Diabetes occurs when the pancreas fails to produce and secrete sufficient insulin for the maintenance of glucose homeostasis. Although many factors required for β-cell development have been elucidated, we know surprisingly little regarding the mechanisms that maintain the differentiated state of adult β-cells. Despite years of fruitful research, many hurdles remain before we can replace failing β-cells in patients. Efforts to produce new β-cells will benefit from detailed knowledge of their differentiation, maturation, maintenance, heterogeneity, and plasticity. Studies of single cells and conditional knockout mice reveal surprising relationships between the different islet cell types and previously unappreciated roles for transcription factors and soluble factors in β-cell maintenance. Herein we highlight numerous innovative efforts made to identify the core mechanisms responsible for β-cell phenotypic maintenance and compare these with other long-lived cell types.
ORIGINS OF ADULT β-CELLS AND THE REGULATION OF ADULT β-CELL MASS
In the adult pancreas, physical β-cell mass is determined by the balance of β-cell birth, differentiation, size, and death. The prenatal origins and development of β-cells (1,2), the molecular mechanisms of adult β-cell proliferation (3,4), and programmed β-cell death (5) have all been reviewed recently and will not be revisited here. The maintenance and adaptation of β-cell mass after birth involves the addition of new β-cells (6). Cells with robust staining for insulin have been shown to slowly proliferate in vivo and in vitro (7–9). It has been reported that β-cells have an extremely long life span under typical conditions and proliferation plays a diminishing role with advancing age (7,10,11). The apparent low rate of β-cell replication has led some groups to suggest that adult β-cells arise from a pool of non–β-cell progenitors (12). Because this topic has been hotly debated and reviewed recently (13), we will only briefly discuss studies relevant to other aspects of this review.
Several approaches have been undertaken to identify a population of resident pancreatic stem cells. Although some progenitor cell markers can be histochemically identified in the adult pancreas, especially after severe injury (14), evidence that these progenitor/precursor cells differentiated from a stem cell population has been difficult to obtain. In vivo analyses indicated that no population of cells resides in the adult pancreas that proliferates at the high rates characteristic of stem cells in gut or skin (15), but do not formally exclude dedicated progenitor cells with a slower mitotic rate. Lineage tracing experiments in mice using a rat Ins2 promoter CreER transgenic line have provided evidence that new β-cells are primarily derived from cells with at least some Ins2 promoter activity (6) (Fig. 1). The interpretation of these studies depends formally on one’s definitions of a “β-cell” and a “progenitor cell”. Is a β-cell a cell with any amount of insulin promoter activity or insulin gene expression? It is well known that Ins2 promoter activity or gene expression is not fully restricted to β-cells, unlike the Ins1 promoter that is activated later in development and marks a majority of mature β-cells (16,17). Cre-Lox lineage-tracing systems are exceptionally sensitive and binary in nature (4 Cre recombinase proteins can theoretically activate the system [18]). Might islet stem/progenitor/precursor cells express low levels of insulin? In vivo and ex vivo studies, showing that adult rodent and human pancreata contain rare insulin-expressing multipotent cells that can form islet and neural cells in culture, hint at this possibility (19). Thus, insulin expression–dependent lineage labeling of β-cells cannot differentiate between self-replication of existing mature β-cells and proliferation and differentiation from insulin expressing stem cells. Another caveat is that inducible Cre systems have the potential of incomplete Cre activation as a result of dosing effects of tamoxifen. Work from Butler and colleagues, who used immunohistochemical staining for insulin (above a threshold) in rat pancreas followed by mathematical modeling, implied a contribution to β-cell expansion from sources other than mature β-cells (12). It remains possible that a specialized population of progenitor/precursor cells with low insulin expression (i.e., below a staining threshold) and relatively low proliferative capacity plays a dynamic role in the renewal of adult β-cell mass. It is also important to note that because it is not possible to perform the same types of experiments on rodents and humans, interspecies conclusions should be made with caution.
