients (a case of goitre in the 2-step group) compared with five serious events in the placebo group.
Table 2.
Number (%) of patients with TEAEs occurring in ≥5% (preferred term) in any one group and symptomatic hypoglycemia in the safety population
The most common TEAEs were gastrointestinal in nature, with nausea being the most frequent (Table 2). Nausea was reported more frequently in patients treated with lixisenatide compared with patients treated with placebo (24.2, 20.2, and 4.1% of patients in the lixisenatide 2-step and 1-step dose increase and combined placebo group, respectively), more frequently during the first 3 weeks of treatment with a reduced occurrence from week 7 and up to the end of treatment. Most of the events were mild to moderate in intensity and resolved without the need to administer corrective treatment.
Vomiting was also reported more frequently in the lixisenatide-treated group (Table 2). Most of the events occurred during the first 3 weeks of treatment (with a reduced occurrence from week 6 and up to the end of treatment), were mild to moderate in intensity, and resolved without the need to administer corrective treatment.
One placebo-treated patient and eight lixisenatide-treated patients (5 [4.2%] 2-step, 3 [2.5%] 1-step) discontinued treatment because of a TEAE. Gastrointestinal disorders were at least partly responsible for the discontinuations in all eight lixisenatide-treated patients (additional reasons were one case of decreased appetite and one case of hypertension).
A total of six cases (3 [2.5%] lixisenatide 2-step dose increase, 1 [0.8%] lixisenatide 1-step dose increase, and 2 [1.6%] placebo patients) of symptomatic hypoglycemia fulfilling the protocol definition were observed, and none of them was severe or led to treatment discontinuation.
There were no reports of suspected pancreatitis during the study, and no instances of lipase or amylase elevation ≥3×ULN.
Injection site reactions were reported by 11 (4.6%) patients, all in the lixisenatide-treated group. None of the events was serious or severe in intensity or led to treatment discontinuation.
Two patients in the lixisenatide 1-step dose increase arm had allergic events considered as possibly related to therapy (urticaria in one case and angioedema in the other case). None of the events was serious. The patient with urticaria recovered with symptomatic treatment and was able to complete the study without recurrence of the event. For the patient with angioedema, the study treatment was discontinued because of vomiting and abdominal pain. No significant differences in adverse events were observed between the antibody-positive and antibody-negative patient population.
CONCLUSIONS
In the current study involving patients with type 2 diabetes not receiving glucose-lowering therapy, lixisenatide monotherapy administered once daily for 12 weeks and titrated using either 1 step or 2 steps up to the maintenance dose of 20 μg once daily provided significant improvements in HbA1c compared with placebo and allowed more patients to achieve HbA1c goals. The magnitude of improvement in HbA1c (placebo-subtracted change of −0.54 and −0.66% from a baseline of 8.0% ± 0.9 and 8.1% ± 0.9 for the 2-step and 1-step dose increase groups, respectively) is consistent with that seen with exenatide twice daily when administered as monotherapy in treatment-naïve patients (placebo-subtracted change of −0.5% with 5 μg and −0.7% with 10 μg twice-daily exenatide from a baseline of 7.8%) (12). No other placebo-controlled studies are available for GLP-1 receptor agonist monotherapy in a similar patient population. The only available study with liraglutide monotherapy used the active comparator glimepiride, and many patients had been treated previously with oral agent monotherapy (13).
In the current study, there was a pronounced effect of lixisenatide on PPG associated with the morning meal, with statistically significant and clinically meaningful improvement of both 2-h PPG (−4.5, −5.5, and −0.7 mmol/L for the 2-step and 1-step dose increase and placebo groups, respectively) and blood glucose excursions (−3.8, −4.4, and −0.7 mmol/L for the 2-step and 1-step dose increase and placebo groups, respectively) measured during a standardized meal test. This represents a 75% reduction in the postprandial glucose excursion. These results are consistent with those of previous studies of lixisenatide (6–8) and greater than those observed for monotherapy with liraglutide 1.2 mg and 1.8 mg (1.7 and 2.1 mmol/L decreases in PPG averaged across all meals, respectively), although breakfast PPG was not reported specifically in that study (13). The importance of targeting PPG is now recognized by international organizations and the International Diabetes Federation recommends that patients with diabetes manage their HbA1c levels to <6.5% by addressing both FPG and PPG, with 2-h PPG level targets of <7.8 mmol/L (140 mg/dL) (14).
