Abstract
Stressors contribute to thrombosis and insulin resistance. Since obesity-related adipose inflammation is also involved in these pathological states, we assumed that stress correlates with adipose inflammation. Male mice were subjected to 2-week intermittent restraint stress. Expression of plasma lipids, monocyte/macrophage markers (CD11b, CD68, and F4/80), proinflammatory cytokines (monocyte chemoattractant protein-1 [MCP-1], tumor necrosis factor-α, and interleukin-6), adiponectin, heat shock protein 70.1 (HSP70.1), and coagulation factors (plasminogen activation inhibitor-1 [PAI-1] and tissue factor [TF]) in blood and inguinal white adipose tissue (WAT) was determined using immunohistochemistry, enzyme-linked immunosorbent assay, and RT-PCR, respectively. Glucose metabolism was assessed by glucose tolerance tests (GTTs) and insulin tolerance tests, and expression of insulin receptor substrate-1 (IRS-1) and glucose transporter 4 (GLUT4) in WAT. To examine effects of MCP-1 blockade, animals were treated with control or neutralizing antibody, or transplanted with control or 7ND (dominant-negative form of MCP-1)-overexpressing adipose-derived stromal cells (ADSCs). Stress increased monocyte accumulation, free fatty acids, proinflammatory cytokine, and HSP70.1 and reduced adiponectin. Adipose stromal cells highly expressed MCP-1. The stress-induced adipose inflammation increased PAI-1 and TF but did not give rise to thrombus formation. Without any changes in GTT, stress worsened insulin sensitivity and decreased IRS-1 and GLUT4 in WAT. Neutralizing antibody and 7ND-ADSCs reversed stress-induced adipose inflammation, procoagulant state, and insulin resistance. Stress evoked adipose inflammation to increase coagulation factors and impair insulin sensitivity through adipose-derived MCP-1.
The organic response to stress implicates two major components of the stress system: the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis. Activation of both systems promotes the secretion of adrenal catecholamines and glucocorticoid to the alternate systemic hormonal and immune responses (1,2). Chronic psychological stress has been linked to a number of negative health consequences, including effects on the cardiovascular, endocrine, and immune systems. As stress is known to induce thromboembolism and perturb glucose metabolism, leading to the onset of type 2 diabetes (3–5), it is important to understand how stressors perturb homeostasis of the coagulation system and glucose metabolism.
Adipose tissue has long been known to act as a heat insulator and storage site of excess free fatty acids (FFAs) for release when needed. However, adipose tissue is also a regulatory organ that plays a critical role in various abnormalities such as dyslipidemia, insulin resistance, type 2 diabetes, and cardiovascular disease (6). Obesity and metabolic syndrome are associated with dysfunctional adipose tissue composed of enlarged adipocytes and chronic inflammation, resulting in insulin resistance and a prothrombotic state (7). Macrophage accumulation in white adipose tissue (WAT) plays a role in the chronic low-grade inflammatory response via secretion of FFAs followed by lipolysis (8) and inflammatory molecules including tumor necrosis factor-α (TNF-α) (9), interleukin-6 (IL-6) (10), monocyte chemoattractant protein-1 (MCP-1) (11), and plasminogen activation inhibitor-1 (PAI-1) (12). These adipose tissue–derived proinflammatory cytokines act synergistically to produce a state of imbalance in both the coagulation system and glucose metabolic pathways. In particular, adipose-derived MCP-1 is critical in exacerbating insulin resistance and a prothrombotic state in the adipose tissue of obese individuals (13,14). Thus, chronic adipose inflammation evoked by MCP-1 is one of the most important mechanisms underlying obesity-related diseases.
There are common consequences of the pathological reactions to stressors and obesity-induced adipose inflammation, including a decline in insulin sensitivity, prothrombotic state, and increased plasma MCP-1 levels (15). Under stressful conditions, heat shock proteins (HSPs), which function as a molecular chaperone intercellularly, are also released as a “danger signal” to immune cells to promote immune responses and protect cells. Indeed we reported that restraint stress induced HSP70.1 in adipose tissue (16). Furthermore we also reported that acute restraint stress causes a thrombotic tendency with the induction of PAI-1 and tissue factor (TF) derived from inguinal WATs (16,17). Based on this finding, we tested the hypothesis of the involvement of adipose inflammation in the pathological reactions to stress.
The current study showed that chronic restraint stress evoked an inflammatory response in adipose tissue, resulting in perturbation of glucose metabolism and a procoagulant state. We focused on the effects of adipose tissue–derived MCP-1 and examined whether 7ND, a deletion mutant of human MCP-1 (lacking the amino-terminal amino acids 2–8) (18), could suppress the stress-induced inflammatory responses in adipose. To provide stable 7ND secretion in vivo, we implanted 7ND-overexpressing, adipose-derived stromal cells (7ND-ADSCs), which are capable of intraperitoneal engraftment and are a reliable carrier of 7ND (19,20). Furthermore, we examined whether MCP suppression with 7ND-ADSCs improved insulin resistance and the prothrombotic state.
RESEARCH DESIGN AND METHODS
Animals and restraint stress procedure.
Eight-week-old male C57BL/6J mice (Chubu Kagaku Shizai Co. Ltd., Nagoya, Japan) were housed two per cage under standard conditions (23 ± 1°C, 50 ± 5% humidity), with a 12-h light/dark cycle in a viral pathogen–free facility and handled properly according to our previous study (16). Animals were randomly assigned to the control or stress group (n = 12, respectively). Control mice were left undistributed and allowed contact with each other, while stressed mice were individually subjected to 2 h/day of immobilization stress for 1 or 2 weeks (between 10:00 a.m. and 12:00 p.m., 6 days/week), as described previously (16). In brief, chronic stress was applied using a 50-mL conical centrifuge tube with multiple punctures that allowed for a close fit to mice. Body weight and food intake were monitored during this period. After the 2-week restraint, the mice were killed to collect blood, inguinal adipose, and skeletal muscle samples for total RNA extraction and paraffin embedding. Plasma total cholesterol, triglyceride, and FFA levels were measured with a commercially available enzymatic kit (Wako, Osaka, Japan) (21). All studies were completed between 10:00 a.m. and 12:00 p.m. The animals were handled in accordance with the guidelines of the Institutional Animal Care and Use Committee of Nagoya University.
