Abstract
The expansion of lower-body adipose tissue (AT) is paradoxically associated with reduced cardiovascular disease and diabetes risk. We examined whether the beneficial metabolic properties of lower-body AT are related to the production and release of the insulin-sensitizing lipokine palmitoleate (16:1n-7). Using venoarterial difference sampling, we investigated the relative release of 16:1n-7 from lower-body (gluteofemoral) and upper-body (abdominal subcutaneous) AT depots. Paired gluteofemoral and abdominal subcutaneous AT samples were analyzed for triglyceride fatty acid composition and mRNA expression. Finally, the triglyceride fatty acid composition of isolated human preadipocytes was determined. Relative release of 16:1n-7 was markedly higher from gluteofemoral AT compared with abdominal subcutaneous AT. Stearoyl-CoA desaturase 1 (SCD1), the key enzyme involved in endogenous 16:1n-7 production, was more highly expressed in gluteofemoral AT and was associated with greater enrichment of 16:1n-7. Furthermore, isolated human preadipocytes from gluteofemoral AT displayed a higher content of SCD1-derived fatty acids. We demonstrate that human gluteofemoral AT plays a major role in determining systemic concentrations of the lipokine palmitoleate. Moreover, this appears to be an inherent feature of gluteofemoral AT. We propose that the beneficial metabolic properties of lower-body AT may be partly explained by the intrinsically greater production and release of palmitoleate.
Fat distribution is a major determinant of health (1). Abdominal adipose tissue (AT) accumulation increases cardiovascular disease and type 2 diabetes risk, whereas expansion of gluteofemoral AT protects from metabolic-related diseases (2,3). The mechanisms surrounding this paradox remain to be elucidated. Evidence from in vivo and in vitro rodent models suggests that AT-derived circulating palmitoleate (16:1n-7) acts as an insulin-sensitizing lipokine that improves insulin signaling in muscle and liver (4,5). A strong positive relationship has recently been demonstrated between the proportion of 16:1n-7 in the plasma nonesterified fatty acid (NEFA) pool and insulin sensitivity in humans (6). Considering this, we proposed that site-specific differences in the release of 16:1n-7, via stearoyl-CoA desaturase 1 (SCD1) activity, may provide an important mechanistic explanation for the differing contributions of distinct AT depots to metabolic health. Using venoarterial difference sampling, we investigated the release of 16:1n-7 from lower-body (gluteofemoral) and upper-body (abdominal subcutaneous) AT depots. AT fatty acid (FA) and mRNA profiling were combined with in vitro studies to investigate tissue-specific FA metabolism in humans.
RESEARCH DESIGN AND METHODS
All studies were approved by the Oxfordshire Clinical Research Ethics Committee, and all volunteers gave written, informed consent.
In vivo measurements of FA release from human AT.
After an overnight fast, FA release was measured in 48 healthy individuals (28 men, BMI 20–31 kg/m2; and 20 women, BMI 18.8–39.3 kg/m2) recruited for previous and on-going studies of AT venoarterial difference measurements (7). For metabolic characteristics see Supplementary Table 1. NEFA was extracted from plasma and analyzed by gas chromatography (GC) as described below.
AT FA composition and transcriptional profiling.
The study recruited 36 healthy individuals, BMI 24–46 kg/m2 (18 men) and 21–40 kg/m2 (18 women), from the Oxford Biobank (8). For metabolic characteristics see Supplementary Table 1. Paired AT samples were taken by needle biopsy (8) from the periumbilical and upper buttock areas. mRNA was extracted from the tissues and reversed transcribed, and quantitative PCR assays were performed (9). Expression values were calculated by the ΔCT transformation method (ΔCT = efficiency[calibrator Ct−sample Ct]) and normalized to PPIA and PGK1 (9). For FA profiling, total lipids were extracted from the lipid layer obtained during RNA extraction (10). The triglyceride (TG) fraction was separated and analyzed by GC, as previously described (10). Analytic accuracy was assessed using external and in-house quality control samples (AOCS std#6, Thames Restek UK Ltd; Seven Seas Ltd, Hull, U.K.). GC results were converted to mol%. The between-assay precision (CV [%], n = 33) for TG was 16:0 (4.0), 16:1n-7 (5.7), 18:0 (6.2) and 18:1n-9 (4.2). The mean (mol %) and within-assay precision (CV [%], n = 22) was calculated on replicate measurements of human AT as follows: 16:0, 22.5 (1.6); 16:1n-7, 5.3 (1.4); 18:0, 4.4 (2.3); and 18:1n-9, 46.0 (1.03).
Isolation and culture of human preadipocytes.
