The upregulation of plasma inflammatory biomarkers in individuals with metabolic syndrome implies that activation of the innate immune response contributes to the pathogenesis of type 2 diabetes (1). Today, a large array of studies has demonstrated that activation of inflammatory pathways underlies obesity-associated insulin resistance at the liver and fat (2–4). However, little is known regarding whether obesity induces inflammation in the brain thereby disrupting the ability of insulin to control glucose homeostasis.
Hotamisligil et al. (5) first established a link between obesity and the increased production of inflammatory molecules by demonstrating that tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, is overexpressed in the adipose tissue of obese mice. This finding was confirmed in humans with obesity and insulin resistance (6,7). TNF-α induces peripheral insulin resistance in rodents (8,9) and alters insulin sensitivity and glucose homeostasis in humans (10,11). In fact, subjects with chronic inflammatory disease who are treated with TNF inhibitor show a 60% reduction in diabetes rates (12). Downstream of the inflammatory process lies the inhibitor of κB kinase (IKK-β) complex and its target, nuclear factor-ĸB (NF-ĸB), a transcription factor that regulates the expression of inflammatory genes (2–4) and mediates peripheral insulin resistance associated with overnutrition (2–4). In parallel, the c-Jun amino-terminal kinase (JNK), which can be activated in response to TNF-α or other stressors, is also implicated in insulin resistance of diabetic mice (2–4).
NF-ĸB–mediated gene expression is regulated in part through the Toll-like receptors (TLRs), which serve to activate proinflammatory signaling cascades upon recognition of pathogen-associated molecular patterns (2–4). Of these, TLR4 mediates fatty acid–induced peripheral insulin resistance (13), thereby highlighting its importance in inflammation and metabolic dysfunction. In parallel, overnutrition induces endoplasmic reticulum (ER) stress followed by a triggering of the compensatory unfolded protein response (UPR) (2–4). Chronic activation of ER stress in the liver triggers proinflammatory signals and induces insulin resistance, while UPR activates JNK and NF-ĸB to impair insulin action (2–4).
Although much remains to be explored, these findings collectively highlight a crucial role of ER stress and inflammation in liver and fat to impair insulin signaling and dysregulate glucose homeostasis in obesity and diabetes. The key question that remains to be addressed is whether overnutrition/obesity induces ER stress and inflammation in the central nervous system to disrupt the ability of insulin to control glucose homeostasis. If this is the case, do any of the key players that are highlighted above play a role in this dysregulation?
In fact, high-fat feeding induces ER stress and UPR as well as the IKK-β/NF-κB proinflammatory pathway in the hypothalamus of rodents (14,15). The activation of hypothalamic ER stress and inflammation impair the ability of central insulin and leptin to inhibit appetite. TNF-α induces ER stress in the hypothalamus (16), while fatty acids activate hypothalamic TLR4 to impair the anorectic effect of central leptin (17). In fact, hypothalamic leptin’s ability to inhibit food intake is restored in mice with neuronal-specific knockout of the TLR adaptor protein MyD88 (18), while anti-inflammatory cytokines such as interleukin (IL)-10 reduce hypothalamic inflammation and mediate the ability of exercise to improve the anorectic control of central insulin and leptin in diet-induced obese rats (19). Although mounting evidence indicates that high-fat feeding induces hypothalamic ER stress and inflammation, the metabolic consequence has been limited to the dysregulation of food intake.
In this issue of Diabetes, Milanski et al. (20) have linked hypothalamic inflammation to a disruption of the brain-liver axis that controls glucose homeostasis in obese rodents through well-designed and executed experiments. The authors first confirm that consumption of a high-fat diet increased hypothalamic expression of the inflammatory cytokines TNF-α and IL-1β in rats, then demonstrate that pretreatment with central anti-TLR4 antibody or an anti–TNF-α monoclonal antibody significantly reduced expression of these cytokines and inhibited NF-ĸB in the hypothalamus. Neutralization of hypothalamic TLR4 or TNF-α in obese rats improved glucose tolerance (as assessed by intraperitoneal glucose tolerance test), and this was associated with improved hepatic insulin signal transduction (insulin receptor substrate → Akt → FoxO1). Next, the authors reproduced earlier findings that TLR4 and TNF-α receptor 1 knockout mice were protected against diet-induced insulin resistance. This was further confirmed by the fact that both TLR4 and TNF-α receptor 1 knockout mice were protected from hypothalamic fatty acid–induced hepatic insulin resistance, suggesting that hypothalamic events may represent an important portion of the total body phenotype of TLR4 and TNF-α receptor 1 knockout mice.
To assess whether changes in hepatic insulin signaling are responsible for the improved glucose tolerance, the authors performed a pyruvate tolerance test, a hyperinsulinemic-euglycemic clamp, and assessed changes in hepatic gluconeogenic gene expression (PEPCK and glucose-6-phosphatase [G6Pase]) in obese rats with hypothalamic inflammatory neutralization. Inhibition of hypothalamic inflammation reduced pyruvate-induced gluconeogenesis and normalized insulin-mediated suppression of glucose production and hepatic PEPCK and G6Pase gene expression in obese rats. In addition, both vagotomy and pharmacological inhibition of muscarinic receptors reversed the metabolic benefits resulting from hypothalamic anti-inflammation, indicating a brain-liver neural axis is required to restore hepatic insulin sensitivity.
