Abstract
Vascular endothelial growth factor (VEGF)–induced breakdown of the blood-retinal barrier requires protein kinase C (PKC)β activation. However, the molecular mechanisms related to this process remain poorly understood. In this study, the role of occludin phosphorylation and ubiquitination downstream of PKCβ activation in tight junction (TJ) trafficking and endothelial permeability was investigated. Treatment of bovine retinal endothelial cells and intravitreal injection of PKCβ inhibitors as well as expression of dominant-negative kinase was used to determine the contribution of PKCβ to endothelial permeability and occludin phosphorylation at Ser490 detected with a site-specific antibody. In vitro kinase assay was used to demonstrate direct occludin phosphorylation by PKCβ. Ubiquitination was measured by immunoblotting after occludin immunoprecipitation. Confocal microscopy revealed organization of TJ proteins. The results reveal that inhibition of VEGF-induced PKCβ activation blocks occludin Ser490 phosphorylation, ubiquitination, and TJ trafficking in retinal vascular endothelial cells both in vitro and in vivo and prevents VEGF-stimulated vascular permeability. Occludin Ser490 is a direct target of PKCβ, and mutating Ser490 to Ala (S490A) blocks permeability downstream of PKCβ. Therefore, PKCβ activation phosphorylates occludin on Ser490, leading to ubiquitination required for VEGF-induced permeability. These data demonstrate a novel mechanism for PKCβ targeted inhibitors in regulating vascular permeability.
Vascular hyperpermeability in the retina contributes to macular edema, associated with loss of vision in retinal diseases including diabetic retinopathy (DR) (1), uveitis, and retinal vein occlusion. Despite its clinical significance, the molecular mechanisms that cause the breakdown of the blood-retinal barrier (BRB) remain poorly defined. Vascular endothelial growth factor (VEGF) was originally isolated as a vascular permeability factor (2) and contributes to vascular leakage in multiple pathologies including retinal vascular diseases (1). VEGF additionally functions as a potent inducer of angiogenesis, and its neutralization has been reported to provide clinical benefits in intraocular angiogenic diseases, such as DR and age-related macular degeneration (3,4). Recent clinical trials demonstrating the effectiveness of anti-VEGF antibody therapy in promoting visual acuity in conjunction with laser treatment attests to the importance of this cytokine in DR (5). VEGF activates several intracellular signal transduction cascades including protein kinase C (PKC), which induces BRB breakdown (6). A clinical trial with the PKCβ-specific inhibitor, ruboxistaurin, has demonstrated beneficial effects for DR and macular edema (7–9). The clinical data have been supported by experimental evidence reporting that this inhibitor reduces VEGF-induced vascular permeability and neovascularization (10,11). Despite the contribution of PKC to VEGF signaling, the effectors that lead to the changes in intercellular junctions and BRB breakdown remain unknown.
The BRB tightly regulates transport between blood and neural parenchyma under physiological conditions (2,12). An important component of the BRB is the endothelial tight junction (TJ) complex. Proteins associated with TJ include transmembrane, scaffolding, and signaling proteins (13). In particular, the transmembrane proteins occludin, tricellulin, the claudin family, and junction adhesion molecules, along with the scaffolding zonula occludens proteins (ZO-1, −2, −3), play major roles in the formation and regulation of the TJ barrier.
Although many of the proteins that constitute the TJ have been identified, the function of specific junctional proteins and regulation of the junctional complex in response to external signals remains an area of intense research. Claudins create a barrier to paracellular permeability, and claudin-5 gene deletion is lethal because of loss of blood-brain barrier integrity (14). Although cells do not require occludin for formation of TJ (15), recent reports have demonstrated a number of phosphorylation sites on occludin that regulate barrier properties. Phosphorylation of threonines 403/404 by PKCη and threonines 424/438 by PKCζ promotes occludin localization to TJ (16,17). Meanwhile, Src-induced tyrosine phosphorylation on Tyr398 and Tyr402 regulates hydrogen peroxide–induced alterations to the junctional complex and permeability (18), and CKII-dependent phosphorylation of Ser408 alters occludin complex formation, allowing claudin pore formation and ion permeability (19). In vascular endothelial cells rho kinase phosphorylates occludin on Thr382 and Ser507, which can be observed in brains of humans with human immunodeficiency virus-1 encephalitis (20).
VEGF treatment of vascular endothelial cells and diabetes increases occludin phosphorylation (21) associated with altered distribution from cell border to intracellular puncta (22). Through a mass spectrometry analysis, multiple occludin phosphorylation sites were identified in VEGF-treated retinal endothelial cells. One of these sites, Ser490, is phosphorylated in a VEGF-dependent manner altering the interaction with ZO-1 and allowing subsequent ubiquitination (23,24) This ubiquitination induces endocytosis of occludin (25) in a pathway similar to that identified for some receptor tyrosine kinases (26). The phosphorylation of Ser490 and occludin ubiquitination has been shown to be necessary for VEGF-induced permeability to 70 kDa dextran and ion flux in retinal endothelial cells in culture (25).
Here we report that VEGF-induced PKCβ activation regulates occludin phosphorylation on Ser490 and allows ubiquitination of occludin leading to TJ disruption and increased vascular permeability in retinal endothelial cells. Furthermore, we demonstrate that this phosphorylation and ubiquitination occur in the retinal vasculature in vivo in response to VEGF treatment. These studies provide a molecular mechanism of action of PKCβ in regulation of vascular permeability in response to VEGF and demonstrate a role for occludin in regulation of vascular permeability.
RESEARCH DESIGN AND METHODS
Materials.
Recombinant human VEGF165 was purchased from R&D Systems (Minneapolis, MN), and Complete Protease Inhibitor cocktail tablets were from Roche (Indianapolis, IN). LY379196 was obtained from Lilly Research Laboratories (Indianapolis, IN), and a PKCβ-specific inhibitor, 3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione (PKCβI), was from Calbiochem (San Diego, CA). Recombinant PKCβII was purchased from Enzo (Farmingdale, NY). All other chemical reagents were from Calbiochem or Sigma-Aldrich (St. Louis, MO).
Cell culture.
