Abstract
Metabolite associations with insulin resistance were studied in 7,098 young Finns (age 31 ± 3 years; 52% women) to elucidate underlying metabolic pathways. Insulin resistance was assessed by the homeostasis model (HOMA-IR) and circulating metabolites quantified by high-throughput nuclear magnetic resonance spectroscopy in two population-based cohorts. Associations were analyzed using regression models adjusted for age, waist, and standard lipids. Branched-chain and aromatic amino acids, gluconeogenesis intermediates, ketone bodies, and fatty acid composition and saturation were associated with HOMA-IR (P < 0.0005 for 20 metabolite measures). Leu, Ile, Val, and Tyr displayed sex- and obesity-dependent interactions, with associations being significant for women only if they were abdominally obese. Origins of fasting metabolite levels were studied with dietary and physical activity data. Here, protein energy intake was associated with Val, Phe, Tyr, and Gln but not insulin resistance index. We further tested if 12 genetic variants regulating the metabolites also contributed to insulin resistance. The genetic determinants of metabolite levels were not associated with HOMA-IR, with the exception of a variant in GCKR associated with 12 metabolites, including amino acids (P < 0.0005). Nonetheless, metabolic signatures extending beyond obesity and lipid abnormalities reflected the degree of insulin resistance evidenced in young, normoglycemic adults with sex-specific fingerprints.
Development of type 2 diabetes is commonly preceded by insulin resistance manifested by increased insulin release to maintain glucose homeostasis (1). Already in young adulthood, impaired insulin sensitivity is associated with increased risk for diabetes (2). In addition, insulin resistance in young adults is often accompanied by a dyslipidemic profile (3,4). Prevailing theories for the pathogenesis of insulin resistance focus on lipid-mediated mechanisms (5); however, the etiology also involves, for example, obesity-induced metabolic by-products and inflammatory signaling (6). The metabolic abnormalities related to insulin resistance are to some extent reflected in the circulating levels of metabolites, and detailed metabolic profiling is therefore increasingly used to gain insight into the complex pathophysiology of diabetes (7).
Metabolic profiling studies conducted in middle-aged and older individuals have demonstrated metabolic signatures of insulin action in obese individuals (8,9). Branched-chain (Leu, Ile, and Val) and aromatic (Phe and Tyr) amino acids were recently associated with the risk for future diabetes in the Framingham Heart Study (10). The underlying mechanisms remain to be established, in particular whether the amino acids are contributing to the disease development in a causal manner. In the same study population, comprehensive lipid profiling demonstrated that fatty acids of shorter carbon chain length and lower double bond content were associated with insulin resistance index and an increased risk of diabetes (11). These results indicate that metabolic profiling can inform about the etiology of insulin resistance and the risk for development of diabetes.
Lifestyle and genetic variation affect insulin sensitivity as well as contribute to determining metabolite concentrations during fasting. Environmental determinants of metabolite levels are increasingly being investigated (12,13), and the genetic architecture of metabolites is gradually being uncovered (14,15); however, the influence of metabolites on insulin resistance remains incompletely understood. To identify putative metabolic pathways in the pathophysiology of insulin resistance, we applied nuclear magnetic resonance (NMR) spectroscopy to quantify circulating metabolites in population-based cohorts of young adults. The objectives were 1) to investigate metabolites associated with insulin resistance index independent of established risk factors, 2) to assess lifestyle contributions to fasting metabolite levels from diet and physical activity, and 3) to elucidate the relationship between metabolites and insulin resistance using novel genetic variants affecting the metabolite levels.
RESEARCH DESIGN AND METHODS
Study population.
The study comprised two population-based Finnish cohorts, the Northern Finland Birth Cohort 1966 (NFBC) (16) and the Cardiovascular Risk in Young Finns Study (YFS) (17). Out of 7,718 individuals with metabolite data, 170 individuals missing glucose or insulin measurements or with concentrations outside the boundaries of the homeostasis model assessment of insulin resistance (HOMA-IR) index were excluded. In addition, individuals diagnosed with type 1 or type 2 diabetes (n = 56), those taking lipid medication (n = 7) or antihypertensive medication (n = 150), and pregnant women (n = 237) were removed, leaving 7,098 individuals for analyses. Participants gave written informed consent, and the study protocols were approved by the local ethics committees.