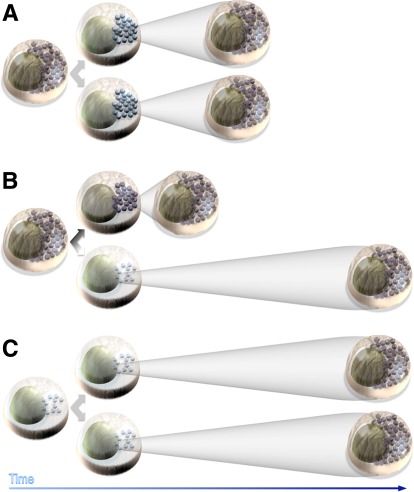
FIG. 1.
Maturation during the life span of single adult β-cells. We present three theoretical possibilities for how maturation kinetics of newly formed β-cells might relate to adult β-cell proliferation events. Here, immature β-cells are drawn with lighter nuclei (denoting reduced expression of key maturity genes, such as insulin) and fewer granules (denoting functional immaturity), but this concept can be potentially extended to any feature of a functionally mature β-cell. The horizontal axis is time. A: Possibility 1: Symmetrical division and maturation of mature adult β-cells. B: Possibility 2: Asymmetrical division and maturation of mature adult β-cells. C: Possibility 3: Symmetrical division and maturation of immature adult β-cells. The last possibility includes scenarios in which cells might dedifferentiate before proliferating. It is also clear from this illustration that the ratio of mature to immature β-cells would depend on the relative contribution of these modes.
Pancreatic duct cells represent a frequently studied candidate for islet cell progenitors. This hypothesis was attractive because insulin-positive cells are often found in proximity to ducts (4) and such a model has morphological similarities to the developmental model of islet genesis (20). Multiple experiments using tamoxifen-inducible Cre-mediated lineage tracing have been conducted to test this hypothesis in adult tissues, using Cre driven by the carbonic anhydrase-II promoter, the Hnf1β promoter or the Muc1 promoter (21–23). In the first study, no increase in labeling (0.9 ± 0.5%) above background (1.3 ± 1.2%) was observed in 10-week-old CAII-CreER mice treated with tamoxifen starting at 4 weeks (21), indicating that neogenesis from duct cells does not play a measurable role in the young adult pancreas. On the other hand, the same CreER experiment showed that 23.6% of the β-cells in the ligated portion of the pancreas were β-galactosidase positive and had therefore arisen from progenitors with CAII promoter activity (21). The latter two investigations, using Hnf1β-CreERT2 and Muc1-CreERT2 mice, both concluded that duct cells do not convert into islet cells in the uninjured pancreas after birth (22,23). Thus, although the origin of new β-cells under physiological conditions in adult animals appears not to involve neogenesis, there may be important roles for embryonic-like repair pathways under stressed conditions. Investigators using injury models, ranging from moderate to severe, have presented data to suggest that non–β-cells can adopt a progenitor role. We will not review those studies here in detail (reviewed in [4]), except to comment on evidence suggesting that the progenitor population activated during injury expresses the gene for Ngn3 (Neurog3) (24). The interpretation of this study is complicated by the demonstration that small amounts of Ngn3 protein are present in adult β-cells (25–28), meaning that the data cannot exclude the possibility that the suspected neogenesis may have arisen in whole or in part via Neurog3-expressing islet cells. We have shown that stresses such as growth factor withdrawal, ER stress, or Notch inhibition reactivate Ngn3 in β-cell lines (28) and islets (25). The evidence that a fraction of β-cells are produced from cells expressing the glucagon promoter under extreme injury conditions further suggests that β-cell progenitors can have an islet origin (29,30).