Primary bovine retinal endothelial cells (BREC) were isolated and cultured as described previously (25). When BREC reached confluence, medium was replaced with MCDB-131 supplemented with 1% fetal bovine serum, 0.01 mg/mL antibiotic-antimycotic, and 100 nmol/L hydrocortisone for 24 h before the experiment.
Animals.
Male Sprague-Dawley rats weighing 150 to 175 g from Charles River Laboratories (Wilmington, MA) were housed in accordance with the Institutional Animal Care and Use Committee guidelines as well as the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research (27). Intravitreal injection was performed using a hamilton syringe through a hole made previously with a 33-gauge needle. After rats were given a lethal dose of pentobarbital, retinas were harvested.
Immunoblot analysis.
Western blot using NuPAGE system (Invitrogen) was performed as described previously (25). Rat retinas were harvested and quickly frozen in liquid nitrogen, followed by lysis with the same buffer. After centrifugation, supernatants were applied to 10% SDS-polyacrylamide gels, followed by transfer to nitrocellulose membrane.
After blocking with 5% milk, 2% enhanced chemiluminescence (ECL) blocking reagent, or 1% BSA in Tris-buffered saline with 0.1% Tween-20, immunoblotting was performed using anti-claudin-5 (Invitrogen), anti-Eps15 (Santa Cruz Biotechnology), and anti-ubiquitin (Enzo) antibodies. Mouse anti-occludin antibody (Invitrogen 33–1500) was used unless otherwise indicated. A specific antibody against occludin phosphorylated on Ser490 was described previously (23). After incubation with secondary horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG, signal was detected with chemiluminescence with ECL Plus or ECL Advance (GE Healthcare, Piscataway, NJ).
Detection of ubiquitination.
BREC or retinal lysate was prepared using immunoprecipitation (IP) buffer (1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS, 150 mmol/L NaCl, 50 mmol/L Tris [pH 7.5], 2 mmol/L N-ethylmaleimide, 1 mmol/L NaVO4, 10 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 mmol/L benzamidine, and complete protease inhibitor cocktail) or coIP buffer (1% Nonidet P-40, 10% glycerol, 50 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 2 mmol/L EDTA, 2 mmol/L N-ethyl-maleimide, 1 mmol/L NaVO4, 10 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 mmol/L benzamidine, and complete protease inhibitor cocktail) as described previously (25). Lysate was incubated with primary antibody for 2 h at 4°C, followed by additional incubation with protein G for 1 h. Precipitated proteins were eluted and subjected to Western blotting.
Immunostaining.
Immunohistochemistry was performed as described previously (28). Eyecups from Sprague-Dawley rats were fixed in 2% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature and blocked in 10% donkey serum with 0.3% Triton X-100. Retinas were incubated with mouse anti-occludin (Invitrogen) or rabbit anti-claudin-5 (Invitrogen) antibodies for 3 days, followed with secondary fluorescent antibodies. All retinas were flat mounted with coverslip (Aqua poly/mount; Polysciences, Warrington, PA).
Expression constructs and mutants.
Occludin mutants were described previously (25). In brief, Ser490 of human occludin in pENTR221 (Invitrogen, Rockville, MD) was substituted to alanine. After the addition of V5-His tag in COOH terminus, these mutants were transferred into pmaxCloning expression vector (AmaxaBiosystems, Gaithersburg, MD). For the occludin-ubiquitin chimera construct, the ubiquitin sequence was subcloned into COOH terminus of occludin mutants in pmaxCloning expression vector. All constructs were confirmed by sequencing. The PKCβII kinase dead mutant m218 (29) is a 13 nucleotide truncation mutation we showed previously to act as a dominant negative (DN) form in BREC.
Transfection.
Transfection of plasmid containing PKC-β and/or occludin mutants was achieved using the nucleofection technique (AmaxaBiosystems), according to the manufacturer’s instructions (6). In brief, BREC were resuspended in human coronary artery endothelial cells nucleofection solution (AmaxaBiosystems) supplemented with 3 µg of each plasmid and applied to electroporation (setting S-005; AmaxaBiosystems). Transfected cells were grown in growth medium, and cells were incubated in MCDB-131 supplemented with 1% fetal bovine serum, 0.01 mg/mL antibiotic-antimycotic, and 100 nmol/L hydrocortisone for the last 24 h.
Permeability assays.
BREC were grown on 0.4-µm pores filters (Transwell; Corning Costar, Acton, MA), and 10 μmol/L rhodamine B isothiocyanate-dextran (70 kDa) were added to the apical chamber of inserts. Aliquots were removed from the basolateral chamber every 30 min for 4 h for quantification, and an aliquot from the apical chamber was taken at the end of the experiment. The rate of diffusive flux (Po) was calculated as described previously (30). Transendothelial electrical resistances (TERs) were measured using an EVOM with a STX2 Electrode (World Precision Instruments, Sarasota, FL), just after the dextran permeability experiment (28).
Retinal vascular permeability was assessed in rat retinas as described by Xu et al. (31). Under ketamine/xylazine anesthesia (67/6.7 mg/kg body weight i.m.), VEGF and/or proteasome inhibitors were intravitreally administrated. Four hours later, animals received femoral vein injection of 45 mg/kg body weight of Evan's blue under anesthesia. After 2 h of circulation, the animals were anesthetized again with ketamine/xylazine, blood was drawn, and the circulation was cleared of dye by transcardiac perfusion for 2 min with citrate buffer (50 mmol/L, pH 3.5, 37°C) containing 1% paraformaldehyde (Fisher, Pittsburgh, PA). Retinas were harvested from both eyes and dried in a Savant Speed-Vac (Thermo Scientific, Waltham, MA). Evan's blue was extracted by incubating each retina in 150 μL of formamide at 70°C overnight. The extract was transferred to a Ultrafree-MC filter tube (Millipore) and centrifuged at 5,000 g for 2 h. Plasma and retina extract samples were assayed in triplicate. The retinal Evan’s blue accumulation was calculated by normalizing to plasma concentration and retina dry weight and expressed as microliters per grams dry retina weight per hour circulation.
In vitro kinase assay.