Lixisenatide monotherapy also provided improvements in FPG (−0.9 mmol/L for the 2-step and −1.1 mmol/L for the lixisenatide 1-step dose increase arms [both P values <0.001]). This is comparable with changes observed with liraglutide monotherapy (1.2 mg = −0.8 and 1.8 mg = −1.4 mmol/L) (13). In this study, lixisenatide provided a mean weight loss of ∼2 kg, which is comparable with what was reported after 12 weeks of treatment with other GLP-1 receptor agonists when administered as monotherapy (12,13).
The glucose-lowering efficacy of lixisenatide can be considered independently of any potential impact because of weight loss since, in the current study, the weight loss observed was not different to that seen with placebo. The patients included in the study had a short duration of diabetes and were not receiving any glucose-lowering therapy and were therefore probably the most sensitive and compliant to dietary interventions, which can explain the weight loss observed with the placebo in this 12-week study. This weight change in the placebo group is compatible with that seen in other studies where lifestyle changes occur in the placebo group, but are usually short lived (15,16).
We found that lixisenatide was well tolerated, with no difference between the 2-step and 1-step dose increase regimens. As expected, the most frequent adverse events were gastrointestinal in nature: mainly nausea, with low rates of vomiting. Most of these events were transient, mild to moderate in intensity, and resolved spontaneously. Only very few led to treatment discontinuation. The nausea frequency (22% for the combined lixisenatide groups) is consistent with the frequency observed in the lixisenatide dose–ranging study (25.5% with 20 μg once-daily dose) and in studies conducted with other GLP-1 receptor agonists when used in monotherapy (13–29%) (12,13). Rates of symptomatic hypoglycemia associated with lixisenatide in the current study were low (with no severe events) and similar to placebo. Although this study is only of 12 weeks’ duration, additional Phase III studies have had a main 24-week treatment period and the majority has also included long-term extension periods of at least another year to assess long-term safety and tolerability. Antibody concentration was very low in the majority of patients, and available data suggest that HbA1c reduction and overall safety profile are similar in antilixisenatide antibody-positive and -negative patients (8). The potential influence of antibodies in the longer term will be addressed in publications of longer-term trials from the GetGoal program.
The current study shows that lixisenatide once daily is effective and well tolerated when used as monotherapy in treatment-naïve patients. The impact of lixisenatide on hyperglycemia around the morning meal may also be advantageous, since this component of glycemia appears to be relatively resistant to oral therapies (17). Recent data in metformin-treated patients also suggest that lixisenatide once daily is noninferior to exenatide twice daily in terms of HbA1c reduction, but has the advantages of less hypoglycemia and fewer gastrointestinal adverse events (18).
In conclusion, the results from this first completed study in the Phase III clinical trials program for lixisenatide have demonstrated that lixisenatide monotherapy administered once daily provides significant improvements in glycemic control with a pronounced postprandial effect. In addition, lixisenatide was safe and well tolerated, and there was no relevant difference in the safety and tolerability of the 1-step and 2-step dose increase regimens. The results support a role for once-daily lixisenatide monotherapy using a 1-step dose increase regimen in patients not controlled on lifestyle interventions and highlight the potential of lixisenatide for further development as a glucose-lowering compound to treat patients with type 2 diabetes.
Acknowledgments
The study was funded by sanofi-aventis, the manufacturer of lixisenatide. The investigators and representatives from sanofi-aventis were responsible for the study design, protocol, statistical analysis plans, analysis, and reporting of the results. Final responsibility for the decision to submit the manuscript for publication was made jointly by all authors.