The NFBC was initiated to study factors affecting preterm birth and subsequent morbidity (http://kelo.oulu.fi/NFBC/). Data collection included clinical examination and 12-h fasting blood sampling at age 31 for 6,007 individuals, of which 5,471 had a fasting metabolite profile measured. Data from this time point were used for the current study. Attendees in the 31-year field study were representative of the original birth cohort. Plasma glucose concentrations were measured by glucose dehydrogenase (Granutest 250; Diagnostica Merck), and insulin concentrations were measured by radioimmunoassay (Pharmacia Diagnostics). Physical activity was assessed for 4,538 individuals by the metabolic equivalent of task (MET) index based on questionnaire data on frequency, intensity, and duration of physical activity as described previously (18).
The YFS was designed to study associations of childhood risk factors to cardiovascular disease in adulthood (http://med.utu.fi/cardio/youngfinnsstudy/). The baseline study in 1980 included 3,596 children aged 3 to 18. Data used in the current study are from the follow-up in 2001 that included 2,247 individuals with a metabolite profile, and these were representative of the baseline cohort (17). Blood samples were drawn after a 12-h fast. Plasma glucose concentrations were analyzed enzymatically (Olympus AU400), and insulin concentrations measured by microparticle enzyme immunoassay kit (Abbott Laboratories). Dietary information on food consumption was collected for 911 individuals from 48-h recalls by dietitian interviewers. Information on the types and amounts of foods and beverages consumed by the subjects in the 2 days prior to the interview was recorded and converted to intakes of energy using the National Food Composition Database (19). Physical activity was calculated for 1,685 individuals by the MET index from a questionnaire (20).
Metabolite quantification.
A high-throughput NMR platform operating at 500 MHz was used for serum metabolite quantification (21). Proton NMR spectra were recorded from native serum as well as lipid extracts to enable quantification of various amino acids, glycolysis intermediates, and fatty acid composition and degree of saturation. Metabolites tested for association with HOMA-IR are listed in Supplementary Table 1. Details of the NMR experimentation and metabolite quantification have been described previously (21–23).
Genotyping.
Genotyping of NFBC (n = 4,537) and YFS (n = 1,867) were conducted on HumanHap 370k and 670k Illumina platforms, respectively. We studied 12 single nucleotide polymorphisms (SNPs) recently associated with circulating amino acids and fatty acid composition measures in a genome-wide association study (15). Variants not directly genotyped were imputed based on HapMap 3 and 1000 Genomes imputation references (National Center for Biotechnology Information Build 36) as described previously (15). Descriptive data for the 12 tested variants are given in Supplementary Table 2.
Statistical analyses.
Insulin resistance was estimated with the HOMA-IR index using the HOMA2 calculator (www.dtu.ox.ac.uk/homacalculator/; accessed June 2011). Metabolites as well as HOMA-IR were log transformed prior to analyses. Clinical characteristics for men and women were compared using t tests.
Metabolite associations with insulin resistance.
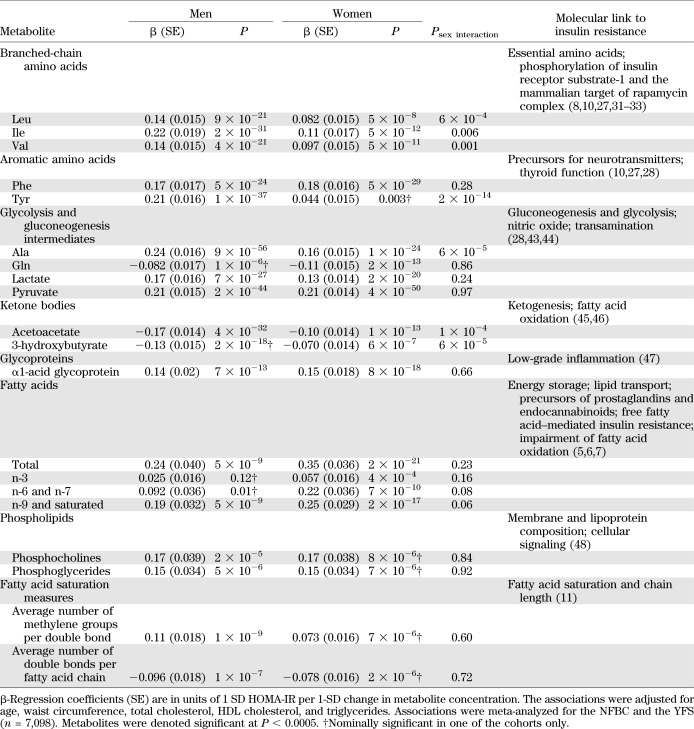
For each metabolite, a multiple linear regression model was fitted with HOMA-IR as outcome and the metabolite as explanatory variable with age, waist circumference, triglycerides, total cholesterol, and HDL cholesterol as covariates. Several associations exhibited metabolite × sex interaction, and analyses were therefore stratified by sex. Results were analyzed separately for the two cohorts and combined using inverse variance–weighted meta-analysis assuming fixed effects. Metabolites were denoted significant if the meta-analyzed P value was <0.0005 to account for multiple testing. To ensure replication, it was furthermore a criterion that each metabolite was nominally significant (P < 0.05) in both cohorts. To ease comparison of effects, association magnitudes are reported in units of 1 SD HOMA-IR per 1-SD increase in each metabolite. The variance of HOMA-IR