COOH-terminal fragments of occludin (aa 413–522, 13.3 kDa or aa 383–522, 16.6 kDa, 10 μg) were incubated with 500 ng PKCβII (Enzo) in 25 mmol/L MOPS (pH 7.2), 12.5 mmol/L β-glycerophosphate, 5 mmol/L EGTA, 2 mmol/L EDTA, 25 mmol/L MgCl2, 0.25 mmol/L dithiothreitol (added fresh), 100 μg/mL phosphatidylserine, 20 μg/mL diacylglycerol, and 0.2 mmol/L ATP for 3 h at 30°C. Samples were then mixed with SDS-PAGE loading buffer, and immunoblot anaylsis for phospho-Ser490 was performed.
Statistical analysis.
All experiments were repeated three or more times. Unless otherwise noted, statistical analysis was performed as follows: a two-tailed t test (two conditions) or ANOVA (three or more conditions) was performed using software for statistical analysis (InStat 3.05; GraphPad) and P < 0.05 was considered significant.
RESULTS
VEGF-induced PKCβ activation is required for occludin Ser490 phosphorylation and ubiquitination in BREC.
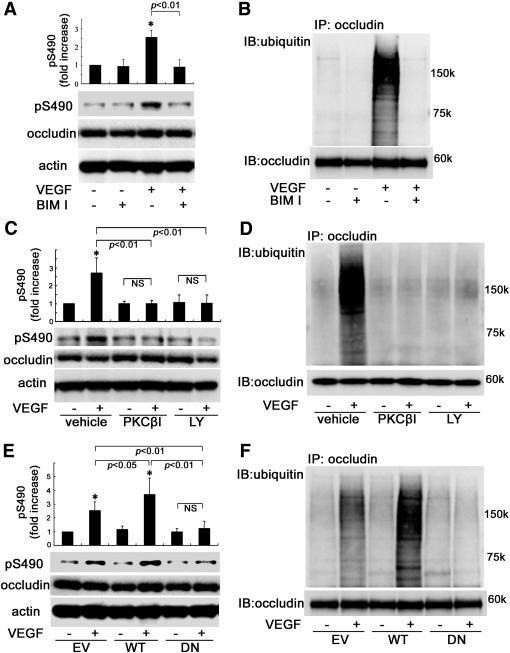
To determine the involvement of classical PKC isoforms in VEGF-induced occludin Ser490 phosphorylation, BREC were incubated with the classical PKC inhibitor bisindolylmaleimide I HCl (BIM 1; 5 μmol/L) followed by VEGF treatment (50 ng/mL, 15 min). Occludin was blotted for Ser490 phosphorylation (Fig. 1A) or immunoprecipitated and blotted for ubiquitination (Fig. 1B). BIM I prevented the VEGF-induced occludin Ser490 phosphorylation (Fig. 1A) and ubiquitination (Fig. 1B), suggesting that these modifications are mediated via classical PKC activation. Given the role of PKCβ in VEGF-induced occludin phosphorylation and endothelial permeability (6), the role of PKCβ specifically in the phosphorylation of occludin on Ser490 was determined. BREC were pretreated for 30 min with either PKCβ inhibitor (PKCβI) at 50 nmol/L, a dose above the half-maximal inhibitory concentration for PKCβ isoforms I and II but still highly specific, or LY379196 at 500 nmol/L, a dose that inhibits classical PKC isoforms and some novel forms, before treatment with VEGF (50 ng/mL) for 15 min. These PKC inhibitors blocked the VEGF-induced phosphorylation on Ser490 and ubiquitination of occludin (Fig. 1C and D). To further establish a role for PKCβ in Ser490 phosphorylation and ubiquitination of occludin, BREC were transfected with empty vector (EV), wild-type (WT)-PKCβ, or a kinase dead 13 nucleotide truncation mutant of PKCβ that we showed previously to act in a DN fashion in BREC (6) (DN)-PKCβ followed by VEGF stimulation. Transfection of WT-PKCβ augmented VEGF-induced Ser490 phosphorylation and ubiquitination of occludin compared with EV, whereas DN-PKCβ blocked these events (Fig. 1E and F). These data indicate that occludin phosphorylation on Ser490 and ubiquitination require VEGF-induced PKCβ activation in BREC.
FIG. 1.
The effects of PKCβ on occludin phosphorylation on Ser490 and ubiquitination in BREC (A–D). Pretreatment with BIM I, PKCβ inhibitor (PKCβI), or LY379196 (LY) suppressed VEGF-induced occludin phosphorylation of Ser490 and ubiquitination as determined by blotting with phospho-Ser490–specific antibody (A and C) or immunoprecipitation followed by ubiquitin blot (B and D). E and F: WT-PKCβ augmented and DN-PKCβ blocked these posttranslational modifications (n = 3–6); *P < 0.01 vs. control. IB, immunoblot; NS, not significant.
PKCβ and occludin phosphorylation/ubiquitination regulates VEGF-induced endothelial permeability.
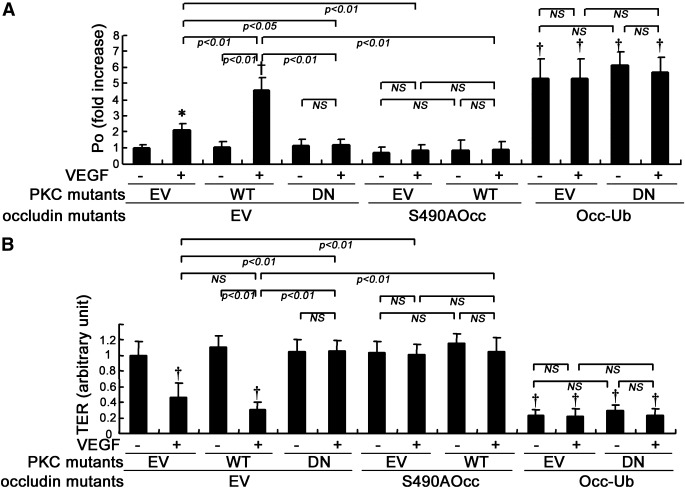
The data shown above suggest that PKCβ is an upstream regulator for occludin phosphorylation and ubiquitination, posttranslational modifications that have been shown to regulate TJ trafficking and permeability (25). The requirement for occludin Ser490 phosphorylation in PKCβ-induced vascular permeability was further investigated. PKCβ mutants and/or occludin mutants were transfected into BREC, and paracellular flux of 70 kDa dextran was evaluated. Transfection with WT-PKCβ significantly augmented VEGF-induced permeability as compared with EV, whereas transfection with DN-PKCβ inhibited VEGF-induced permeability to dextran (Fig. 2A) as reported previously (6) and prevented the VEGF-induced loss of electrical resistance (Fig. 2B). Cotransfection of occludin S490A blocked the VEGF-induced dextran permeability and the change in electrical resistance observed in either EV- or WT-PKCβ transfected BREC, demonstrating that PKCβ-mediated occludin phosphorylation on Ser490 is required for VEGF-induced vascular permeability. Additionally, cotransfection of the occ-Ub chimera with DN-PKCβ increased dextran permeability and reduced electrical resistance in BREC, suggesting that occludin ubiquitination is a downstream effector of PKCβ and sufficient for VEGF-induced hyperpermeability.