V.A.F. received research support from Novo Nordisk, sanofi-aventis, Eli Lilly, Daiichi-Sankyo, Pamlabs, Reata, and Halozyme and honoraria from GlaxoSmithKline, Takeda, Novo Nordisk, sanofi-aventis, Eli Lilly, Daiichi-Sankyo, Pamlabs, Xoma, and AstraZeneca for consulting and lectures. R.A.-R. received grant/research support from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, sanofi-aventis, Public Health Research Institute, and Roche and honoraria from Pfizer, sanofi-aventis, AstraZeneca, and Merck Sharp & Dohme for consulting and advisory board attendance. G.B. and P.M. are employees of sanofi-aventis. J.E.G. has sat on advisory boards/provided consultancy for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, MannKind Corporation, Merck, and sanofi-aventis and is on the Speakers’ Bureau for Bristol-Myers Squibb/AstraZeneca, Boehringer Ingelheim, Merck, and sanofi-aventis and on the Data and Safety Monitoring Board for sanofi-aventis.
No other potential conflicts of interest relevant to this article were reported.
V.A.F. and J.E.G. researched data, reviewed and edited the manuscript, and contributed to the discussion of the data. R.A.-R. and D.R. reviewed and edited the manuscript and contributed to the discussion of the data. G.B. researched data, wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion of the data. P.M. researched data. V.A.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
The authors thank all of the investigators, coordinators, and patients who took part in this study. Editorial assistance was provided by Susan Crawford, Absolute Healthcare Communication and funded by sanofi-aventis.
Footnotes
Clinical trial reg. no. NCT00688701, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1935/-/DC1.
References
- 1.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed]
- 3.Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)—preclinical and clinical results. Best Pract Res Clin Endocrinol Metab 2009;23:463–477 [DOI] [PubMed] [Google Scholar]
- 4.Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract 2010;64:1402–1414 [DOI] [PubMed] [Google Scholar]
- 5.Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab 2008;34(Suppl. 2):S73–S77 [DOI] [PubMed] [Google Scholar]
- 6.Distiller LA, Ruus P, ACT6011 Study Group Pharmacokinetics and pharmacodynamics of a new GLP-1 agonist AVE0010 in type 2 diabetes patients (Abstract). Diabetes 2008;57(Suppl. 1):A154–A155 [Google Scholar]
- 7.Ratner RE, Rosenstock J, Boka G, Silvestre L. Post-meal pharmacodynamic profile of AVE0010, a once-daily GLP-1 receptor agonist, in patients with type 2 diabetes inadequately controlled on metformin (Abstract). Diabetologia 2009;52(Suppl. 1):S60 [Google Scholar]
- 8.Ratner RE, Rosenstock J, Boka G, DRI6012 Study Investigators Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010;164:58–64 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y-H, Ruus P. Pharmacokinetics and safety of the GLP-1 agonist AVE0010 in patients with renal impairment (Abstract). Diabetes 2009;59(Suppl. 1):A149–A150 [Google Scholar]
- 11.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 12.Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008;30:1448–1460 [DOI] [PubMed] [Google Scholar]
- 13.Garber A, Henry R, Ratner R, et al. LEAD-3 (Mono) Study Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabet Med 2008;25:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH. Lau J. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;1:CD004096 [DOI] [PMC free article] [PubMed]
- 16.Norris SL, Zhang X, Avenell A, et al. Long-term non-pharmacological weight loss interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2:CD004095 [DOI] [PMC free article] [PubMed]
- 17.Monnier L, Colette C, Rabasa-Lhoret R, et al. Morning hyperglycemic excursions: a constant failure in the metabolic control of non-insulin-using patients with type 2 diabetes. Diabetes Care 2002;25:737–741 [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once-daily vs. exenatide twice-daily in type 2 DM inadequately controlled on metformin (GetGoal-X) (Abstract). Diabetes 2011;60(Suppl. 1A):LB10 [Google Scholar]