DYNAMICS OF ADULT β-CELL MATURATION AT THE SINGLE-CELL LEVEL
Adult β-cells are heterogeneous, with differences in glucose-responsiveness, insulin promoter activity, and insulin protein levels (31,32). Heterogeneity may be linked to differential susceptibility to death under conditions of type 1 diabetes (33) or stress induced by misfolded insulin proteins (34). The observation of heterogeneity can be interpreted as independent subpopulations of β-cells that stably express different amounts of insulin. In this scenario, it could be possible that one of these populations represents a group of specialized β-cell progenitors or cells with enhanced plasticity. An alternate interpretation is that some or all of the observed β-cell heterogeneity represents an instantaneous cross-section through a population of cells captured at different stages in their life cycle, with each stage exhibiting a distinct gene expression pattern (Fig. 1). Both concepts are consistent with the observation that extremely rare (or extremely fleeting) adult insulin-positive (Glut2low) cells can be derived from cultures of islets leading to clusters of multipotent, self-renewing cells (19). The latter hypothesis was untested until the recent development of single-cell techniques for analyzing β-cell maturation kinetics, by simultaneously tracking Ins1 and Pdx1 promoter activities (17,35). At any given time point, ∼20–30% of β-cells with Pdx1 promoter activity did not show Ins1 promoter activity. It should be noted that nearly normal levels of Ins2 mRNA, which also comes on earlier in development and accounts for two-thirds of islet insulin production (16), are still found in these immature cells. Time-lapse imaging demonstrated that Pdx1+/Ins1low islet cells could convert to Pdx1+/Ins1+ cells without cell division (17,35). Genes involved in the mature β-cell phenotype (e.g., Glut2, MafA) were expressed at higher levels in Pdx1+/Ins1+ cells relative to Pdx1+/Ins1low cells. Conversely, genes implicated in early β-cell development (MafB, Nkx2.2) were enriched in Pdx1+/Ins1low cells. Genomic analysis of these two cell stages confirmed that the Pdx1+/Ins1low cells were enriched for genes associated with progenitor cells. On the other hand, the Pdx1+/Ins1+ cells expressed the expected repertoire of β-cell genes needed for functional maturity (17,35). These results suggest that adult β-cells can pass through distinct maturation states that are similar (perhaps identical) to those transitions observed at the population level during embryonic development. Additional dual-reporters (e.g., MafA/MafB) should be generated to further this concept and expand the number of visualized transitions. It is plausible that these maturation events happen en masse in the neonatal period when the functional and genomic status of mature β-cells is finalized (36). We propose that the term β-cell maturation be extended to both embryonic and adult case subjects in the absence of evidence that they are mechanistically distinct. Efforts to harness these immature cells might enable the repopulation of nonfunctional or destroyed β-cells of the diabetic pancreas.
MAINTENANCE OF IDENTITY/MATURITY IN β-CELLS AND OTHER LONG-LIVED CELL TYPES: ACTIVE OR PASSIVE CONTROL?
In addition to β-cell birth and death, functional β-cell mass is potentially influenced by the differentiation status of individual β-cells, including their ability to produce mature insulin and respond to an increase in blood glucose with appropriate insulin secretion. Once progenitors or immature cells differentiate into mature insulin-secreting β-cells, how is their gene expression profile maintained? This is a general problem in biology, encountered by all postmitotic cell types, most notably long-lived cells like pancreatic β-cells (10). Decades of work have uncovered many transcription factors and the mechanisms by which they steer developing pancreatic progenitors toward the generation of multiple, highly differentiated pancreatic cell subtypes (20). Lineage progression toward ever increasing cellular diversity is often viewed as a ratchet mechanism of irreversible steps resulting in the specification and then terminal differentiation of cell subtype identities. From this viewpoint, terminally differentiated cells have long been considered as irreversibly locked into their identity (Fig. 2). However, the discovery that cells can be reprogrammed to an induced pluripotential stem cell state (37) challenges this assumption. In a landmark article, Blau and Baltimore (38) postulated that the identity of a cell, or differentiated status, requires persistent active regulation, rather than lapsing into a passive locked-in state. Although little genetic evidence was available at the time, sufficient evidence has since accumulated to propose that the terminally differentiated state indeed requires active maintenance (Fig. 2). This carries with it profound implications. It suggests that degradation of active maintenance mechanisms may contribute to progressive degeneration of cell identity and function. It also suggests that gene regulation in mature cells may be sufficiently plastic for therapeutic intervention.
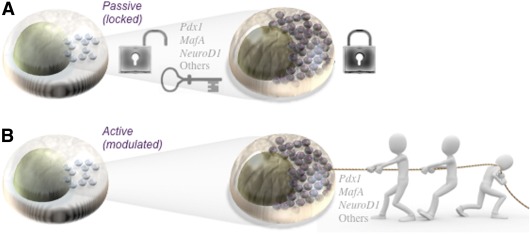
FIG. 2.