FIG. 2.
The effects of PKCβ and occludin (Occ) mutants on endothelial permeability in BREC. After transfection with PKCβ and/or occludin mutants, VEGF-induced permeability in BREC was evaluated using 70 kDa dextran flux (A) or TER (B) (n = 11 to 12); *P < 0.05 vs. control; †P < 0.01 vs. control. NS, not significant.
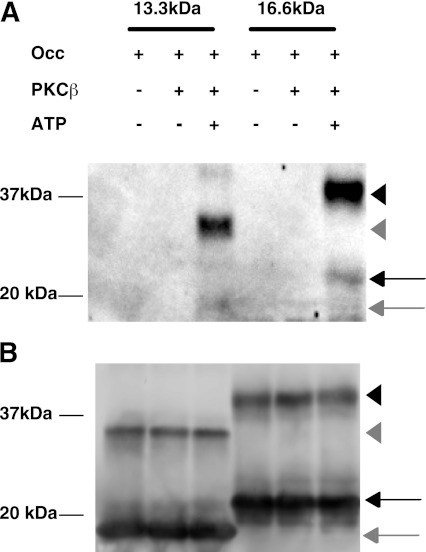
A direct effect of PKCβ on occludin Ser490 phosphorylation was investigated. COOH-terminal fragments of occludin (amino acids 413–522 or amino acids 383–522) were incubated with recombinant PKCβII and ATP followed by immunoblotting for occludin phospho-Ser490. PKCβII was able to phosphorylate occludin on Ser490 in both COOH-terminal fragments, indicating that PKCβII can directly phosphorylate occludin Ser490 (Fig. 3).
FIG. 3.
PKCβII phosphorylates COOH-terminal fragments of occludin (Occ) in vitro. A: Both a 13.3-kDa (aa 413–522) and a 16.6-kDa (aa 383–522) fragment of the COOH-terminal fragment of occludin were phosphorylated by PKCβII only in the presence of ATP as observed using the occludin phospho-Ser490–specific antibody. Phosphorylation was observed on the monomer (arrow) but predominantly on the dimer (arrowhead) for the larger (black) and smaller (gray) occludin fragments. B: Occludin immunoblot with polyclonal occludin antibody.
VEGF increases occludin phosphorylation and ubiquitination in rat retinas.
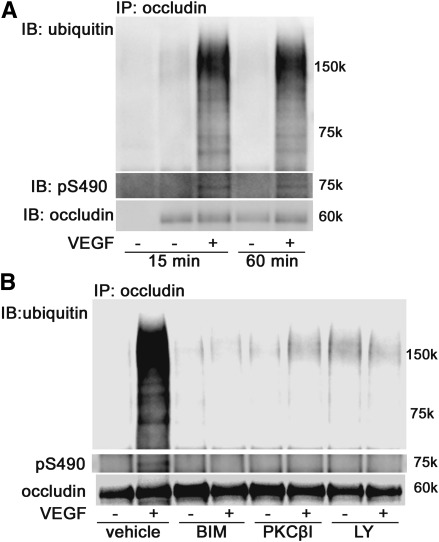
VEGF-induced occludin phosphorylation and ubiquitination in rat retinas was determined in vivo. VEGF (1.5 ng) or vehicle was injected intravitreally, and retinas were isolated and lysed at the indicated time points. Occludin was immunoprecipitated and blotted with antibodies to phospho-Ser490 or ubiquitin as above. Both the phosphorylation on Ser490 and ubiquitination were increased by VEGF treatment at either 15 or 60 min, compared with the vehicle (Fig. 4A). To evaluate the PKC inhibitors in vivo, VEGF and/or each inhibitor (150 pmol BIM I, 1.5 pmol PKCβI, 15 pmol LY379196) were intravitreally injected and retinas were harvested after 30 min. All three different inhibitors blocked both the VEGF-induced occludin Ser490 phosphorylation and ubiquitination (Fig. 4B), suggesting that these posttranslational modifications are mediated via activation of PKCβ in the rat retinal vasculature.
FIG. 4.
PKCβ-mediated occludin modifications in rat retinas. A: Occludin IP and phospho-Ser 490 or ubiquitin blot demonstrate time course effect of VEGF treatment on these posttranslational modifications. B: PKC inhibitors BIMI, PKCβI, and LY379196 blocked VEGF-induced occludin phosphorylation on Ser490 and ubiquitination in rat retinas. These experiments were repeated three times. IB, immunoblot.
VEGF-induced TJ trafficking requires PKCβ activation in rat retinal vasculature.