explained by the metabolites was estimated from the global R2 by including all significant metabolites (Table 2) along with age, waist, total and HDL cholesterol, triglycerides, and MET index in the model. The additional variance explained was the increase in R2 over the variance explained by established risk factors alone. Metabolite × waist interactions were assessed by ANCOVA to test for differences in slopes across tertiles of waist circumference. To address the number of independent metabolites, a backward stepwise regression model with all 20 significant metabolites was fitted with HOMA-IR as outcome. Essentially identical results were obtained throughout with additional adjustment for all components defining metabolic syndrome and further adjustment for BMI, weight and height, and smoking. Similar results were found when limiting analyses to individuals with fasting glucose <5.6 mmol/L. All findings were essentially identical when replacing HOMA-IR for fasting plasma insulin as outcome in the models.
TABLE 2.
Associations of circulating metabolites with HOMA-IR
Dietary and physical activity associations with metabolites.
Associations of relative energy intake measures and MET index with the metabolites were assessed using linear regression models adjusted for age and sex. Associations were further tested conditioned on HOMA-IR. Physical activity data were meta-analyzed for the two cohorts (n = 6,223), whereas dietary data were available only for a subset of the YFS cohort (n = 911).
Associations of genetic variants regulating metabolite levels with insulin resistance.
A total of 12 preselected SNPs affecting the metabolites were assessed for association with insulin resistance index. The SNPs were tested as predictors of HOMA-IR in linear regression models adjusted for age, sex, waist, and 10 principal components accounting for population structure. The 12 SNPs were further analyzed for association with the metabolites. Genetic associations were meta-analyzed using inverse variance weighting (n = 6,344).
RESULTS
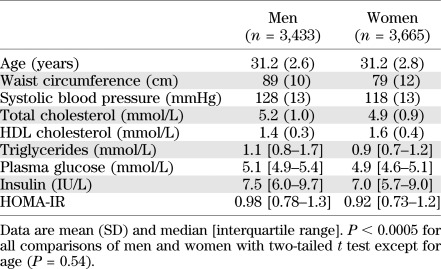
Clinical characteristics.
The study comprised 7,098 young adults (mean age 31 years, range 24–39). Clinical characteristics of the population are shown in Table 1. The study population represents metabolically healthy individuals from the general Finnish population; only 11% had impaired fasting glucose (≥5.6 mmol/L), and the 80th percentile of HOMA-IR index was 1.3, corresponding to 77% HOMA insulin sensitivity (24).
TABLE 1.
Characteristics of the study population
Metabolite associations with HOMA-IR.
To elucidate metabolic pathways characterizing or contributing to insulin resistance, 39 circulating metabolites and lipid measures from high-throughput profiling were studied. A list of analyzed metabolites is given in Supplementary Table 1. In total, 20 metabolite measures were found to be associated with HOMA-IR at P < 0.0005 for either men or women and nominally significant in both cohorts as listed in Table 2. The metabolites include amino acids, intermediates of glycolysis and gluconeogenesis, and fatty acid composition and saturation measures. Gln and ketone bodies (3-hydroxybutyrate and acetoacetate) exhibited inverse associations, as did the average number of double bonds per fatty acid chain. Branched-chain amino acids, Tyr, Ala, and ketone bodies displayed sex-dependent effects (P < 0.001 for metabolite × sex interaction) with stronger associations observed for men. Furthermore, association magnitudes (e.g., β = 0.24 SD HOMA-IR per 1 SD Ala concentration, corresponding to 1.0 IU/L higher insulin per 1 SD Ala) in men were similar for amino acids and total fatty acids, whereas in women, fatty acid associations tended to be stronger. The metabolites explained 44 and 38% of variance in HOMA-IR for men and women, respectively, in combination with established risk factors (age, waist, standard lipids, and physical activity), and added an additional respective 12 and 8% to the variance explained by established risk factors alone. In a stepwise model, most amino acids and gluconeogenesis intermediates remained significant, while several fatty acid composition measures were not independently associated with HOMA-IR (Supplementary Table 3).