Maintenance of functional maturity in adult β-cells: active or passive control? Two alternate, but not mutually exclusive, theories on the maintenance of differentiated phenotypes in long-liver cells like pancreatic β-cells are shown. A:Possibility 1: Key transcription factors are transiently activated and lock the cell into a mature state. B: Possibility 2: Key transcription factors are required constantly at some level to maintain differentiation. In this mode, the differentiation state of the cell is more malleable and subject to modulation by intrinsic, including reductions in key transcription factors, and extrinsic factors.
Differentiation is the process by which specified cell types start to express their type-specific battery of terminal differentiation genes: those genes that execute the form and function of that cell type. The combinatorial action of multiple sequence-specific transcription factors at the cis-regulatory sequences of their target genes lays the foundation for differentiation. These factors then recruit the mediator complex and/or chromatin-modifying enzymes to either increase or suppress the transcriptional activity of bound genomic regions (39). But once cell identity is attained, how expression of terminal differentiation genes is faithfully maintained throughout the lifetime of the cell is unresolved. For long-lived cell types such as β-cells, neurons, and certain plasma cells, organismal homeostasis requires that cell type–specific maintenance occur over many decades. Increasingly, a persistent role for developmental transcription factors in the maintenance of subtype-specific gene expression profiles has been downplayed in favor of distinct, dedicated maintenance mechanisms. These maintenance mechanisms include the following: CpG DNA methylation that suppresses transcription; polycomb and trithorax group complexes that persistently suppress inappropriate gene expression or maintain active gene expression, respectively (40,41); and histone modifications that influence the efficiency of gene transcription (42).
However, these mechanisms are best understood for inheritance of cell-type gene expression patterns in dividing cells, and less well understood for persistent maintenance of cell-type gene expression patterns in long-lived nondividing cells (43). DNA methylation functions in the inheritance of β-cell–specific identity and gene expression after cell division. Bhushan and colleagues showed, using Ins2-Cre mediated ablation of β-cell DNA methyltransferase 1 (Dnmt1), that dividing β-cells no longer generated only new β-cells but also α-cells (44). The underlying mechanism involved derepression of the pro–α-cell transcription factor Arx (45), as a result of the loss of CpG methylation of surrounding cis-regulatory sequences. As a necessary and sufficient regulator of α-cell differentiation within the pancreatic lineage, expression of Arx increased α-cell gene expression such as glucagon and MafB at the expense of β-cell genes including Pdx1, Pax4, and insulin (44,45). Intriguingly, however, Dnmt1 ablation only derepressed Arx after β-cell division, and not in nondividing long-lived β-cells, since Dnmt1 principally acts to maintain DNA methylation during cell division (44). Regardless, these studies point to the importance of epigenetic factors in maintaining adult β-cell identity and show how perturbations in appropriate chromatin state can result in increased cellular plasticity.
An accumulating set of studies using conditional genetic techniques in a range of cell types has directly implicated persistent roles for sequence-specific developmental transcription factors in the long-term maintenance of cell identity (46–52). The ETS domain transcription factor Pet1 is required for differentiation of serotonergic neurons of the raphe nucleus acting as a critical activator of serotonergic genes (53). Pet1 ablation selectively in adult mice resulted in a profound downregulation of these same serotonergic genes, demonstrating a role for Pet1 in long-term maintenance of serotonergic identity. Pet genes have recently been implicated in β-cell adaptation during pregnancy (54). Similarly, conditional knockout of Nurr1 in adult midbrain dopaminergic neurons resulted in progressive loss of dopaminergic neuronal markers (49). Similar results have been borne out by analysis of critical developmental transcription factors in neonatal or adult sympathetic neurons (48), lymphatic endothelial cells (55), as well as B-cells and other long-lived plasma cells (50,51). More recently, Eade et al. (56) have shown for two distinct Drosophila neuronal subtypes that the entire subtype-specific network of transcription factors is subsequently required to maintain the fully differentiated state of those neurons throughout life. Thus, substantial evidence suggests that developmental transcription factor networks are required to maintain the identity of mature neurons, a cell type with increasingly apparent parallels with islet cells (57).