Paired gluteal and abdominal subcutaneous AT biopsy specimens were obtained, as described above, from five healthy women (BMI 20–24 kg/m2). Stromal-vascular cells were isolated, as previously described (11). Fully confluent cells were stimulated for 14 days with an adipogenic cocktail (11). For the first 4 days, 0.25 mmol/L 3-isobutyl-1-methylxanthine and 2 μmol/L troglitazone were added to the adipogenic medium. Differentiated cells were harvested for FA composition analysis.
Calculations.
Release of 16:1n-7 from abdominal and gluteofemoral AT was calculated relative to 16:0 and the essential FA linoleate (18:2n-6). 18:2n-6 is not endogenously synthesized and displayed no significant difference in abundance (mol %) between AT depots. Relative release of 16:1n-7 was calculated as venoarterial difference of 16:1n-7 (μmol/L)/venoarterial difference of 16:0 or 18:2n-6 (μmol/L). Insulin resistance was calculated using the homeostatic model assessment of insulin resistance (HOMA-IR) (12). Product-to-precursor ratios were used as measures of enzyme activity. For SCD1 activity, the desaturation indices of 16:0 and 18:0 were calculated from relative abundance (mol %) in the particular lipid fraction (ratio of 16:1n-7 to 16:0 and 18:1n-9 to 18:0). For elongase activity, the ratios of 18:0 to 16:0 and 18:1n-7 to 16:1n-7 were calculated.
Statistics.
Data were analyzed using SPSS 15 software (SPSS UK, Chertsey, U.K.). All data are presented as means ± SEM unless otherwise stated. All data sets were tested for normality according to the Shapiro-Wilk test, and log transformed where appropriate. Comparisons were made between AT depots using a paired t test or a Wilcoxon signed rank test. Sex analysis was performed using the independent t test. Correlations were assessed with the Pearson correlation coefficient.
RESULTS
A major role for gluteofemoral AT in the release of 16:1n-7 in humans.
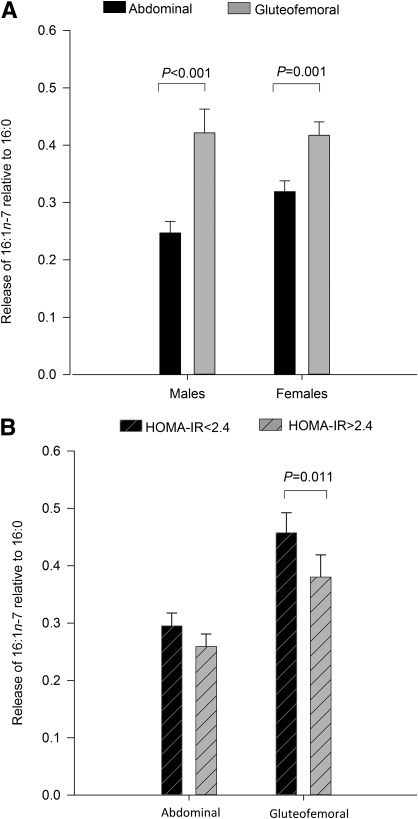
To investigate regional variation in the release of 16:1n-7 from AT, FA release from the gluteofemoral subcutaneous AT (GSAT) and abdominal subcutaneous AT (ASAT) depots was directly assessed using venoarterial sampling. When calculated relative to its precursor palmitate (16:0), the release of 16:1n-7 was significantly higher from GSAT than ASAT in both sexes (Fig. 1A). To confirm this was not driven by regional differences in 16:0 abundance, 16:1n-7 release was also calculated relative to 18:2n-6. Relative to 18:2n-6, 16:1n-7 release was significantly higher from GSAT compared with ASAT in men (0.47 ± 0.04 vs. 0.36 ± 0.03; P < 0.001) and women (0.54 ± 0.04 vs. 0.41 ± 0.02; P < 0.001). This is the first report that individual AT depots differ in their contribution to circulating 16:1n-7 levels.
FIG. 1.
Depot-specific release of 16:1n-7 from lower- and upper-body subcutaneous AT depots. A: The release of 16:1n-7 relative to 16:0 was greater from gluteofemoral AT compared with abdominal AT in both sexes (P ≤ 0.001). B: When grouped according to insulin-resistance status (as calculated by HOMA-IR), the release of 16:1n-7 relative to 16:0 from the gluteofemoral depot was higher in individuals with a HOMA-IR<2.4 (P < 0.05). Release of 16:1n-7 relative to 16:0 from abdominal AT was not different between HOMA-IR groups. All data are represented as mean ± SEM.