Anti–MCP-1 treatment with neutralizing antibody.
To determine the effects of blocking MCP-1, nonstressed and stressed mice (n = 5, respectively) received three intraperitoneal injections of 50 μg per dose of anti–MCP-1 monoclonal antibody (MAB479) or the nonspecific, isotype-matched control antibody (both obtained from R&D Systems) at day 0, 4, and 8.
Quantitative PCR.
Total RNA extraction, reverse transcription, and quantitative PCR were performed as described previously (22). The primer sequences used in this study are listed in Supplementary Table 1. RNA amounts were each normalized to their respective β-actin mRNA.
Histological analysis of inguinal adipose tissue.
The inguinal WAT was processed, and sections (5-μm thickness) were stained with hematoxylin-eosin according to standard histological procedures. Stained slides were viewed blindly and independently by two investigators under a microscope at ×200 magnification. The size of inguinal adipocytes was estimated using Win ROOF version 5.02 (MITANI Co., Fukui, Japan).
Immunohistochemistry and enzyme-linked immunosorbent assay.
Immunohistochemistry using antibodies for CD11b (1:100; Abcam), MCP-1 (1:200; RSD), fibrin (10 μg/mL) (22), and FLAG (1:500; CST) was performed on the adipose tissue using standard protocols (23).Two investigators blindly and independently counted CD11b-positive and -negative cells under a microscope at ×200 magnification. The rate of CD11b-positive cells was calculated as the sum of the number of nuclei of CD11b-positive cells divided by the total number of nuclei in a slide. Ten microscopic fields were chosen in three different sections per mouse for examination.
Mouse MCP-1, 7ND, insulin, TNF-α, and IL-6 levels in the serum were quantified using mouse and human CCL2 ELISA Ready-SET-Go! (human CCL2 for detection of 7ND; eBioscience, Kobe, Japan), mouse insulin (Mercodia), and TNF-α and IL-6 ELISA kits (R&D Systems), respectively, according to the instructions provided by the manufacturer.
Intraperitoneal glucose and insulin tolerance tests.
After 2 weeks of daily stress, the mice were subjected to intraperitoneal glucose and insulin tolerance tests (GTTs and ITTs) according to standard methods. In brief, for GTT, mice were fasted overnight and then challenged with 2 g/kg d-glucose (Sigma-Aldrich, St. Louis, MO), followed by serial assessment of blood glucose up to 120 min using a blood glucose level monitor (Glutest Ace; Sanwa Kagaku Kenkyusho Co., Nagoya, Japan). For ITT, the mice were fasted for 16 h before testing. Insulin (0.75 units/kg; Actrapid Penfill, NovoNordisk) was injected intraperitoneally, and blood glucose was measured.
Preparation of control and 7ND-ADSCs.
Mouse ADSCs were established as described previously (24). Primary ADSCs were maintained in α-minimal essential medium (αMEM; Gibco-BRL, Carlsbad, CA) containing 10% FBS. Cultures at passages four to eight were used. Lentiviral construct (EGFP-pBGJR, a gift from Dr. S. Rivella, Cornell University, New York, NY) containing mouse FLAG-tagged deletion mutant MCP-1 (7ND; a gift from K.E.) or control vector was transduced into ADSCs. Transfection was confirmed by the presence of EGFP or 7ND production before use. Control and 7ND-ADSCs were cultured in αMEM containing 10% FBS and 1% penicillin/streptomycin. Control ADSCs and 7ND-ADSCs (1 × 106 cells) were suspended in 200 μl PBS and injected intraperitoneally into control and stressed mice on days 0 and 7 (n = 8–10).
Statistical analysis.
Data are expressed as mean ± SD in all other analyses. Differences were compared by one-way ANOVA followed by Fisher least significant differences test and considered significant at P < 0.05. Frequencies were analyzed with the χ2 test.
RESULTS
Stress induces monocyte accumulation in the adipose tissue.
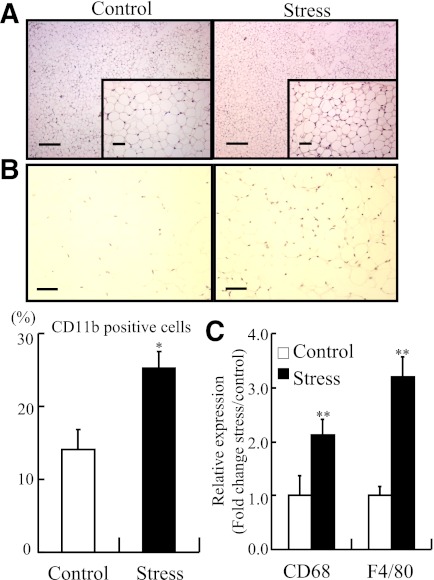
To determine whether restraint stress evokes an inflammatory response in WAT, we subjected 8-week-old male C57BL/6J mice to 1 or 2 weeks of daily restraint stress. Acute restraint stress increased mononuclear cells in WAT within 2 h, but this effect was transient. Negligible accumulation of mononuclear cells was observed in the adipose tissues of control mice and mice stressed for 1 week (data not shown). However, mononuclear cell infiltration was noted in adipose tissues from the mice that underwent restraint stress over 2 weeks (Fig. 1A). Subsequent expression analysis of macrophage surface markers demonstrated that 2 weeks of stress significantly increased the proportion of CD11b-positive cells in adipose tissues compared with tissues from control mice (Fig. 1B). Similarly, the expression levels of CD68 and F4/80 were also significantly increased in the adipose tissue of stressed mice (Fig. 1C).
FIG. 1.