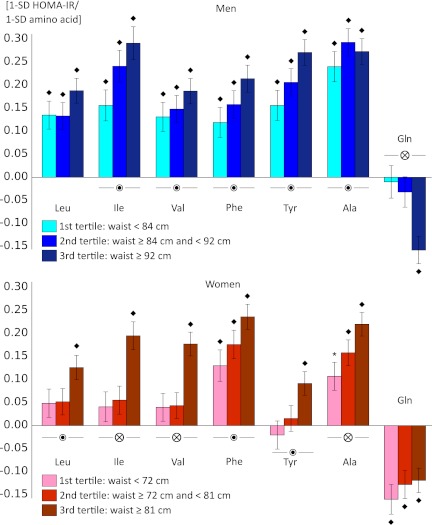
Both insulin resistance and circulating metabolite levels are linked with abdominal obesity (1,6,7). Therefore, we assessed whether the metabolite associations were consistent across tertiles of waist circumference. Results for amino acids are illustrated in Fig. 1; for the remaining metabolites, results are shown in Supplementary Fig. 1. These analyses revealed metabolite × waist interaction for most of the metabolites (14 and 12 associations with P < 0.05 for metabolite × waist tertile for men and women, respectively), with stronger associations observed for more abdominally obese individuals. It is notable that for women, associations of branched-chain amino acids and Tyr with HOMA-IR were significant only in the upper tertile of waist circumference. In contrast, men displayed associations for branched-chain and aromatic amino acids throughout the range of waist circumference; however, the sex interactions persisted even for obese individuals (data not shown). Correspondingly, Gln was inversely associated with HOMA-IR for abdominally obese men only, whereas the association was present across tertiles of waist circumference for women.
FIG. 1.
Associations of amino acids with HOMA-IR across tertiles of waist circumference for men and women. Linear regression and ANCOVA models were adjusted for age and waist circumference, total cholesterol, HDL cholesterol, and triglycerides. Association magnitudes are in units of 1-SD increased HOMA-IR per 1-SD increase in amino acid level, and error bars indicate SE. Associations were meta-analyzed for the two cohorts (n = 7,098). *P < 0.05; ♦P < 0.0005 for association of amino acid with HOMA-IR.  P < 0.05; ⊗P < 0.0005 for amino acid × waist interaction, indicating different slopes across tertiles of waist circumference.
P < 0.05; ⊗P < 0.0005 for amino acid × waist interaction, indicating different slopes across tertiles of waist circumference.
Dietary composition, physical activity, and metabolites.
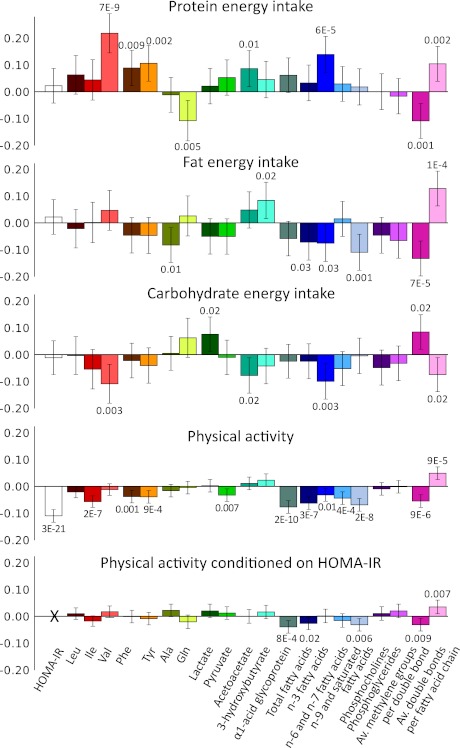
Associations of relative dietary energy intake and physical activity (MET index) with metabolites linked with HOMA-IR are shown in Fig. 2. Dietary composition was associated with several fasting metabolite levels but not with insulin resistance index. Protein energy intake per total energy intake was directly associated with Val, Phe, and Tyr and inversely associated with Gln. Relative protein as well as fat energy intake were directly associated with less fatty acid saturation. Dietary associations with the metabolites were essentially unaltered when conditioned for HOMA-IR (data not shown). In contrast to dietary measures, physical activity was inversely associated with insulin resistance index as well as several metabolites, including Ile, Phe, Tyr, α1-acid glycoprotein, total fatty acids, and fatty acid saturation measures. The physical activity associations with metabolites were of smaller magnitude than with HOMA-IR and were attenuated or rendered nonsignificant upon conditioning on HOMA-IR.