Model systems have also begun to shed light on the mechanisms that maintain long-lived cell identity. For example in Caenorhabditis elegans, Che1, Ast1, or the combination of Ttx3 and Ceh10 act as sequence-specific transcription factors required for the full differentiation of their specific neuronal subtype (52,58,59), playing both differentiation and maintenance roles. These studies also found that these transcription factors autoregulate their own expression (52,58), creating an elegantly simple self-perpetuation loop that maintains subtype-specific transcription factor expression and subsequently terminal differentiation gene expression. Although such simple autoregulation is not observed for all systems (56), it is noteworthy that Pdx1 and several other key pancreatic transcription factors also use autoregulatory feedback loops that help to reinforce the β-cell phenotype (60). Insulin itself may participate in an autocrine feedback loop to maintain β-cell insulin expression and secretion (61). Thus, evidence demonstrates that mature cells maintain their type-specific gene expression programs at least in part by maintaining expression of developmental transcription factor networks. We propose that a similar maintenance mechanism is active in pancreatic β-cells.
ACTIVE MAINTENANCE OF β-CELL FUNCTION: EVIDENCE FROM CONDITIONAL MUTANTS AND HUMAN GENETICS
Extensive analyses of endocrine pancreas development have elucidated many of the gene networks required for the formation of β-cells from progenitors. However, how β-cell identity is maintained and how β-cells adapt to environmental stresses in adulthood are less clear. Conditional gene ablation in mice now permits testing of the hypothesis that regulatory networks controlling β-cell differentiation continue to play important roles in maintaining β-cell identity and, hence, function in the adult. Support for this hypothesis comes from studies on maturity-onset diabetes of the young (MODY), a group of autosomal-dominant disorders that typically manifest in adolescents, predominantly resulting from heterozygous mutations in islet developmental transcription factors (HNF4α, HNF1α, HNF1β, IPF1/PDX1, NEUROD1, and perhaps others) (62).
The homeobox gene Pdx1, one of the most intensively studied pancreatic islet transcription factors, acts both during pancreatic lineage determination and during terminal differentiation of β-cells. Humans and mice lacking the Pdx1 gene are born without a pancreas, and Pdx1 acts at the insulin promoter to induce its expression and maintenance in β-cells (63). Pdx1 heterozygosity leads to a rare monogenic form of type 2 diabetes, termed MODY4 (62). The disease manifests many years after β-cell differentiation, suggesting a persistent role for Pdx1 in maintaining the normal function and survival of β-cells. Inducible approaches have been used to demonstrate that Pdx1 deletion in adult islet cells impairs β-cell function, providing direct evidence for such a maintenance role. With the use of Ins2-Cre, floxed Pdx1 was ablated in β-cells at a late stage of differentiation. This resulted in a 40% loss of β-cell mass, substantial downregulation of amylin/IAPP, a 90% loss of insulin, elimination of the glucose transporter Glut2, as well as a degree of lineage confusion wherein 22% of insulin-positive cells also expressed glucagon, a differentiation marker for pancreatic α-cells (64). Similar results were obtained in a subsequent study utilizing induced Pdx1 RNAi expression (64) or in doxycycline-treated inducible transgenic Pdx1tTA/tTA:TgPdx1 mice (65–67). Indeed, forced Pdx1 expression can cause α-cell to β-cell reprogramming (68). Although these studies point to a maintenance role for Pdx1 in mature β-cells, it is currently difficult to interpret the exact nature of the dedifferentiated cells. Additional analysis of characteristic markers of β-cell identity within the pancreas will be required to unravel the phenotype and understand the impact of Pdx1 ablation in β-cells. It is intriguing that soluble factors including insulin and incretin hormones can act on the adult β-cell to dynamically regulate Pdx1 activity (69,70), but whether they actively contribute to the maintenance of differentiation is less well understood.