Monocytes accumulated in inguinal adipose of stressed mice. The stressed mice were individually subjected to 2 h/day of immobilization stress for 2 weeks. Inguinal adipose tissues from stressed and control mice were analyzed by hematoxylin-eosin staining, CD11b immunostaining, and quantitative RT-PCR for CD68 and F4/80. A: Mononuclear cells accumulated in inguinal adipose tissues after the 2-week restraint stress. Original magnification ×40; bar, 250 μm (top). Original magnification × 200; bar, 50 μm (bottom). B: Accumulation of CD11b-positive cells (monocytes) increased in adipose tissue from the stressed mice (original magnification ×200; bar, 50 μm) (top). Quantitative analysis of CD11b-positive cells relative to total nuclear number (bottom). Data are expressed as mean ± SD. *P < 0.05, compared with the control mice; n = 12 in each group. C: Quantitative analysis of CD68 and F4/80 expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the control mice; n = 12, respectively. (A high-quality digital representation of this figure is available in the online issue.)
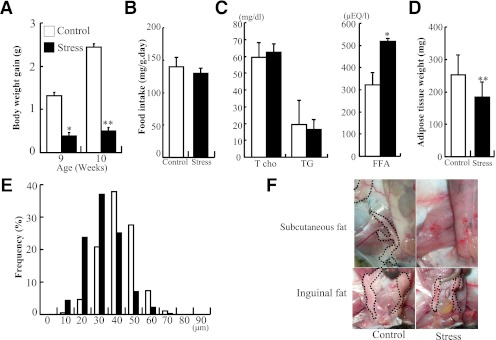
Body weight gain was significantly reduced in the stressed mice compared with the control, even after 1 week of daily stress (Fig. 2A), even though the mice in both groups consumed the same amount of food (Fig. 2B). Analysis of plasma lipid composition in stressed mice showed increase in FFA concentration and no change in total cholesterol and triglyceride levels (Fig. 2C). The weight of inguinal adipose tissue was significantly lower in the stressed mice (weight, 182 ± 46 vs. 252 ± 58 mg; n = 12 for each group) (Fig. 2D). The stressed mice also showed a higher frequency of smaller adipocytes in the inguinal adipose tissue compared with the control mice (average adipocyte diameter, 36.4 ± 11 vs. 46.3 ± 10 μm) (Fig. 1A and Fig. 2E). Subcutaneous adipose tissue was hardly detectable in inguinal subcutaneous fat pad, and visceral inguinal adipose shrank in the stressed mice (Fig. 2F). The results indicate that restraint stress increases FFAs followed by lipolysis and evokes an inflammatory response in adipose tissue of nonobese subjects without alterations in diet.
FIG. 2.
Restraint stress reduced weight gain and adipose. Body weight and inguinal adipose of the control and stressed mice were accurately weighed, and cell sizes in the collected adipose were estimated under a microscope at ×200 magnification using image analysis software. A: Body weight gain of the control and stressed mice. B: Food intake was comparable between the control and stressed mice; n = 12 in each group. C: Plasma fat and fatty acid composition of control and stressed mice. D: Weight of inguinal adipose tissue was decreased in the stressed mice. E: Distribution of adipocyte size in inguinal adipose from the control and stressed mice. Adipose cell size was decreased in the stressed mice. F: Subcutaneous and inguinal fat pad. Circle dot lines denote adipose tissue. Data are expressed as mean ± SD. *P < 0.05 and **P < 0.01, compared with the control mice; n = 12 in each group. (A high-quality digital representation of this figure is available in the online issue.)
Stress induces MCP-1 and proinflammatory cytokines in adipose tissues.
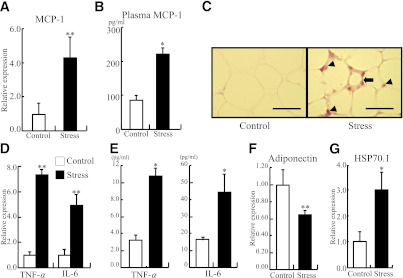
Expressions of MCP-1, TNF-α, and IL-6 were significantly increased in adipose tissues after the 2-week restraint stress compared with the control animals (MCP-1, 100 ± 61 vs. 440 ± 120%; TNFα, 100 ± 20 vs. 732 ± 110%; IL-6, 100 ± 50 vs. 493 ± 92%, respectively) (Fig. 3A and D). Plasma MCP-1, TNF-α, and IL-6 levels were also elevated in the stressed group over those in control mice (Fig. 3B and E). MCP-1 was highly expressed in stromal cells and monocytes in adipose tissue from the stressed mice (Fig. 3C). However, the expression of the anti-inflammatory adipokine, adiponectin, was significantly reduced in the stressed mice compared with the control (100 ± 17 vs. 64.9 ± 4.6%) (Fig. 3F). The expression of HSP70.1 was increased in adipose of stressed mice, which is concordant with our previous report (Fig. 3G) (16). These data indicated that adipose tissues of the stressed mice produced high levels of proinflammatory cytokines, including MCP-1.
FIG. 3.
Restraint stress induced proinflammatory cytokine expression and reduced adiponectin expression in adipose tissue. Inguinal adipose tissues from the stressed and control mice were analyzed by quantitative RT-PCR for MCP-1, TNF-α, IL-6, and adiponectin, and by immunohistochemistry for MCP-1. Plasma level of MCP-1 from both groups was also analyzed using a mouse CCL2 ELISA kit. A: Quantitative analysis of MCP-1 expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the control mice; n = 12 in each group. B: Plasma MCP-1 level was elevated in the stressed group. Data are expressed as mean ± SD. *P < 0.05, compared with the control mice. C: MCP-1 was highly expressed in stromal cells (arrow) and monocytes (arrowheads) in adipose tissues from the stressed mice. Original magnification ×400; bar, 25 μm. D: Quantitative analysis of TNF-α and IL-6 expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the control mice. E: Plasma TNF-α and IL-6 levels were elevated in the stressed group. Data are expressed as mean ± SD. *P < 0.05, compared with the control mice. F: Quantitative analysis of adiponectin expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the control mice. G: Quantitative analysis of HSP-70.1 expression in adipose tissue. Data are expressed as mean ± SD. *P < 0.05, compared with the control mice. (A high-quality digital representation of this figure is available in the online issue.)
Stress causes deterioration of glucose metabolism and increase in procoagulant factors.