An inverse association was observed between insulin resistance, as defined by HOMA-IR, and both the proportion of 16:1n-7 (mol %) in the plasma NEFA pool (r = −0.50, P = 0.0003) and the plasma NEFA 16:1n-7 concentration (r = −0.40, P = 0.006). Using the group median, subjects were classified by HOMA-IR (<2.4 or ≥2.4). Relative to 16:0, the release of 16:1n-7 from GSAT was higher in the more insulin-sensitive (HOMA-IR <2.4) compared with insulin-resistant individuals (Fig. 1B). Release of 16:1n-7 relative to 16:0 from ASAT was not different between HOMA-IR groups. Similarly, when expressed relative to 18:2n-6, 16:1n-7 release from GSAT was higher in insulin-sensitive compared with insulin-resistant individuals (0.46 ± 0.03 vs. 0.38 ± 0.04; P = 0.015), suggesting a link between gluteofemoral release of 16:1n-7 and insulin sensitivity.
Depot-specific production of 16:1n-7 is an inherent feature of AT.
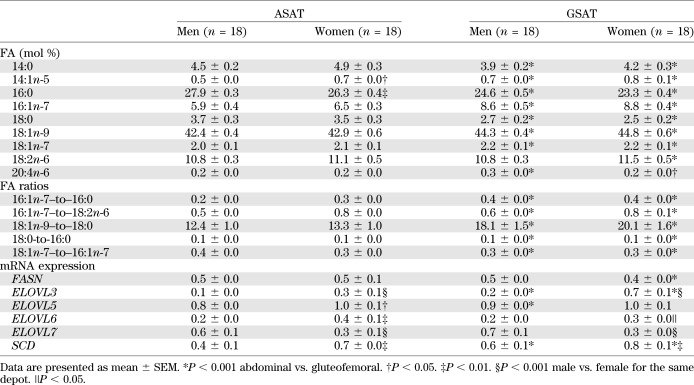
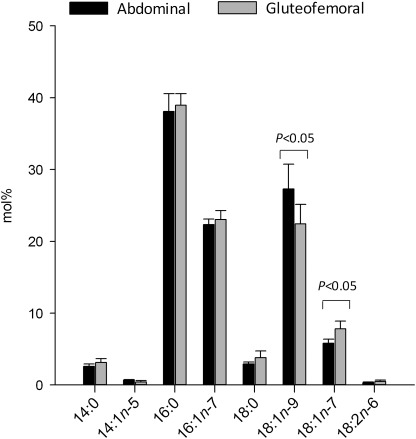
Regional variation in the release of 16:1n-7 from AT may reflect differences in endogenous production. By analyzing AT TG-FA composition, we found the proportion of 16:1n-7 and its elongation product 18:1n-7 was significantly higher in GSAT in both sexes (Table 1). This is consistent with depot-specific differences in AT FA composition described previously (13). To explore whether this tissue phenotype is a consequence of the local milieu or an inherent feature, human primary preadipocytes were isolated from both depots and differentiated in vitro. In support of intrinsic differences between the tissues, there was a tendency for higher 16:1n-7 and a significant enrichment of its elongation product 18:1n-7 (P < 0.05) in cells derived from GSAT compared with ASAT (Fig. 2).
TABLE 1.
TG-FA composition (mol %) and mRNA expression of lipid metabolism genes in GSAT and ASAT
FIG. 2.
FA profiling of isolated human preadipocytes derived from GSAT and ASAT. After a 14-day adipogenic time-course, TG-FA composition (mol %) of gluteofemoral-derived preadipocytes displayed enrichment in 18:1n-7, the elongation product of 16:1n-7. P < 0.05 gluteofemoral compared with abdominal preadipocytes. Data are represented as mean ± SEM.
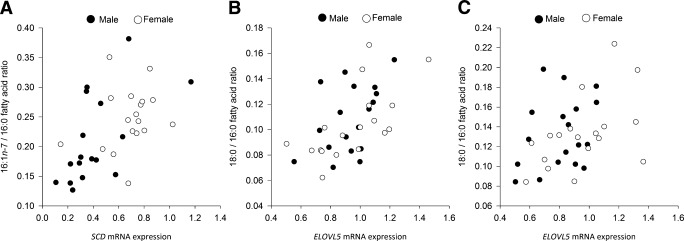
SCD1 mRNA expression was significantly different between depots and sexes (P < 0.001). Within-person SCD1 expression was consistently higher (P = 1.6 × 10−7) in GSAT than in ASAT in both sexes and higher in women than in men in both AT depots (Table 1). This sexual dimorphism for SCD1 mRNA expression has not previously been reported. Product-to-precursor ratios (desaturation indices) provided evidence for significantly higher desaturation in GSAT compared with ASAT in both sexes (Table 1). We also investigated whether differences in the SCD1 desaturation index (16:1n-7/16:0) reflect depot-specific SCD1 mRNA expression. In ASAT, there was a strong (r = 0.64, P = 0.005) association between SCD1 mRNA expression and the 16:1n-7–to–16:0 ratio (Fig. 3A), but this was not found in GSAT (data not shown).