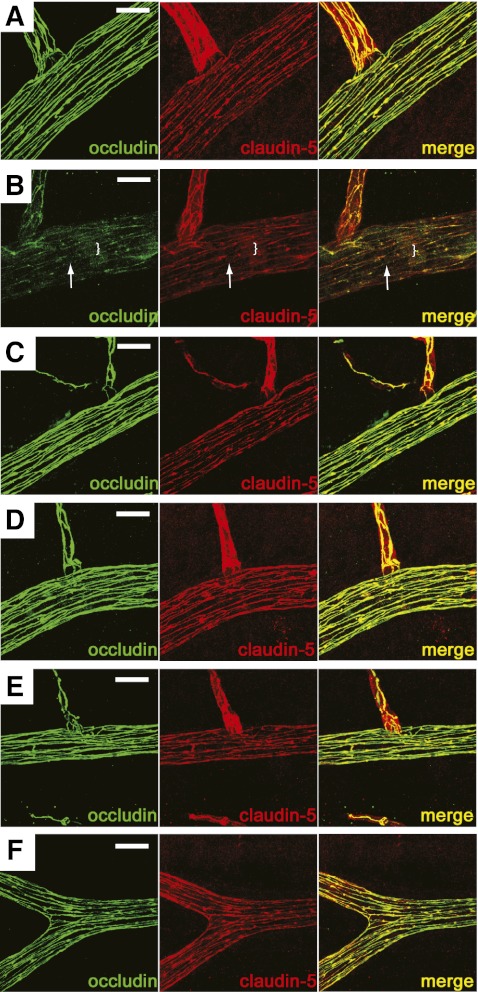
The effect of PKCβ activation on TJ protein organization at the cell border in rat retinas was examined. Thirty minutes after injection of VEGF or coinjection of VEGF with inhibitor, the retinas were isolated and fixed, followed by immunostaining for occludin or claudin-5. When compared with vehicle-injected retinas, intravitreal VEGF injection decreased the occludin immunoreactivity at the cell border and increased cytoplasmic staining (Fig. 5A and B). Claudin-5 cell border immunoreactivity also appeared reduced after VEGF injection, as compared with vehicle, albeit with a less dramatic change than occludin. Additionally, administration of the PKCβ inhibitors, PKCβI or LY379196, blocked the VEGF-induced changes in occludin and claudin-5 localization as determined by immunocytochemistry (Fig. 5C–F and Supplementary Figs. I and II). These data suggest that VEGF-induced PKCβ activation is necessary for occludin and claudin-5 protein trafficking in rat retinal vasculature.
FIG. 5.
PKCβ-mediated TJ redistribution in rat retinal arterioles. Retinal whole mount immunostaining for occludin (green) or claudin-5 (red) in control (A), VEGF-treated (B), PKCβI (C), VEGF and PKCβI (D), LY379196 (E), or LY379196 and VEGF (F). VEGF reduced cell border staining (arrow) and increased a diffuse cytoplasmic staining (bracket) of TJ proteins that was prevented by the PKCβ inhibitors. Scale bar = 20 μm. (A high-quality digital representation of this figure is available in the online issue.)
PKCβ regulates occludin ubiquitination and proteosomal degradation.
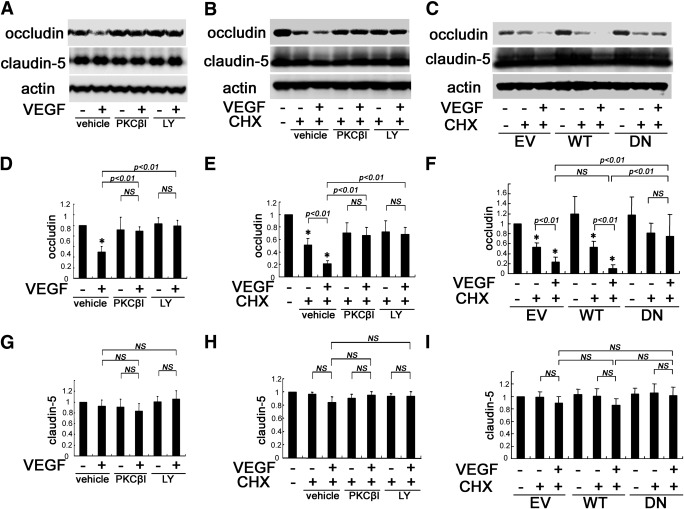
Occludin phosphorylation and ubiquitination were shown previously to regulate endocytosis and disruption of the TJ. Over time, the ubiquitinated occludin is degraded. Here we demonstrate that occludin ubiquitination and degradation require PKCβ activation. Rats were injected intravitreally with VEGF and either PKCβI or LY379196. Retinas were harvested (1.5 h), and protein was extracted followed by immunoblot analysis for occludin. As expected, VEGF treatment reduced retinal occludin content as compared with controls. Treatment with PKCβ inhibitors blocked the VEGF-induced reduction in occludin content, without altering the basal levels (Fig. 6A and D). However, retinal claudin-5 protein levels were not changed by either VEGF or PKCβ inhibitor treatment (Fig. 6G). To test whether the reduction in occludin content occurs as a result of decreased synthesis or increased degradation, protein synthesis in BREC was inhibited using cycloheximide (CHX). After pretreatment with CHX (10 μg/mL), PKCβ inhibitors and VEGF were added simultaneously, and BREC were lysed 3 h later. CHX treatment alone reduced occludin protein levels compared with controls, and addition of VEGF to the CHX-treated samples resulted in a further reduction in occludin levels as compared with CHX treatment alone. Moreover, the VEGF-induced reduction of occludin was blocked by treatment with PKCβ inhibitors (Fig. 6B and E). In addition, transfection of BREC with DN-PKCβ, but not EV or WT-PKCβ, blocked the VEGF-induced decrease in occludin protein levels (Fig. 6C and F). Collectively, these results indicate that VEGF increases occludin degradation via PKCβ activation.
FIG. 6.
PKCβ-mediated occludin reduction by VEGF. A: VEGF-induced loss of occludin content in retinas was prevented by PKCβ inhibitors. The contents of occludin (D) and claudin-5 (G) were quantified after treatment with VEGF and PKCβI or LY379196 (n = 8). B, E, and H: Occludin reduction under CHX treatment was increased by VEGF in BREC, whereas PKCβ inhibitors blocked this effect. LY, LY379196; n = 6. C, F, and I: WT-PKCβ transfection augmented VEGF-induced occludin reduction under CHX treatment in BREC, whereas DN-PKCβ transfection blocked this effect (n = 6); *P < 0.01 vs. control. NS, not significant.
VEGF-induced occludin degradation is mediated via the proteasome.