FIG. 2.
Associations of dietary composition and physical activity with circulating metabolites. All associations were adjusted for age and sex. Physical activity associations are shown with additional adjustment for HOMA-IR as well. Association magnitudes are in units of 1-SD change in metabolite concentrations per 1-SD change in lifestyle measure. Error bars indicate 95% CIs and numbers indicate P values of association. Protein, fat, and carbohydrate energy intake is per total energy intake. Dietary energy intake was derived from 48-h dietary interviews (n = 911), and physical activity was quantified as MET index based on questionnaires (n = 6,223). Av., average.
Genetic variants, metabolites, and insulin resistance.
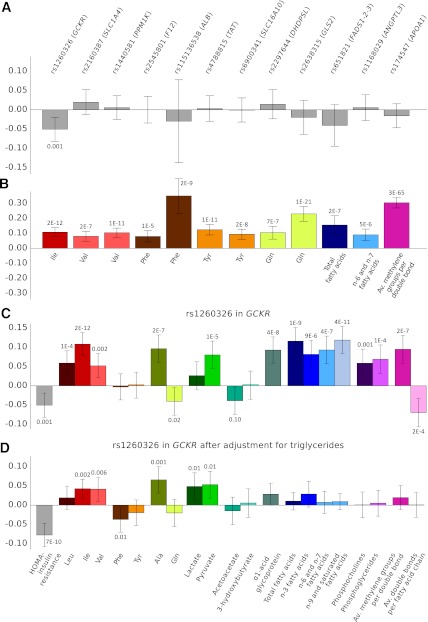
To gain insight into the direction of effect underlying the metabolite associations, we tested if genetic variants regulating the metabolite levels were also modifying HOMA-IR. Associations of the SNPs with HOMA-IR are shown in Fig. 3A. None of the SNPs affecting the metabolites were associated with HOMA-IR (P > 0.05), with the exception of a variant in GCKR previously associated with insulin resistance and other metabolic traits (15,25,26). The lack of associations with HOMA-IR was despite the fact that the analyzed genetic variants were significant determinants of the metabolites (Fig. 3B). The SNP rs1260326 in GCKR was associated with insulin resistance index (P = 0.001) and additionally associated with 12 of the 20 metabolites (P < 0.0005), as shown in Fig. 3C. Most pronounced associations were found for Ile, Ala, α1-acid glycoprotein, total fatty acids, and n-9 and saturated fatty acids (P < 1 × 10−7 for all). Of note, the insulin resistance–lowering allele was associated with the metabolite levels in the opposite direction to those observed between the metabolite levels and HOMA-IR. The associations were essentially unaltered when adjusting for HOMA-IR (data not shown); however, upon conditioning on triglycerides, they were largely attenuated, while the association with HOMA-IR was enhanced (Fig. 3D).
FIG. 3.
Associations of genetic variants regulating metabolite levels with HOMA-IR (A) and the strongest circulating metabolite measure (B). Associations for rs1260326 in GCKR with the metabolites before (C) and after (D) adjustment for triglycerides. Error bars indicate 95% CIs and numbers indicate P values of association. All associations were adjusted for sex, age, waist, and population structure and meta-analyzed for the two cohorts (n = 6,343). Association magnitudes are in units of 1 SD HOMA-IR or metabolite concentration per allele copy. Av., average.
DISCUSSION
This study demonstrates that the systemic metabolite profile strongly reflects the degree of insulin resistance evidenced in young, apparently healthy adults. The metabolic signatures of insulin resistance were different for men and women and modulated by obesity. Analyses of genetic and lifestyle determinants of the metabolite profile did not lend support to an etiological role of the metabolites in the pathogenesis of insulin resistance; however, the observed pleiotropy for insulin resistance, lipids, and amino acids for a variant in GCKR illustrates how altered glucose sensing may widely affect the metabolite profile.
High-throughput metabolic profiling identified 20 metabolite measures associated with HOMA-IR. A variety of metabolites comprising amino acids, glycolysis intermediates, ketone bodies, and lipid constituents displayed pronounced associations independent of the established dyslipidemic pattern of insulin resistance (3,4). These findings provide hypotheses implicating known and novel metabolites as markers of early stage insulin resistance. Potential mechanisms underpinning the associations are given in Table 2. The prominent imprint of insulin resistance on the metabolite profile substantiates and extends findings from smaller profiling studies (8,9,27,28) by providing quantitative information on individual metabolites in population-based cohorts of young adults. The 12 and 8% of additional variance in HOMA-IR explained by the metabolites for men and women, respectively, emphasizes metabolic signatures beyond obesity and lipid abnormalities and further contrasts findings from genome-wide association studies where genetic variants have explained only a minor fraction of the variance in insulin resistance (26).