FIG. 3.
Associations between mRNA expression of lipid metabolism genes and TG-FA acid product-to-precursor ratios. A: SCD1 mRNA expression in abdominal AT was strongly associated with the 16:1n-7–to–16:0 ratio in men (r = 0.64, P = 0.005) but not in women (r = 0.23, NS). B: ELOVL5 mRNA expression in gluteofemoral AT was significantly associated with the 18:0-to-16:0 ratio in women (r = 0.64, P = 0.004) and men (r = 0.48, P = 0.045). C: ELOVL5 mRNA expression in abdominal AT was significantly associated with the 18:0-to-16:0 ratio in women (r = 0.50, P = 0.03).
Greater elongation of 16:0 to 18:0 was observed in ASAT compared with GSAT as determined by the 18:0-to-16:0 ratio (Table 1). The mRNA expression of several ELOVL genes, which may act at this step, varied between depot and sex (Table 1). There was a striking association between ELOVL5 mRNA expression and the 18:0-to-16:0 ratio in GSAT in women (r = 0.64 P = 0.004) and men (r = 0.48, P = 0.045; Fig. 3B). There was also a strong (r = 0.50, P = 0.03) association between ELOVL5 mRNA expression and the 18:0-to-16:0 ratio in ASAT in women but not in men (Fig. 3C). Elongases play a critical role in FA modification, but their regulation remains unclear (14). These findings suggest a significant role for ELOVLs and SCD in depot-specific AT FA metabolism that may influence the FA composition of stored TG.
DISCUSSION
Gluteofemoral AT is sometimes regarded as a quiescent “metabolic sink” that retains excess FA (1), but we provide evidence that it may play a more active role. This study has revealed AT depot-specific and sexual dimorphic desaturation and elongation of FA. This enables tissue-specific release of palmitoleate, which may constitute an insulin-sensitizing lipokine signal (4,5). Indeed, we have previously reported a case of human partial lipodystrophy resulting from a mutation (P467 L) in the PPARG gene that was characterized by insulin resistance and extremely low plasma NEFA 16:1n-7. Upon insulin-sensitization with rosiglitazone, a marked increase in the 16:1n-7–to–16:0 ratio was observed in this individual (10).
Previous reports of an association between plasma palmitoleate and insulin sensitivity are conflicting (6,15). In agreement with Stefan et al. (6) we demonstrate a positive relationship between the proportion of 16:1n-7 in the plasma NEFA pool and insulin sensitivity. The 16:1n-7 content of plasma lipid fractions other than NEFA are difficult to assess in this context (16,17) because they do not reflect AT well (18). The plasma NEFA pool is strongly determined by FA release from subcutaneous AT (19). ASAT and GSAT are exposed to the same circulating dietary FA in vivo. A plausible explanation for the notable differences in tissue 16:1n-7 enrichment is the desaturation of 16:0 via the enzyme SCD1, although differences in FA uptake and storage may play a role. However, there is evidence that 16:1n-7 uptake by human AT does not differ from the uptake of other major FAs (20). In contrast, the proportional uptake of 18:0 and 22:6n-3 (docosahexaenoic acid) is lower than for other FAs (20,21). In principle, partitioning of 16:1n-7 toward other lipid pools, such as phospholipids, is unlikely to explain regional differences in FA composition, because phospholipids not only comprise a minor proportion of AT FA (<0.1%) (18) but also display a lower enrichment of 16:1n-7 than TG (22).
When examining regional differences in FA release, depot size and sexual dichotomy of body fat distribution should be given careful consideration. In lean individuals, the amount of fat in upper- and lower-body depots is comparable (23–25). Contrary to this, in common obesity, the amount of upper-body fat is usually greater than lower-body fat (26,27). Even in individuals defined as lower-body obese, the amount of fat in the upper-body depot is almost twice that of the lower-body depot (27). Thus, it could be speculated that the association between AT-derived 16:1n-7 and insulin sensitivity is lost in obese cohorts because there would be a greater contribution to the systemic NEFA pool from the upper-body ASAT, which is more enriched with saturated FA. Quantifying ASAT is challenging with conventional methods such as dual-energy X-ray absorptiometry (DEXA) because discrimination between this depot and visceral AT is not possible. The more routine use of imaging modalities, such as computed tomography combined with DEXA (28), to dissect anatomically distinct regions will enable more comprehensive studies on regional FA trafficking. To date, several studies have shown that 16:1n-7 enrichment is similar between ASAT and visceral AT (13,29,30). Although visceral fat accumulation is associated with increased metabolic risk, it would seem unlikely that a low or high delivery of 16:1n-7 from visceral fat would have a significant impact on peripheral insulin resistance because the contribution of this depot to the systemic NEFA pool is small (19).