The MODY6 transcription factor Neurod1 (bHLH factor) is also essential for β-cell differentiation. Recently, postdifferentiation ablation of floxed Neurod1 (using Ins2-Cre or Pdx1-CreER) resulted in glucose intolerance, defective insulin secretion, and reduced Ins1 expression (47). These authors also demonstrated that although the Ins1 gene was downregulated, the Ins2 gene was not, in line with the distinct regulation of the two mouse insulin genes during development (16). It is interesting that the opposite is true for the transcription factor FoxO1 and its upstream regulator Raf1, which regulate the Ins2 gene and not Ins1 (61,71). Neurod1 and FoxO1 are proposed to function in the differentiation and maintenance of mature β-cells. Although a survey of all developmental transcription factors in mature β-cells is not available, the results of these studies on Pdx1 and Neurod1, combined with the identity of MODY genes HNF1α, HNF1β, and HNF4α, make it reasonable to propose that elaborate networks of developmentally required transcription factors are retained in mature β-cells and that they are essential, in combination, to maintain β-cell gene expression and function. A prediction of this model would be that hypomorphic aberrations in multiple transcription factors would synergistically abrogate normal β-cell function. Indeed, there is some support for this hypothesis. Pdx1+/− heterozygotes exhibit reduced glucose-stimulated insulin secretion but neither Hnf3β+/− nor Hnf4α+/− heterozygotes show any obvious defect (72). However, both Pdx1+/−:Hnf3β+/− and Pdx1+/−:Hnf4α+/− compound heterozygotes display β-cell functional deficits that are greater than the sum of either single heterozygote alone (72,73). Moreover, this careful study also found subtle differences in the sets of genes that were disrupted in the different heterozygous backgrounds, suggesting that each transcription factor acts in a partially overlapping manner to maintain a subset of the overall battery of β-cell terminal differentiation genes. Whether these phenotypes arise from developmental defects or a requirement for these genes in maintaining adult β-cell differentiation remains to be tested.
CONTROL OF ADULT β-CELL FATE AND FUNCTION BY DEVELOPMENTAL FACTORS: THE NOTCH/NGN3 EXAMPLE
With the concept of active maintenance of cellular identity in mind, a number of laboratories have studied in adult islets the role of essential developmental pathways, including networks involving MODY factors (e.g., Pdx1 [65–68]), gene systems required for α-cell versus β-cell fate (e.g., Arx/Pax4 [45]), and regulatory networks in the maintenance of a precursor cell pool (e.g., Notch/Ngn3 signaling). Although the expression of some genes, such as Ngn3, is much higher during development, published evidence suggests that many of the genes essential for embryonic development of the islet are maintained at low levels in the adult β-cell. For example, Ngn3 is necessary for development of all endocrine cell lineages in the pancreas, and Ngn3-positive cells represent embryonic stage/niche-dependent, individually unipotent progenitor cells (74,75). During embryonic development, Ngn3 and the pool of endocrine progenitor cells is controlled by Notch via Hes1 (27,76). We demonstrated that small amounts of Ngn3 protein are present in adult human and mouse islets (25), and although this observation was controversial at the time, it has recently been extended by others (26,27). Indeed, conditional knockout of Ngn3 in adult islets using the Pdx1-CreER deleter mouse demonstrated a role for Ngn3 in β-cell function and maturation (26). Decreasing Notch signaling with the inhibitor DAPT increased Ngn3 expression and induced apoptosis in adult human and mouse islets under low glucose conditions (25), indicating that this gene network is critically important for β-cell survival in the adult. However, DAPT protected islets under high glucose conditions (25), suggesting the energy-dependent regulation of apoptosis by Notch. It is interesting that presenilin genes, which are required to activate Notch, are regulated by glucose in adult β-cells (77). It is also notable that, in our hands, robust overexpression of Ngn3 induced β-cell apoptosis (25), consistent with Ngn3 being a negative target of prosurvival Notch signaling. Thus, emerging evidence suggests that the Notch/Ngn3 pathway plays important roles in the adult β-cell and that these roles may be different from the roles of these genes during development. Little is known about the factors that regulate Notch and Ngn3 signaling in the adult islet, although we recently found roles for the Musashi family of translational suppressors, which are known to control Notch signaling, in β-cell survival and differentiation (28).