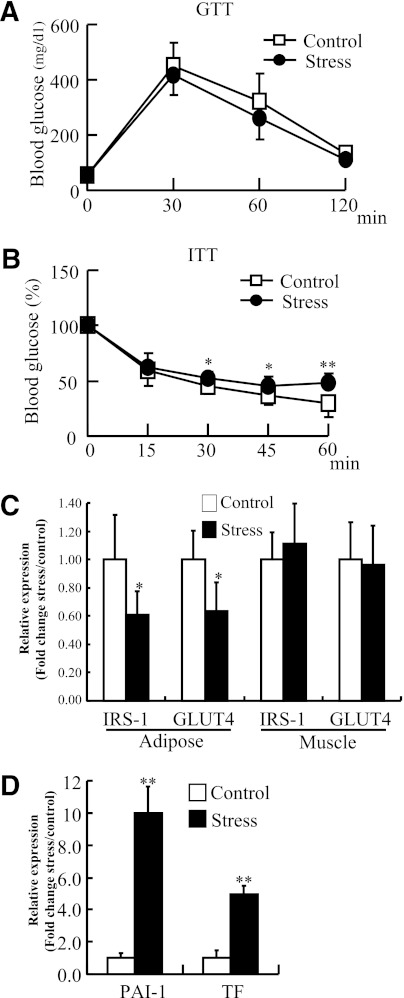
There was no significant difference in glucose tolerance between the control and stressed groups (Fig. 4A). However, insulin tolerance was significantly worse in the stressed mice at all time points after 30 min (Fig. 4B). We examined the mechanism underlying this effect on insulin tolerance in the stressed mice by measuring the expressions of insulin receptor substrate-1 (IRS-1) and glucose transporter 4 (GLUT4) in adipose tissue and skeletal muscle. Restraint stress reduced both expressions in adipose tissues compared with the levels in control mice (IRS-1, 100 ± 32% in control vs. 60.6 ± 18%; GLUT4, 100 ± 23% in control vs. 63.6 ± 21%), but not in skeletal muscle (Fig. 4C). Plasma insulin concentrations were unchanged after repeated stress (control and stressed, 143 ± 53 and 150 ± 55 pg/mL).
FIG. 4.
Restraint stress worsened glucose metabolism and promoted a prothrombotic state. After 2 weeks of daily stress, intraperitoneal glucose tolerance and insulin tolerance testing were performed according to standard methods. The expressions of IRS-1, GLUT4, PAI-1, and TF in adipose tissues were analyzed with quantitative RT-PCR. A: Glucose tolerance was comparable between the control and stressed mice. B: Insulin tolerance was significantly deteriorated in the stressed mice after 30 min. Data are expressed as mean ± SD. *P < 0.05 and **P < 0.01; n = 12 in each group. C: Quantitative analysis of IRS-1 and GLUT4 expression in adipose tissue and skeletal muscle. Data are expressed as mean ± SD. *P < 0.05, compared with the control mice; n = 12, respectively. D: Quantitative analysis of PAI-1 and TF expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the control mice.
We next analyzed the expressions of PAI-1 and TF in the mouse adipose tissue and showed that daily restraint stress significantly increased the expression levels of prothrombotic factors in adipose tissues over those in control tissues (PAI-1, 100 ± 26 vs. 998 ± 180%; TF, 100 ± 63 vs. 494 ± 65%, respectively) (Fig. 4D), similar to acute restraint stress (16). However, thrombus formation was not detected in adipose tissue and kidney using immunohistochemistry for fibrin (data not shown). These results indicate that a 2-week daily restraint stress regimen could induce adipose inflammation that worsens insulin tolerance and increases procoagrant factors, which did not give rise to thrombus formation.
MCP-1–neutralizing antibody suppresses adipose inflammation in stressed mice.
To determine the effects of blocking MCP-1 on adipose inflammation, we quantified monocyte infiltration in adipose tissues of the nonstressed and stressed mice. Neither control nor MCP-1 antibody altered monocyte infiltration in adipose tissues of nonstressed mice (data not shown). Control antibody treatment did not suppress monocyte accumulation induced by the stress. Meanwhile, MCP-1 antibody treatment reduced stress-induced accumulation of CD11b-positive cells (control vs. MCP-1 antibody, 24.8 ± 2.8 vs. 14.7 ± 1.5%; P < 0.05).
7ND-ADSCs intraperitoneally implanted continuously release 7ND in vivo.
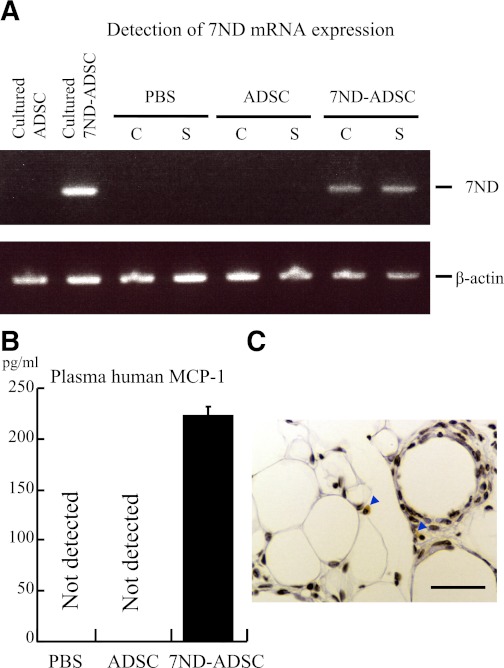
PBS, control ADSCs, or 7ND-ADSCs were intraperitoneally injected on day 0 and 7 of the daily restraint stress. The release of 7ND in vivo was then examined in the stressed and control mice on day 14. These treatments did not affect body weight gain, food intake, or weight of adipose tissues (data not shown). In addition, expression of 7ND was detectable in inguinal adipose tissues and peripheral blood of 7ND-ADSC–treated mice with or without daily restraint stress. Meanwhile, the PBS- and control ADSC–treated groups showed a minimal background (Fig. 5A and B). A few FLAG tag–positive cells were detected in perivascular areas of inguinal adipose tissue of 7ND-ADSC–implanted mice (Fig. 5C). The results indicate that the transplanted 7ND-ADSCs release 7ND in vivo during the stress period.