Men and women displayed significant differences in their metabolite profiles despite a similar range of HOMA-IR in this study (Supplementary Table 1 and Table 1). While sex differences in metabolite concentrations are well known (29), this study revealed novel sex-dependent associations for amino acids and ketone bodies with insulin resistance (Table 2). For instance, Tyr displayed five times higher magnitude of association for men, whereas Phe did not exhibit different effects for men and women. This could indicate involvement of sex hormones in the regulation of these precursors of thyroid hormones and neurotransmitters. The associations were also modified by obesity, with Tyr and branched-chain amino acids being significant for women only if they were abdominally overweight (Fig. 1). Sex differences in metabolite associations with insulin action have previously been observed in a small study with stronger associations of a cluster of large neutral amino acids for overweight men (9). Differences in adipose tissue composition and adipokine levels could underpin these observations; however, the molecular mechanisms remain to be investigated. Although the prevalence of diabetes is similar among men and women, there are differences in the development of insulin resistance, with young men being more insulin resistant after puberty in conjunction with an adverse lipid profile (3,4). In addition, some studies suggest greater protein turnover rates and lesser insulin sensitivity of protein anabolism for women (30). Several studies on amino acids in relation to insulin resistance have been conducted for men only (28,31,32); however, our results indicate that future studies should account for sex- and obesity-specific differences.
Assessment of lifestyle effectors and genetic determinants of metabolite levels may illuminate the etiology underpinning the metabolic signatures of insulin resistance. Both diet and physical activity were associated with the fasting metabolite profiles (Fig. 2). None of the dietary measures were linked with insulin resistance index; however, the elevation of several amino acids by increased protein energy intake could potentially be detrimental for insulin sensitivity, as suggested by several studies (8,10,31–33). On the other hand, high protein intake was also associated with an increase in n-3 fatty acids and a higher number of double bonds per fatty acid, thus pointing to a beneficial role of a protein-rich diet. Lipids of high double bond content were recently linked with decreased risk for incidence of diabetes (11), in accordance with the inverse association between average number of double bonds per fatty acid chain and insulin resistance index found in this study.
Physical activity was inversely associated with insulin resistance index as well as lipids and amino acids; however, the associations were less pronounced for the metabolites. Furthermore, the associations diminished when conditioning on HOMA-IR, indicating that the metabolite associations are not independent of insulin resistance. Because physical activity is known to improve insulin sensitivity (34), these results could indicate that physical activity is primarily affecting insulin resistance and the associations observed with the metabolite profile could be secondary hereto.
The question whether amino acids contribute to the pathogenesis of insulin resistance and type 2 diabetes in a functional manner remains unsettled (35). Experimental studies suggest that branched-chain amino acids may promote insulin resistance (8,31), yet direct evidence in humans is lacking. Dietary composition is likely to influence both metabolite levels and the development of insulin resistance in a causal manner (8,33), yet we did not find concomitant associations with dietary measures and HOMA-IR despite the observation that protein energy intake was associated with fasting amino acid levels. These results, therefore, do not suggest an etiological role of amino acids in the disease pathogenesis. The cross-sectional dietary associations, however, may potentially be confounded by other environmental factors. To circumvent such bias, we tested genetic variants determining metabolite levels. Such analyses may serve to elucidate direction of effect, since insulin resistance can be affected by genetic variants, whereas the genes are not influenced by insulin resistance (36). We found that 11 out of 12 genetic variants, including 6 regulating branched-chain and aromatic amino acids, were not associated with HOMA-IR (Fig. 3A). Thus, these analyses on the direction of effect do not lend support to the notion of a causal role of amino acids in the development of insulin resistance. On the other hand, the modest effects of each genetic variant and the limited range of HOMA-IR in the young study population may partly account for the lack of associations, and further studies are required to determine the etiological roles of amino acids in the pathogenesis.