Although pharmacological inhibition of hypothalamic TLR4—but not TNF-α—led to a substantial weight loss in obese rats, inhibition of hypothalamic TLR4 and TNF-α both restored the ability of insulin to inhibit glucose production through the brain-liver axis, suggesting that enhancement of hepatic insulin sensitivity was not secondary to weight loss. Neutralization of hypothalamic TLR4 or TNF-α in obese rats also reduced hepatic steatosis, which could have led to an enhancement in hepatic insulin sensitivity. However, despite the fatty liver phenotype of LDL receptor knockout mice, inhibition of hypothalamic inflammation in LDL receptor knockout mice fed a high-fat diet showed improved hepatic insulin signal transduction. Thus, these data collectively suggest that independent of changes in body weight and hepatic lipid accumulation, inhibition of diet-induced hypothalamic inflammation restores the ability of insulin to stimulate hepatic signal transduction and suppress glucose production in obese rodents.
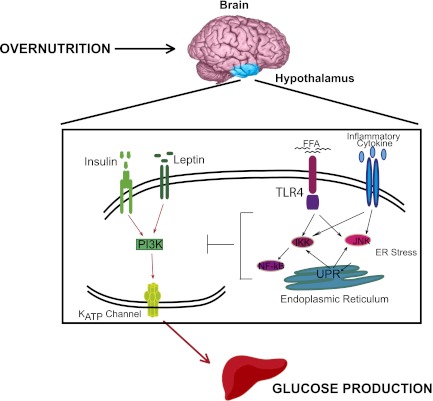
Of note, insulin triggers signaling cascades and activates ATP-sensitive potassium (KATP) channels in the hypothalamus to inhibit glucose production (21), while central leptin similarly enhances insulin-mediated inhibition of glucose production in normal rats (22). Thus, it remains to be assessed whether inhibition of hypothalamic inflammation restores the central ability of insulin and/or leptin signaling to activate a brain-liver circuit to inhibit glucose production in obese rodents. In addition, it would be important to investigate the mechanistic links between hypothalamic TLR4 or TNF-α signaling and central insulin/leptin resistance, and to further assess whether hypothalamic activation of ER stress, UPR, JNK, and/or NF-ĸB impair central insulin/ leptin signaling to dysregulate glucose production (Fig. 1).
FIG. 1.
Working hypothesis. Overnutrition induces ER stress and inflammation in the hypothalamus and activates downstream signaling effectors and processes such as IKK/NF-ĸB, JNK, and UPR to impair insulin and/or leptin signal transduction to activate KATP channels and inhibit hepatic glucose production. FFA, free fatty acid; PI3K, phosphatidylinostiol 3-kinase.
Lastly, obesity is not only associated with hypothalamic inflammation and gliosis in rodents but also induces gliosis in the hypothalamus of humans (23). Given that intranasal insulin delivery lowers plasma glucose levels in humans (24,25) while activation of KATP channels in the brain of humans is implicated to lower glucose production (26), a possibility remains that obesity induces insulin resistance in the brain and consequently dysregulates glucose homeostasis in humans as in rodents.
ACKNOWLEDGMENTS
The laboratory of T.K.T.L. is supported by grants from the Canadian Diabetes Association (OG-3-10-3048), the Canadian Institutes of Health Research (MOP-86554 and MOP-82701), the Canada Research Chair in Obesity, the John Kitson McIvor Endowed Chair in Diabetes Research, and the Early Researcher Award from the Ontario Ministry of Research and Innovation (ER08-05-141). P.I.M. is supported by an Ontario Graduate Scholarship and a Banting and Best Diabetes Centre/University Health Network graduate award. T.K.T.L. holds the John Kitson McIvor Endowed Chair in Diabetes Research and the Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1455.
REFERENCES
- 1.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–1292 [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363–374 [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 1995;95:2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 9.Ventre J, Doebber T, Wu M, et al. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 1997;46:1526–1531 [DOI] [PubMed] [Google Scholar]
- 10.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis 2005;64:765–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley TL, Zanni MV, Johnsen S, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 2011;96:E146–E150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 2011;305:2525–2531 [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis RG, Arruda AP, Romanatto T, et al. TNF-α transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus. Neuroscience 2010;170:1035–1044 [DOI] [PubMed] [Google Scholar]
- 17.Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 2009;29:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinridders A, Schenten D, Könner AC, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 2009;10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Milanski M, Arruda AP, Coope A, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 2012;61:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 [DOI] [PubMed] [Google Scholar]
- 22.German J, Kim F, Schwartz GJ, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 2012;61:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippi BM, Mighiu PI, Lam TK. Is insulin action in the brain clinically relevant? Diabetes 2012;61:773–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishore P, Boucai L, Zhang K, et al. Activation of K(ATP) channels suppresses glucose production in humans. J Clin Invest 2011;121:4916–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]