FIG. 5.
Intraperitoneally implanted 7ND-ADSCs continuously released 7ND in vivo. PBS, ADSCs, and 7ND-ADSCs (106 cells) were injected into control and stressed mice on day 0 and 7 intraperitoneally (n = 8–10 in each group.). 7ND mRNA in inguinal adipose tissues was detected by RT-PCR using 40 cycles. Plasma level of 7ND was analyzed with human CCL2 ELISA kit. 7ND-ADSCs were detected in inguinal cells using immunohistochemistry for FLAG. A: 7ND mRNA was detected in inguinal adipose tissues from the 7ND-ADSC–treated mice but not from PBS- or ADSC-treated mice. B: 7ND protein was solely detected in peripheral blood from the 7ND-ADSC–treated mice. Data are expressed as mean ± SD. C: FLAG tag–positive cells were detected in inguinal fat of 7ND-ADSC–implanted mice. Arrowheads denote FLAG tag–positive cells. Original magnification ×400; bar, 25 μm. (A high-quality digital representation of this figure is available in the online issue.)
7ND-ADSC provides protection against adipose inflammation in stressed mice.
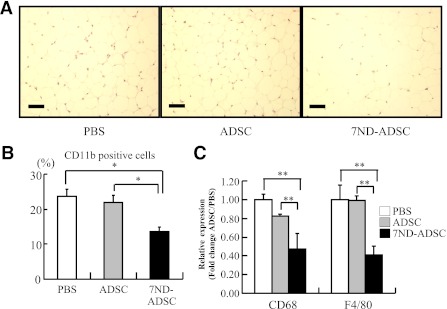
To determine the effects of blocking MCP-1 with the 7ND mutant, we quantified monocyte infiltration in adipose tissues of the nonstressed and stressed mice. The 7ND-ADSC treatment reduced stress-induced accumulation of CD11b-positive cells in the adipose tissues compared with the PBS- and control ADSC–treated mice, respectively (PBS, control ADSCs, and 7ND-ADSCs, 23.8 ± 1.8 vs. 21.8 ± 2.0 vs. 13.6 ± 1.2%, respectively) (Fig. 6A and B). Meanwhile, neither the PBS nor ADSC treatment affected monocyte accumulation induced by the stress. Indeed, the expression levels of CD68 and F4/80 were significantly decreased in adipose tissues of the 7ND-ADSC–treated mice in parallel with the CD11b-positive cell result (PBS, control ADSCs, and 7ND-ADSCs: CD68, 100 ± 2.3 vs. 82.2 ± 0.8 vs. 43.7 ± 6.5%; F4/80, 100 ± 6.3 vs. 99.0 ± 3.2 vs. 41.1 ± 4.1%) (Fig. 6 C). Surprisingly, the 7ND-ADSC treatment reversed the stress-induced monocyte accumulation up to the level of the nonstressed group. Each treatment did not alter monocyte count in adipose tissues of the nonstressed group (data not shown).
FIG. 6.
The 7ND-ADSC implant reduced stress-induced monocyte accumulation in the inguinal adipose. A: Mononuclear cellular accumulation was not obvious in inguinal adipose tissues from the 7ND-ADSC–treated mice (original magnification ×200 magnification; bar, 50 μm). B: Accumulation of CD11b-positive cells remarkably decreased in adipose tissue from the 7ND-ADSC–treated mice compared with control mice. Data are expressed as mean ± SD. *P < 0.05, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group. C: CD68 and F4/80 expression was significantly reduced in adipose tissues from the 7ND-ADSC–treated mice. Data are expressed as mean ± SD. **P < 0.01, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group. (A high-quality digital representation of this figure is available in the online issue.)
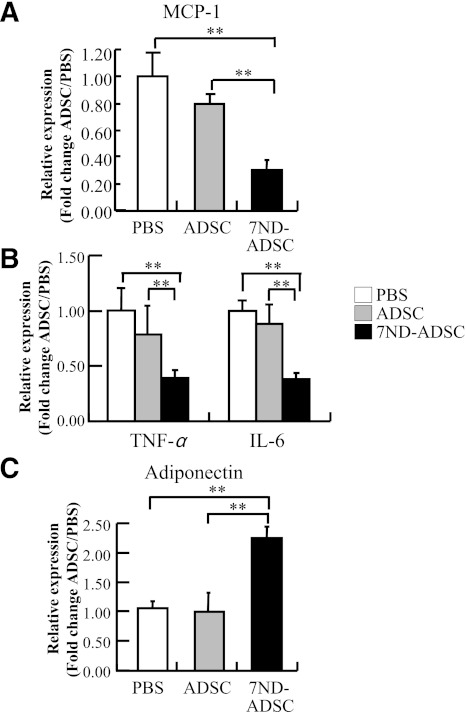
We also examined the effects of 7ND-ADSC on the expressions of stress-induced proinflammatory cytokines in adipose tissues. The expression levels of MCP-1, TNF-α, and IL-6 were significantly decreased in the 7ND-ADSC–treated mice compared with the PBS- and control ADSC–treated mice (P < 0.01, respectively) (Fig. 7A and B). In contrast, the expression level of adiponectin was significantly increased in the 7ND-ADSC–treated mice compared with the PBS- and control ADSC–treated mice (Fig. 7C). Among the nonstressed mice, treatment with PBS, control ADSCs, and 7ND-ADSCs hardly affected the expression levels of proinflammatory cytokines and adiponectin (data were not shown). These results suggest that MCP-1 antagonism with 7ND reduced the stress-induced inflammatory reaction in adipose tissues.
FIG. 7.
The 7ND-ADSC implant suppressed adipose inflammation in the stressed mice. A: Quantitative analysis of MCP-1 expression in adipose tissue. B: Quantitative analysis of TNF-α and IL-6 expression in adipose tissue. C: Quantitative analysis of adiponectin expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group.
7ND-ADSC treatment rescues the stress-induced decline in insulin sensitivity and the prothrombotic state.