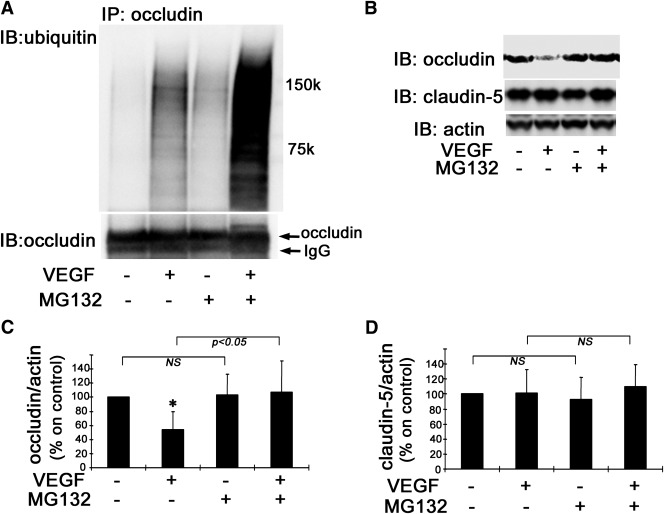
To further investigate these changes in vivo, VEGF and/or the proteasome inhibitor MG132 were intravitreally injected and retinas were isolated 30 min later. VEGF injection increased occludin ubiquitination, and this effect was augmented by MG132 (Fig. 7A). Additionally, MG132 injection blocked the VEGF-induced decrease in occludin content observed 1.5 h after injection (Fig. 7B and C). These treatments had no effect on claudin-5 content (Fig. 7B and D). These data suggest that VEGF-induced occludin degradation requires the ubiquitin-proteasome pathway.
FIG. 7.
Occludin ubiquitination and degradation in vivo. Coinjection of MG132 augments VEGF-induced occludin ubiquitination in vivo as observed by IP and ubiquitin blot, lower panel occludin monoclonal antibody blot (A). Treatment with MG132 prevented VEGF-induced occludin degradation in rat retinas, whereas claudin-5 content was unaffected (B–D) (n = 6–8); *P < 0.01 vs. control. IB, immunoblot; NS, not significant.
The ubiquitin-proteasome system modulates VEGF-induced vascular permeability.
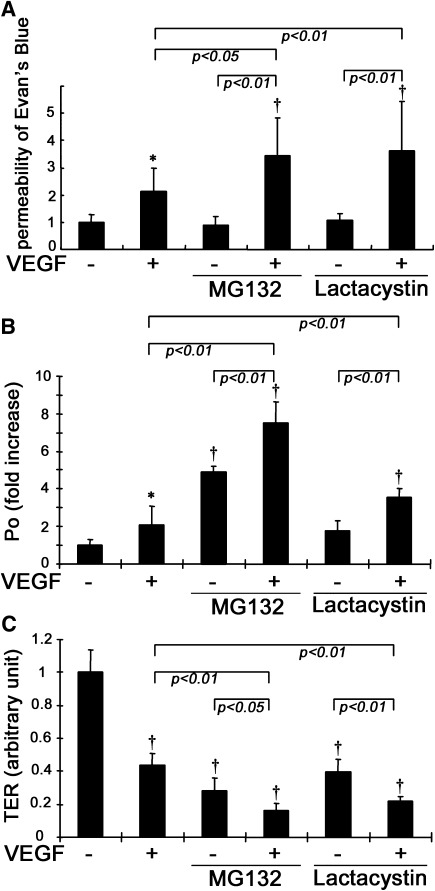
To further understand the role of occludin ubiquitination in vascular permeability, MG132 or lactacystin was intravitreally injected along with VEGF and retinal permeability was assessed by the Evan’s blue assay. MG132 or lactacystin augmented VEGF-induced vascular permeability in rat retinas as measured by dye accumulation, without altering basal levels (Fig. 8A). The flux of 70 kDa dextran in BREC monolayers was also measured after proteasome inhibition. Either VEGF or MG132 alone increased macromolecular flux significantly, and the flux after treatment with both VEGF and MG132 was additive (Fig. 8B). Lactacystin also augmented VEGF-induced macromolecular flux significantly but had no statistically significant effect on flux alone, although a trend toward an increase could be observed. We additionally assessed TER and found that both VEGF and MG132 (or lactacystin) induced a further reduction in TER compared with either VEGF or MG132 (or lactacystin) alone (Fig. 8C). These data suggest that occludin ubiquitination and trafficking rather than proteasomal degradation regulates VEGF-induced vascular permeability both in vivo and in vitro.
FIG. 8.
VEGF-induced vascular permeability was augmented by MG132 or lactacytin. A: MG132 or lactacystin augmented VEGF-induced retinal permeability assessed by the Evan’s blue assay (n = 10–12). B and C: Treatment with both VEGF and MG132 (or lactacystin) resulted in increased endothelial permeability of 70 kDa dextran (B) and TER measurements (C) as compared with VEGF treatment alone in BREC (n = 7 to 8); *P < 0.05 vs. control; †P < 0.01 vs. control.
Proteasome inhibitors augment VEGF-induced TJ trafficking in retinal vasculature.
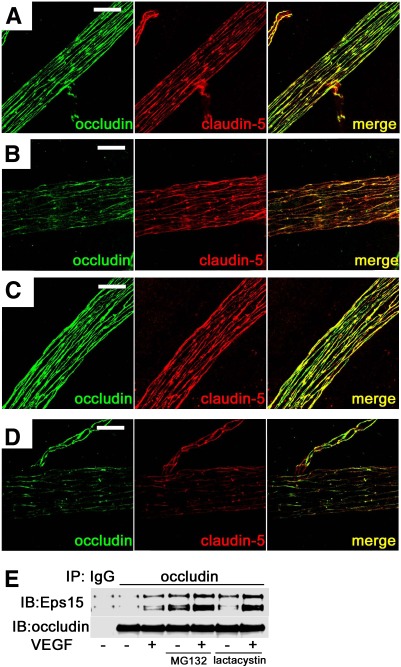
To assess the role of ubiquitination in TJ trafficking in vivo, the effect of MG132 treatment on VEGF-induced TJ redistribution in rat retinal vasculature was assessed. Thirty minutes after intravitreal injection of VEGF, retinas were isolated, fixed, and immunostained for occludin and claudin-5. VEGF decreased the immunoreactivity of occludin at the cell border with increased intracellular punctate staining, as compared with the control (Fig. 9A), and modestly decreased claudin-5 border staining (Fig. 9B). MG132 injection alone did not change the distribution of occludin or claudin-5 as compared with control (Fig. 9C). However, when both VEGF and MG132 were intravitreally injected simultaneously, the cell border staining of occludin and particularly claudin-5 was further decreased compared with VEGF alone (Fig. 9D and Supplementary Figs. III and IV).