A single genetic variant, rs1260326 in the glucokinase regulatory protein gene GCKR, was associated with insulin resistance index. This missense variant has previously been linked with insulin resistance, glycemia, and several other metabolic traits and, recently, amino acids (15,25,26,37,38). The insulin resistance–lowering allele was associated with elevated metabolite levels, in contrast to the direct correlations observed between metabolites and HOMA-IR (Fig. 3C). The pertinent variant has been suggested to mimic the consequences of glucokinase overexpression leading to increased glycolytic flux (25,39,40). Associations with Ala, lactate, pyruvate, and branched-chain amino acids suggest effects on the glucose-alanine cycle as well (38). Because the metabolite associations were largely attenuated upon adjustment for triglycerides (Fig. 3D), we propose a shared origin underlying the associations for amino acids and lipids with GCKR. The pleiotropy observed for GCKR indicates how perturbations in the glucose sensory mechanism may subsequently affect lipid and amino acid levels. Altered glucose utilization could also underlie the metabolite associations with insulin resistance established in this study, and the genetic analyses are therefore compatible with elevated amino acid and lipid levels being predominantly secondary rather than direct contributors to the development of insulin resistance.
The large population studied rendered direct measurement of insulin sensitivity infeasible, and insulin resistance was therefore approximated by the HOMA index (24). While the HOMA model largely reflects hepatic insulin resistance, studies in Finnish populations show that fasting measures are adequate surrogates of clamp test–derived insulin resistance (41). The limited sensitivity of high-throughput NMR confines quantification to highly abundant metabolites, and the wider coverage achieved by mass spectrometry, in particular for molecular lipid species, holds promise to provide further insight into the pathophysiology of insulin resistance (7,11,42). Our study was conducted in a homogenous population, yet the results confirm previous smaller studies in older populations including Asian men (27). Strengths of the study include metabolic profiling in population-based cohorts of young adults and the combination of additional phenotype and genotype data to elucidate origins of the associations.
The diversity of metabolic associations with HOMA-IR highlights metabolic signatures of insulin resistance beyond the characteristics of metabolic syndrome and suggests a strong relation between insulin resistance and the systemic metabolite profile already evidenced in early adulthood. A combination of amino acids, lipids, and intermediates of glycolysis formed sex-specific imprints of insulin resistance on the metabolite profile that warrant attention in future physiological studies. Genetic evidence did not provide support for a functional role of the metabolites in the pathogenesis of insulin resistance, yet irrespective of cause or effect, even modest insulin resistance was associated with an adverse cardiometabolic profile. Understanding the relation between insulin resistance and the systemic metabolite profile in young, normoglycemic adults may help to promote lifestyle habits for prevention of insulin resistance prior to development of hyperglycemia and overt type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland, the Responding to Public Health Challenges Research Programme of the Academy of Finland, and the Academy of Finland Center of Excellence in Complex Disease Genetics; the Instrumentarium Science Foundation; the Sigrid Juselius Foundation; the Paulo Foundation; the Finnish Foundation for Cardiovascular Research; the Emil Aaltonen Foundation; the Paavo Nurmi Foundation; the Jenny and Antti Wihuri Foundation; the Juho Vainio Foundation; the Finnish Cultural Foundation; the Social Insurance Institution of Finland; the Oulu, Kuopio, Tampere, and Turku University Hospital Medical Funds; the European Community’s Seventh Framework Programme Collaborative European Effort to Develop Diabetes Diagnostics; National Heart, Lung, and Blood Institute Grant 5R01-HL-087679-02 through the STAMPEED program; National Institutes of Health/National Institute of Mental Health Grant 5R01-MH-63706-02; the ENGAGE project and grant agreement HEALTH-F4-2007-201413; and the Medical Research Council, U.K.
No potential conflicts of interest relevant to this article were reported.
P.W. researched and interpreted data and wrote the manuscript. V.-P.M., T.Tu., J.K., S.R., and M.A.-K. contributed to interpretation of data and discussion. P.S. performed the NMR experiments. A.J.K. analyzed the NMR spectral data. M.J.S., T.Ta., J.S.V., T.R., M.K., T.L., O.T.R., and M.-R.J. provided clinical data and interpretations. All authors reviewed, commented on, and accepted the manuscript. P.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful for the skilled technical assistance of Tarja Ihalainen, University of Eastern Finland, for lipid extraction.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1355/-/DC1.