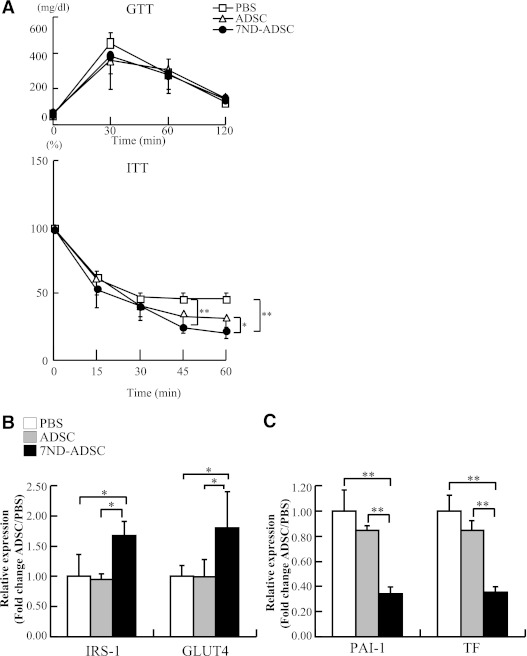
To examine whether the anti-inflammatory effect of 7ND improves stress-induced insulin resistance and increase in procoagrant factors, we analyzed GTT, ITT, and expression levels of IRS-1, GLUT4, PAI-1, and TF in adipose tissues. There was no significant difference in glucose tolerance among the three groups after stress (Fig. 8A). However, insulin tolerance improved significantly at 45 min after the 7ND-ADSC therapy (Fig. 8A). Indeed, 7ND-ADSCs restored the expression levels of IRS-1 and GLUT4 in adipose tissues up to the levels in control mice (Fig. 8B). The expression levels of IRS-1 and GLUT4 in skeletal muscle were not altered by these treatments (data not shown).
FIG. 8.
7ND treatment rescued the stress-induced decline in insulin sensitivity and coagulability. A: Glucose tolerance was comparable among the mice treated with PBS, ADSCs, and 7ND-ADSCs (top). Insulin tolerance was significantly recovered in the 7ND-ADSC–stressed mice (bottom). Data are expressed as mean ± SD. *P < 0.05 at 60 min and **P < 0.01 at 45 and 60 min, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group. B: Quantitative analysis of IRS-1 and GLUT4 expression in adipose tissue. Data are expressed as mean ± SD. *P < 0.05, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group. C: Quantitative analysis of PAI-1 and TF expression in adipose tissue. Data are expressed as mean ± SD. **P < 0.01, compared with the PBS- and ADSC-treated mice, respectively; n = 8–10 in each group.
The expression levels of PAI-1 and TF in adipose tissue were significantly reduced in 7ND-ADSC–treated mice, compared with those treated with PBS or control ADSCs, in the same manner as monocyte accumulation and proinflammatory cytokine induction (Fig. 8C). GTT, ITT, and expression levels of IRS-1, GLUT4, PAI-1, and TF in adipose tissues were unchanged in the nonstressed mice after treatment with PBS, control ADSCs, and 7ND-ADSCs (data not shown).
Considered together, the above findings highlight the important role of MCP-1 expression in adipose tissue in the stress-induced decline of glucose metabolism and prothrombotic state.
DISCUSSION
The current study demonstrated for the first time that despite a decrease in both body weight gain and adipose tissue weight, chronic restraint stress administered over 2 weeks evoked low-grade inflammation in murine adipose tissue, similar to that seen in obesity-related disease. This inflammatory change involved the induction of HSP70.1 and proinflammatory adipokines and a decrease in adiponectin. Further stress-induced adipose inflammation resulted in low insulin sensitivity with reduced adipose IRS-1 and GLUT4, and increased PAI-1 and TF, which did not induce thrombus formation. As the MCP-1–neutralizing antibody treatment suppressed stress-induced adipose inflammation, we focused on the effects of adipocyte-derived MCP-1 among the synergic effects of proinflammatory adipokines. Inhibiting MCP-1 by implanting 7ND-ADSCs suppressed the stress-induced adipose inflammatory changes, reversing both the prothrombotic state and decline in insulin sensitivity.
The inflammatory changes in the mouse adipose tissue were reversible within the 1-week regimen of daily stress, but not after 2 weeks of daily stress administration. The same stressful stimuli applied repetitively enhance activity of the sympathetic system and hypothalamic-pituitary-adrenal axis similarly (25,26) to increase both plasma catecholamine and cortisol levels. A similar pattern of neuroendocrine adaptation might take place with the localized adipose tissue immune response. The synergetic effects of the β-adrenergic pathway and cortisol on adipocytes are inextricably linked and inseparable. As both sympathetic nerve endings and cortisol stimulate lipolysis (27,28), adipose tissues are shrunk to release FFAs after 2 weeks of daily stress administration. Indeed we observed atrophic adipose tissue with reduced cell size and elevated FFA levels after restraint stress without any alteration in food intake. Recently it has been reported that large adipocytes have increased lipolytic capacity and are sensitive to lipolytic stimuli (29). Stress hormone–mediated lipolysis would reduce adipose cell size and increase FFA concentration. As dysregulation of adipose tissue lipolysis results in increased FFAs in diet-induced obese individuals, which activates TLR4 signaling to promote adipose inflammation (8), increased lipolysis and FFAs would also be involved in stress-induced adipose inflammation. The restraint-stressed mice revealed low-grade chronic inflammation without showing an obesity-related phenotype. As far as we know, this is the first report of a lean murine model with low-grade adipose inflammation. Because adipose inflammation is an independent risk factor for evoking low insulin sensitivity and the prothrombotic state followed by metabolic syndrome, this study highlighted that the susceptibility to various stressors would impair health to the same extent as obesity.