Lower-body fat predominantly consists of gluteal and femoral depots. There is evidence to suggest that these AT depots possess distinct functional characteristics (31,32). Although a direct within-subject comparison of 16:1n-7 release from the two lower-body depots has yet to be undertaken, tissue enrichment of 16:1n-7 in these depots is similar (29). We hypothesize that individuals who preferentially accumulate lower-body fat will have a greater net release of 16:1n-7 from this region relative to other fat depots, with a correspondingly greater impact on systemic 16:1n-7 NEFA concentrations.
The finding that gluteofemoral AT plays an important role in 16:1n-7 production in vivo in humans could provide further mechanistic understanding of the metabolically protective properties of this AT depot. We anticipate that this work will be the starting point for more complex in vivo human studies to gain further insight into the function of the lipokine palmitoleate.
ACKNOWLEDGMENTS
This study was funded by the Wellcome Trust and European Union FP7 LipidomicNet (202272).
No potential conflicts of interest relevant to this article were reported.
K.E.P., M.J.N., B.A.F., and L.H. conducted experimental procedures and wrote the manuscript. K.N.F. and F.K. reviewed and edited the manuscript. F.K. and L.H. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Siobhán McQuaid (University of Oxford) and Dr. Konstantinos Manolopoulos (University of Oxford) for clinical assistance with sample retrieval and Sandy Humphreys (University of Oxford) for statistical expertise.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1810/-/DC1.
REFERENCES
- 1.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959 [DOI] [PubMed] [Google Scholar]
- 2.Canoy D, Luben R, Welch A, et al. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens 2004;22:2067–2074 [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649 [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 2006;399:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefan N, Kantartzis K, Celebi N, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010;33:405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010;59:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neville MJ, Collins JM, Gloyn AL, McCarthy MI, Karpe F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity (Silver Spring) 2011;19:888–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risérus U, Tan GD, Fielding BA, et al. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes 2005;54:1379–1384 [DOI] [PubMed] [Google Scholar]
- 11.Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem 2010;285:6044–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 13.Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, Strong JP. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am J Clin Nutr 1989;50:288–291 [DOI] [PubMed] [Google Scholar]
- 14.Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res 2010;49:186–199 [DOI] [PubMed] [Google Scholar]
- 15.Fabbrini E, Magkos F, Su X, et al. Insulin sensitivity is not associated with palmitoleate availability in obese humans. J Lipid Res 2011;52:808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Cao H, King IB, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 2005;82:747–750 [DOI] [PubMed] [Google Scholar]
- 18.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–380 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding BA, Samra JS, Ravell CL, Frayn KN. Metabolism of individual fatty acids during infusion of a triacylglycerol emulsion. Lipids 1999;34:535–541 [DOI] [PubMed] [Google Scholar]
- 21.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res 2003;44:2065–2072 [DOI] [PubMed] [Google Scholar]
- 22.Field CJ, Angel A, Clandinin MT. Relationship of diet to the fatty acid composition of human adipose tissue structural and stored lipids. Am J Clin Nutr 1985;42:1206–1220 [DOI] [PubMed] [Google Scholar]
- 23.Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 2008;51:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A 2010;107:18226–18231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006;291:E1115–E1123 [DOI] [PubMed] [Google Scholar]
- 26.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60:2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santosa S, Hensrud DD, Votruba SB, Jensen MD. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr 2008;88:1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 29.Calder PC, Harvey DJ, Pond CM, Newsholme EA. Site-specific differences in the fatty acid composition of human adipose tissue. Lipids 1992;27:716–720 [DOI] [PubMed] [Google Scholar]
- 30.Garaulet M, Pérez-Llamas F, Pérez-Ayala M, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 2001;74:585–591 [DOI] [PubMed] [Google Scholar]
- 31.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 2008;87:56–63 [DOI] [PubMed] [Google Scholar]
- 32.Votruba SB, Jensen MD. Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol Endocrinol Metab 2007;292:E1823–E1828 [DOI] [PubMed] [Google Scholar]