FIG. 9.
MG132 augmented VEGF-induced TJ trafficking in rat retinal arterioles. VEGF induced TJ disruption at cell borders and increased intracellular staining of occludin and claudin-5 while simultaneous administration of VEGF and MG132 augmented this effect. (A) Controls, (B) VEGF injection, (C) MG132 injection, and (D) VEGF and MG132 injection. E: Coimmunoprecipitation in BREC showed that VEGF increased the interaction between Eps15 and occludin, and MG132 (or lactacystin) augmented this effect. Scale bar = 20 μm. IB, immunoblot. (A high-quality digital representation of this figure is available in the online issue.)
Previous studies have demonstrated that ubiquitinated occludin interacts with proteins containing an ubiquitin interacting motif that chaperone the endocytosis process (25). The interaction between occludin and Eps15, a trafficking modulator containing an ubiquitin interacting motif, in the presence of MG132 was assessed. Coimmunoprecipitation experiments in BREC demonstrate that VEGF (50 ng/mL) treatment for 30 min increased the interaction between occludin and Eps15, and the proteasome inhibitors MG132 or lactacystin augmented this effect (Fig. 9E). These data suggest that proteasome inhibitors enhanced VEGF-induced TJ trafficking and resultant vascular permeability in endothelial cells through increased occludin ubiquitination and endocytosis.
DISCUSSION
VEGF signaling through PKCβ has been targeted for therapeutic intervention for retinal eye disease. Clinical evidence demonstrates that neutralizing antibodies to VEGF improves visual acuity in conjunction with laser photocoagulation for patients with diabetic macular edema (5). Previous publications have shown that PKCβ transduces a signal for VEGF-induced vascular permeability (6,32) and that a PKCβ-specific inhibitor, ruboxistaurin, partially blocks the progression of visual impairment (7–9,33). However, the downstream effectors from this kinase pathway and a mechanistic explanation for PKCβ regulation of permeability were unknown. Recent work from our laboratory has demonstrated that VEGF-induced occludin phosphorylation on Ser490 and subsequent ubiquitination changes the distribution of TJ proteins in retinal endothelial cells from cell border to intracellular puncta, promoting endothelial permeability (25). Here, we demonstrate that VEGF-induced activation of PKCβ results in phosphorylation of the TJ protein occludin on Ser490 and subsequent ubiquitination that is necessary and sufficient to induce permeability. Because gene deletion studies demonstrate occludin is not required for TJ formation, the data suggest that occludin phosphorylation regulates the trafficking dynamics of other TJ proteins such as claudin-5. Furthermore, these events occur in vivo in the VEGF-induced breakdown of the BRB.
The data implicate PKCβ as specifically involved in occludin phosphorylation downstream of VEGF since two PKCβ inhibitors blocked VEGF-induced occludin phosphorylation and PKCβ could phosphorylate an occludin fragment at Ser490 in vitro. The current studies do not exclude other forms of PKC that may contribute to occludin phosphorylation and vascular permeability, especially from different stimuli. PKC substrate specificity may be achieved through localization of the kinase as well as isoform-specific regulation (34). Future knockdown or gene deletion studies may determine whether Ser490 is an exclusive PKCβ substrate or a target for multiple kinases.
The data presented suggest that the ubiquitin-induced trafficking of occludin, not degradation, promotes VEGF-induced vascular permeability since treatment with proteasome inhibitors augmented VEGF-induced occludin ubiquitination and permeability. The role of ubiquitination and endocytosis of occludin in vascular permeability may extend beyond VEGF. The JNK pathway, which is induced in the diabetic state, activates an ubiquitin E3 ligase of occludin, Itch (35,36). Furthermore, occludin endocytosis has been shown previously to be required for TNF-induced TJ breakdown in vivo (32,37,38). A role for occludin ubiquitination as a common pathway in vascular permeability in response to TNF-α–induced or oxidative stress–induced hyperpermeability remains to be determined. Evidence also exists that claudins may be ubiquitinated leading to endocytosis and degradation. A recent study demonstrates that the ubiquitin E3 ligase LNX1p80 directly binds claudin-1 and overexpression of the ligase in MDCK cells reduces claudin-1, -2, and -4 in a lysosomal dependent pathway. It is noteworthy that occludin, ZO-1, and E-cadherin remain unaffected (39).
Accumulating evidence indicates that Rab family small G proteins contribute to epithelial TJ remodeling (40). In particular, Rab13 contributes to endocytic recycling of occludin in epithelial cells (41). However, the role of this junctional Rab, or other Rab proteins, in VEGF-induced occludin endocytosis in endothelial cells has yet to be examined. Furthermore, VEGF-induced signaling through its receptors leads to the activation of several signaling pathways including Src (42), which may contribute to the breakdown of BRB (43). We did not evaluate the role of Src in PKCβ-induced occludin modification and the relationship of this signaling pathway to PKC-induced permeability remains to be determined.
We have shown that PKCβ is able to directly phosphorylate COOH-terminal fragments of occludin on Ser490 by an in vitro kinase assay. It is noteworthy that the purified occludin fragments appeared to be present in both monomer and dimer forms, and phosphorylation of occludin on Ser490 predominantly occurred on the dimer observed by SDS-gel. Whether dimerization of phospho-occludin is a result of the gel conditions used or represents a preferred state of the native protein after phosphorylation remains unclear. However, dimerization of the COOH-terminal coiled-coil region of occludin has been reported previously and implicated in regulation of the TJ complex during oxidative stress (44,45). The data presented here demonstrate that occludin may be a direct substrate of PKCβ. Previous studies suggest Ser490 phosphorylation reduces interaction of occludin with ZO-1 and promotes Itch-dependent ubiquitination (25).