The mechanism by which psychological stress evokes a systemic inflammatory reaction with cross-talk between the central nervous system and immune system remains unknown. HSPs, which are induced by stress to protect organs, would be one of the most potential mediators because they target various immune and vascular cells and augment inflammatory reactions that lead to cytokine production (30,31). Indeed we observed restraint stress induced HSP70.1 in adipose tissue in this study, which is concordant with our previous report (16). Furthermore we observed an increase in inflammatory adipokine and a decrease in adiponectin, indicating that adipose tissue is one of the major target organs of the stress-induced inflammation that exacerbates the systemic reaction. MCP-1 is a trigger of inflammation in adipose tissue, resulting in monocyte accumulation and inflammatory cytokines (11,32). In this study, chronic restraint stress altered pro- and anti-inflammatory adipokines in accordance with MCP-1 induction in adipose stromal cells and accumulated monocytes. Further, the MCP-1–neutralizing antibody and 7ND treatments suppressed monocyte accumulation in adipose tissue, restoring the adipokine expression. Indeed, TNF-α and IL-6 stimulate adipocytes and endothelial cells in the adipose tissue itself to produce MCP-1 (33). Monocytes, recruited into adipose tissue by adipocyte-derived MCP-1, also secrete TNF-α, IL-6, and MCP-1 in response to CC chemokine receptor-2 activation (34). Thus, adipose-derived MCP-1 enhances the amplification cascade underpinning continuous adipose inflammation through autocrine and paracrine interactions between monocytes and adipocytes under stress conditions as well as obese ones.
Overexpression of MCP-1 in adipose tissue evokes chronic inflammation to impair insulin sensitivity systemically (35), and MCP-1 inhibition recovers obesity-induced insulin resistance (36). Therefore, MCP-1 plays a critical role in insulin resistance, which exists in the downstream mechanism of adipose inflammation in obese individuals. We focused on adipose and skeletal muscle to analyze the mechanism of systemic insulin resistance caused by stress-induced MCP-1 because adipose tissue accounts for ∼15–20% of insulin-stimulated glucose uptake, and skeletal muscle is responsible for ∼80% of whole-body, insulin-mediated glucose metabolism (37). As previously described, MCP-1 expression in adipose tissue increases macrophage infiltration and induces TNF-α secretion by interactions between macrophages and adipocytes (38). Reportedly, TNF-α downregulates IRS-1 and GLUT4 expression in adipose tissue, resulting in insulin resistance in terms of adipocyte glucose uptake (39,40). Thus, MCP-1 exacerbates obesity-induced insulin resistance in adipose tissues. In this study we also observed similarly increased expression of MCP-1 and TNF-α, reduced expression of IRS-1 and GLUT4, and declining insulin sensitivity in adipose tissue after restraint stress. This would also alter systemic insulin sensitivity because GLUT4 deficiency in adipose impairs insulin sensitivity in skeletal muscle and liver (41). Furthermore plasma IL-6 would functionally impair insulin signaling in skeletal muscles at IRS-1 function by three mechanisms that involve 1) serine phosphorylation by JNK, 2) impairment on tyrosine phosphorylation by SOCS3, and 3) tyrosine dephosphorylation by PTP1B, which is independent from IRS-1 and GLUT4 expression (42). Decreased adiponectin levels in adipose tissue would also impair systemic insulin sensitivity (43). These effects were recovered by MCP-1 inhibition together with the 7ND treatment. It thus seems that stress-induced adipose inflammation has a similar consequence on insulin sensitivity to that observed in obese subjects, and that MCP-1 could be one of the most important mediators of stress-induced insulin resistance.
Obesity is involved in the prothrombotic state and increases the risk of thrombosis (17,44,45). This is because proinflammatory adipokines, including MCP-1 and TNF-α, induce prothrombotic factors in adipose tissues of obese subjects (17,45). We reported previously that adipose-derived PAI-1 and TF, which stabilize thrombi and initiate the coagulation cascade, respectively, participate in the stress-induced thrombus formation in aged mice (16,17,46). In this study we showed that MCP-1 would play a critical role in stress-induced procoagulant factor production in adipose tissue. Our findings might suggest a new mechanism of stress-induced cardiovascular disease, and it is possible that obesity and stress share common pathological machinery for adipose inflammation. In this study setting, we could not detect thrombus formation itself using immunohistochemistry for fibrin, although we previously reported thrombus formation in adipose and kidney of stressed 24-month-old mice (16). This is because we observed younger mice to exclude the influence of aging and suggest that stress-induced thrombosis requires a severe condition (i.e., aging) more than PAI-1 and TF induction by stress (17).
ADSCs would be an ideal candidate for autologous cell therapy because they are readily accessible and an abundant source of pluripotent adult stem cells, enabling repetitive cell therapy. Reportedly, ADSCs can be settled down in vivo after an intraperitoneal injection (19,47) to work as a cellular vector delivering various bioactive substances for cell-based gene therapy (20). In the current study, we detected a few FLAG-positive cells in perivascular areas of inguinal adipose tissue and observed efficient expression of 7ND derived from the ADSC cellular vectors in adipose tissue and plasma. Injected ADSCs run through blood and lymphatic flow, migrate, and settle down to adipose tissues because ADSCs themselves express a macrophage-lineage marker and show similar cellular behavior. Furthermore the delivery of 7ND-ADSCs once a week decreased stress-induced adipose inflammation. Thus our results provide preclinical support for a model of 7ND-based anti-inflammatory therapy based on the use of ADSCs as cellular vectors (24).
In conclusion, daily restraint stress over a 2-week period evoked the expression of MCP-1 and other inflammatory adipokines in adipose tissue and a low-grade chronic state of adipose inflammation that exacerbated insulin resistance and induced the procoagulant factors. MCP-1 inhibition with 7ND-ADSCs reversed adipose inflammation and these pathological consequences. Stress-induced pathological machinery may therefore be common among obesity-related diseases in terms of the inflammatory response in adipose tissue.
ACKNOWLEDGMENTS
This work was supported by research grants from the Takeda Research Foundation, a grant from the Japan Cardiovascular Research Foundation (Bayer Scholarship for Cardiovascular Research to K.T.), and Grant-in-Aid for Scientific Research (Kakenhi 21590950 to K.T. and 23390208 to T.M.).
No potential conflicts of interest relevant to this article were reported.