REFERENCES
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 3.Raitakari OT, Porkka KV, Rönnemaa T, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The Cardiovascular Risk in Young Finns Study. Diabetologia 1995;38:1042–1050 [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation 2008;117:2361–2368 [DOI] [PubMed] [Google Scholar]
- 5.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 7.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzmann SS, Merrifield CA, Rezzi S, et al. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res 2012;11:643–655 [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med 2010;2:33ra37 [DOI] [PMC free article] [PubMed]
- 14.Suhre K, Shin SY, Petersen AK, et al. CARDIoGRAM Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kettunen J, Tukiainen T, Sarin AP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 2012;44:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatti C, Service SK, Hartikainen AL, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 2009;41:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raitakari OT, Juonala M, Rönnemaa T, et al. Cohort profile: the Cardiovascular Risk in Young Finns Study. Int J Epidemiol 2008;37:1220–1226 [DOI] [PubMed] [Google Scholar]
- 18.Tammelin T, Laitinen J, Näyhä S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes Relat Metab Disord 2004;28:775–782 [DOI] [PubMed] [Google Scholar]
- 19.Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. Br J Nutr 2005;93:923–931 [DOI] [PubMed] [Google Scholar]
- 20.Mansikkaniemi K, Juonala M, Taimela S, et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 1 July 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Soininen P, Kangas AJ, Würtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst (Lond) 2009;134:1781–1785 [DOI] [PubMed] [Google Scholar]
- 22.Tukiainen T, Tynkkynen T, Mäkinen VP, et al. A multi-metabolite analysis of serum by 1H NMR spectroscopy: early systemic signs of Alzheimer’s disease. Biochem Biophys Res Commun 2008;375:356–361 [DOI] [PubMed] [Google Scholar]
- 23.Inouye M, Kettunen J, Soininen P, et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol Syst Biol 2010;6:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 25.Orho-Melander M, Melander O, Guiducci C, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes 2008;57:3112–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuis J, Langenberg C, Prokopenko I, et al. DIAGRAM Consortium. GIANT Consortium. Global BPgen Consortium. Anders Hamsten on behalf of Procardis Consortium. MAGIC Investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 [DOI] [PubMed] [Google Scholar]
- 28.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittelstrass K, Ried JS, Yu Z, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 2011;7:e1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. The influence of sex on the protein anabolic response to insulin. Metabolism 2005;54:1529–1535 [DOI] [PubMed] [Google Scholar]
- 31.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005;54:2674–2684 [DOI] [PubMed] [Google Scholar]
- 32.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002;51:599–605 [DOI] [PubMed] [Google Scholar]
- 33.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007;27:293–310 [DOI] [PubMed] [Google Scholar]
- 34.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 2008;192:127–135 [DOI] [PubMed] [Google Scholar]
- 35.Gerszten RE, Wang TJ. Two roads diverge: weight loss interventions and circulating amino acids. Sci Transl Med 2011;3:80ps15 [DOI] [PubMed]
- 36.De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 2011;60:1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers JC, Zhang W, Sehmi J, et al. Alcohol Genome-wide Association (AlcGen) Consortium. Diabetes Genetics Replication and Meta-analyses (DIAGRAM+) Study. Genetic Investigation of Anthropometric Traits (GIANT) Consortium. Global Lipids Genetics Consortium. Genetics of Liver Disease (GOLD) Consortium. International Consortium for Blood Pressure (ICBP-GWAS) Meta-analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) Genome-wide association study identifies loci influencing plasma levels of liver enzymes. Nat Genet 2011;43:1131–113822001757 [Google Scholar]
- 38.Tukiainen T, Kettunen J, Kangas AJ, et al. Detailed metabolic and genetic characterization reveals new associations for 30 known lipid loci. Hum Mol Genet 2012;21:1444–1455 [DOI] [PubMed] [Google Scholar]
- 39.Beer NL, Tribble ND, McCulloch LJ, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 2009;18:4081–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Doherty RM, Lehman DL, Télémaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 1999;48:2022–2027 [DOI] [PubMed] [Google Scholar]
- 41.Lorenzo C, Haffner SM, Stancáková A, Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic Finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab 2010;95:5082–5090 [DOI] [PubMed] [Google Scholar]
- 42.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med 2011;365:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hankard RG, Haymond MW, Darmaun D. Role of glutamine as a glucose precursor in fasting humans. Diabetes 1997;46:1535–1541 [DOI] [PubMed] [Google Scholar]
- 44.Crawford SO, Hoogeveen RC, Brancati FL, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol 2010;39:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 46.McGarry JD, Foster DW. Effects of exogenous fatty acid concentration on glucagon-induced changes in hepatic fatty acid metabolism. Diabetes 1980;29:236–240 [DOI] [PubMed] [Google Scholar]
- 47.Lee YS, Choi JW, Hwang I, et al. Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J Biol Chem 2010;285:22174–22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Kong H, Guan Y, et al. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal Chem 2005;77:4108–4116 [DOI] [PubMed] [Google Scholar]