In conclusion, VEGF-stimulated PKCβ activation regulates retinal vascular permeability through occludin phosphorylation and ubiquitination in vivo. These studies suggest occludin ubiquitination promotes trafficking of TJ proteins creating regions of broken junctions and supports a role for occludin in the regulation of vascular permeability. Future studies that determine the contribution of PKC and occludin posttranslational modifications to retinal pathology in diabetes, vein and artery occlusions, and uveitis will provide important novel insight into the molecular mechanisms of macular edema.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health Grant EY012021 and support from the Juvenile Diabetes Research Foundation and the Jules and Doris Stein Professorship from Research to Prevent Blindness (all D.A.A.).
No potential conflicts of interest relevant to this article were reported.
T.M. contributed to the study design, performed experiments, and wrote the manuscript. T.F. contributed to performing experiments and wrote the manuscript. C.L. performed experiments. D.A.A. contributed to the study design and interpretation and wrote the manuscript. D.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1367/-/DC1.
T.M. is currently affiliated with the Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, Sakyo, Kyoto, Japan.
C.L. and D.A.A. are currently affiliated with the Department of Ophthalmology and Visual Sciences, Kellogg Eye Center, Ann Arbor, Michigan.
REFERENCES
- 1.Antonetti DA, Barber AJ, Bronson SK, et al. JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 2006;55:2401–2411 [DOI] [PubMed] [Google Scholar]
- 2.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 2004;36:1206–1237 [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 4.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805–2816 [DOI] [PubMed] [Google Scholar]
- 5.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci 2006;47:5106–5115 [DOI] [PubMed] [Google Scholar]
- 7.Davis MD, Sheetz MJ, Aiello LP, et al. PKC-DRS2 Study Group Effect of ruboxistaurin on the visual acuity decline associated with long-standing diabetic macular edema. Invest Ophthalmol Vis Sci 2009;50:1–4 [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Davis MD, Girach A, et al. PKC-DRS2 Group Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology 2006;113:2221–2230 [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Vignati L, Sheetz MJ, et al. Oral protein kinase c beta inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study and the Protein kinase C beta Inhibitor-Diabetic Retinopathy Study 2. Retina 2011;31:2084–2094 [DOI] [PubMed] [Google Scholar]
- 10.Suzuma K, Takahara N, Suzuma I, et al. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA 2002;99:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor. Diabetes 1997;46:1473–1480 [DOI] [PubMed] [Google Scholar]
- 12.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001;2:285–293 [DOI] [PubMed] [Google Scholar]
- 13.Bauer HC, Traweger A, Zweimueller-Mayer J, et al. New aspects of the molecular constituents of tissue barriers. J Neural Transm 2011;118:7–21 [DOI] [PubMed] [Google Scholar]
- 14.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003;161:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000;11:4131–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Suzuki T, Seth A, et al. PKCzeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J 2011;437:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Elias BC, Seth A, et al. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA 2009;106:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias BC, Suzuki T, Seth A, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 2009;284:1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raleigh DR, Boe DM, Yu D, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 2011;193:565–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto M, Ramirez SH, Sato S, et al. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol 2008;172:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999;274:23463–23467 [DOI] [PubMed] [Google Scholar]
- 22.Barber AJ, Antonetti DA, Gardner TW, The Penn State Retina Research Group Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci 2000;41:3561–3568 [PubMed] [Google Scholar]
- 23.Sundstrom JM, Tash BR, Murakami T, et al. Identification and analysis of occludin phosphosites: a combined mass spectrometry and bioinformatics approach. J Proteome Res 2009;8:808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol 2003;4:491–497 [DOI] [PubMed] [Google Scholar]
- 25.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 2009;284:21036–21046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval M, Bédard-Goulet S, Delisle C, Gratton JP. Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. J Biol Chem 2003;278:20091–20097 [DOI] [PubMed] [Google Scholar]
- 27.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci 2008;49:2635–2642 [DOI] [PubMed] [Google Scholar]
- 28.Phillips BE, Cancel L, Tarbell JM, Antonetti DA. Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2008;49:2568–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalfant CE, Ohno S, Konno Y, et al. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol 1996;10:1273–1281 [DOI] [PubMed] [Google Scholar]
- 30.Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. In The Blood-Brain Barrier: Biology and Research Protocols. Nag S, Ed. Totowa, New Jersey, Humana Press, 2003, p. 365–374 [DOI] [PubMed] [Google Scholar]
- 31.Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 2001;42:789–794 [PubMed] [Google Scholar]
- 32.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol 1994;267:L223–L241 [DOI] [PubMed] [Google Scholar]
- 33.Scott IU, Edwards AR, Beck RW, et al. Diabetic Retinopathy Clinical Research Network A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114:1860–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 2008;88:1341–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneto H, Nakatani Y, Miyatsuka T, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 2004;10:1128–1132 [DOI] [PubMed] [Google Scholar]
- 36.Gao M, Labuda T, Xia Y, et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science 2004;306:271–275 [DOI] [PubMed] [Google Scholar]
- 37.Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 2010;189:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med 1989;169:1977–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi S, Iwamoto N, Sasaki H, et al. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J Cell Sci 2009;122:985–994 [DOI] [PubMed] [Google Scholar]
- 40.Nishimura N, Sasaki T. Rab family small G proteins in regulation of epithelial apical junctions. Front Biosci 2009;14:2115–2129 [DOI] [PubMed] [Google Scholar]
- 41.Morimoto S, Nishimura N, Terai T, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem 2005;280:2220–2228 [DOI] [PubMed] [Google Scholar]
- 42.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 2006;312:549–560 [DOI] [PubMed] [Google Scholar]
- 43.Scheppke L, Aguilar E, Gariano RF, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest 2008;118:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter JK, Castro V, Voss M, et al. Redox-sensitivity of the dimerization of occludin. Cell Mol Life Sci 2009;66:3655–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter JK, Rueckert C, Voss M, et al. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann N Y Acad Sci 2009;1165:19–27 [DOI] [PubMed] [Google Scholar]