Y.U. performed experiments and contributed to writing the manuscript. K.T. designed and performed experiments, chaired discussions, and wrote and edited the manuscript. K.Y., T.N., and T.Ma. performed cell treatment experiments. K.E. provided 7ND construct. R.K. and M.N. performed animal experiments. X.W.C. and H.N. performed a pathological analysis. T.Mu. coordinated this project and reviewed the manuscript. K.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Issa F.G. (Word-Medex Pty Ltd., Sydney, Australia) for careful reading and editing of the manuscript, Dr. Mikio Iwashita (Daiichi-Sankyo Co., Ltd., Tokyo, Japan) for suggestions on the statistical evaluations, and all members of the laboratory for sharing reagents and advice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0828/-/DC1.
REFERENCES
- 1.Ziemssen T, Kern S. Psychoneuroimmunology—cross-talk between the immune and nervous systems. J Neurol 2007;254(Suppl. 2):II8–II11 [DOI] [PubMed] [Google Scholar]
- 2.Groeschel M, Braam B. Connecting chronic and recurrent stress to vascular dysfunction: no relaxed role for the renin-angiotensin system. Am J Physiol Renal Physiol 2011;300:F1–F10 [DOI] [PubMed] [Google Scholar]
- 3.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 2004;164:1873–1880 [DOI] [PubMed] [Google Scholar]
- 5.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ 2006;332:521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:2276–2283 [DOI] [PubMed] [Google Scholar]
- 7.Dentali F, Squizzato A, Ageno W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin Thromb Hemost 2009;35:451–457 [DOI] [PubMed] [Google Scholar]
- 8.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 2003;24:278–301 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Mizuarai S, Araki H, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem 2003;278:46654–46660 [DOI] [PubMed] [Google Scholar]
- 12.Shimomura I, Funahashi T, Takahashi M, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 1996;2:800–803 [DOI] [PubMed] [Google Scholar]
- 13.Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology 2010;151:971–979 [DOI] [PubMed] [Google Scholar]
- 14.Mihara M, Aihara K, Ikeda Y, et al. Inhibition of thrombin action ameliorates insulin resistance in type 2 diabetic db/db mice. Endocrinology 2010;151:513–519 [DOI] [PubMed] [Google Scholar]
- 15.Jonsdottir IH, Hägg DA, Glise K, Ekman R. Monocyte chemotactic protein-1 (MCP-1) and growth factors called into question as markers of prolonged psychosocial stress. PLoS ONE 2009;4:e7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto K, Takeshita K, Shimokawa T, et al. Plasminogen activator inhibitor-1 is a major stress-regulated gene: implications for stress-induced thrombosis in aged individuals. Proc Natl Acad Sci USA 2002;99:890–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res 2005;66:276–285 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol 1995;15:4851–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486–2496 [DOI] [PubMed] [Google Scholar]
- 20.Grisendi G, Bussolari R, Cafarelli L, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res 2010;70:3718–3729 [DOI] [PubMed] [Google Scholar]
- 21.Aoyama T, Takeshita K, Kikuchi R, et al. γ-Secretase inhibitor reduces diet-induced atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 2009;383:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeshita K, Yamamoto K, Ito M, et al. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost 2002;28:545–554 [DOI] [PubMed] [Google Scholar]
- 23.Takeshita K, Satoh M, Ii M, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res 2007;100:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito S, Nakayama T, Hashimoto N, et al. Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuate bleomycin-induced lung damage. Am J Pathol 2011;179:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty R, Horwatt K, Konarska M. Chronic stress and sympathetic-adrenal medullary responsiveness. Soc Sci Med 1988;26:333–341 [DOI] [PubMed] [Google Scholar]
- 26.Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol 2009;300:137–146 [DOI] [PubMed] [Google Scholar]
- 27.Fliers E, Kreier F, Voshol PJ, et al. White adipose tissue: getting nervous. J Neuroendocrinol 2003;15:1005–1010 [DOI] [PubMed] [Google Scholar]
- 28.Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology 2010;92(Suppl. 1):86–90 [DOI] [PubMed] [Google Scholar]
- 29.Laurencikiene J, Skurk T, Kulyte A, et al. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab 2011;96:E2045–E2049 [DOI] [PubMed] [Google Scholar]
- 30.Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity [retracted in: Ann N Y Acad Sci 2009;1167:241]. Ann N Y Acad Sci 2007;1113:28–39 [DOI] [PubMed] [Google Scholar]
- 31.Blake MJ, Udelsman R, Feulner GJ, Norton DD, Holbrook NJ. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci USA 1991;88:9873–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasshauer M, Klein J, Kralisch S, et al. Monocyte chemoattractant protein 1 expression is stimulated by growth hormone and interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2004;317:598–604 [DOI] [PubMed] [Google Scholar]
- 34.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem 1996;271:11603–11607 [DOI] [PubMed] [Google Scholar]
- 35.Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006;281:26602–26614 [DOI] [PubMed] [Google Scholar]
- 36.Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol 2008;28:2195–2201 [DOI] [PubMed] [Google Scholar]
- 37.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 1990;322:223–228 [DOI] [PubMed] [Google Scholar]
- 38.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005;25:2062–2068 [DOI] [PubMed] [Google Scholar]
- 39.Ruan H, Miles PD, Ladd CM, et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 2002;51:3176–3188 [DOI] [PubMed] [Google Scholar]
- 40.Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 2008;114:183–194 [DOI] [PubMed] [Google Scholar]
- 41.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001;409:729–733 [DOI] [PubMed] [Google Scholar]
- 42.Benito M. Tissue-specificity of insulin action and resistance. Arch Physiol Biochem 2011;117:96–104 [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Mao X, Wang L, et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 2007;282:7991–7996 [DOI] [PubMed] [Google Scholar]
- 44.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–2971 [DOI] [PubMed] [Google Scholar]
- 45.Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev 2009;10:554–563 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K, Shimokawa T, Yi H, et al. Aging and obesity augment the stress-induced expression of tissue factor gene in the mouse. Blood 2002;100:4011–4018 [DOI] [PubMed] [Google Scholar]
- 47.Lin G, Wang G, Liu G, et al. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev 2009;18:1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]