SOLUBLE REGULATORS OF ADULT β-CELL DIFFERENTIATION STATUS
A number of groups have carried out studies aimed at determining whether soluble growth factors that are known to regulate pancreatic β-cell development may play a role in maintaining adult β-cell identity. Emerging evidence suggests a prominent role for members of the TGFβ superfamily. Using a factorial design high-content screening approach, we have recently found that activin A and follistatin have reciprocal effects on the maturity of adult β-cells (78). BMP4-BMPR1A signaling is also required for maintaining the adult β-cell phenotype and plays an essential role in glucose-stimulated insulin secretion (79). Mice expressing dominant-negative BMPR1A in the Pdx1 promoter domain showed >50% downregulation of Pdx1, insulin, Nkx6.1, PC1/3, PC2, Glut2, glucokinase, and calpain-10, as well as multiple genes required for exocytosis; the converse was observed in mice overexpressing BMP4 in β-cells (79). Such a maintenance role for extrinsic BMP signaling appears to be conserved; in Drosophila, the maintenance of differentiation in adult neurons requires persistent BMP signaling (80). Nutrients, including glucose, also have powerful positive effects on β-cell maturity (78). It is not yet clear whether the effects of glucose are direct or mediated via autocrine insulin signaling. Although these studies point to the feasibility of modulating adult β-cell differentiation status with soluble factors, glucose and insulin are obviously poor therapeutic candidate targets in vivo.
SIGNALING NETWORKS IN ADULT PANCREATIC CELL PLASTICITY
A key prediction of the hypothesis that differentiation requires active maintenance is that adult β-cells would exhibit substantial plasticity should key transcription factor pathways be perturbed. Indeed, surprisingly few factors are required to make induced pluripotent stem cells from multiple tissues, including the pancreas (81). Elegant work showed that Pdx1, Ngn3, and MafA transcription factors are sufficient to convert exocrine cells into insulin-positive cells (82). Forced overexpression of Akt has been shown to transdifferentiate cells with insulin promoter activity into acinar and ductal cells (83). Nevertheless, the extent to which these cells are transdifferentiated and the extent to which adult islet cells exhibit plasticity remains to be assessed. Evidence of plasticity between islet endocrine cell lineages is also growing. For example, in the adult, it has been shown that some insulin-expressing cells also express glucagon and other hormones (35,84). The frequency of these multihormonal cells in the adult may increase with stress (85,86) or when critical transcription factors such as Pdx1 are dysregulated (64,67,87). Indeed, recent lineage-tracing experiments have demonstrated that a fraction of regenerating β-cell passes through a phase with glucagon promoter activity (29). These and other findings (88,89) cast doubt on the concept that β-cell and α-cell lineages are completely distinct, as suggested previously (90). Approaches to track the expression of insulin and glucagon, simultaneously, in living cells could help to resolve the nature of this apparent lineage switch.
CONCLUSIONS
Our understanding of the intrinsic and extrinsic forces that maintain the mature β-cell state or permit lineage plasticity is evolving rapidly. Recent studies point to dynamic regulation of maturity, as well as a surprising level of lineage flexibility in the endocrine pancreas. We suggest that temporally controlled gene targeting and single-cell analysis of cell fate decisions will continue to advance the field. We also expect that parallels between the regulation of adult cell differentiation in islet cells and other tissues will continue to open new avenues for investigation. Future studies will undoubtedly provide a more detailed picture of the plasticity within adult pancreatic endocrine cell types. It is likely that this fundamental β-cell biology research will reveal important insights that can be harnessed as part of efforts to protect, regenerate, or replace β-cells lost in diabetes.
ACKNOWLEDGMENTS
Work in the authors’ laboratories is supported by the Juvenile Diabetes Research Foundation, the Canadian Diabetes Association, the Stem Cell Network, the Canadian Institutes of Health Research, the Child & Family Research Institute, and the Alzheimer Society of Canada.
No potential conflicts of interest relevant to this article were reported.
M.S., F.C.L., B.G.H., T.J.K., D.W.A., and J.D.J. wrote the manuscript and approved the final version. J.D.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank members of their laboratories for helpful discussions. The authors apologize to the many colleagues whose work they did not have space to cite.
REFERENCES
- 1.Rojas A, Khoo A, Tejedo JR, Bedoya FJ, Soria B, Martín F. Islet cell development. Adv Exp Med Biol 2010;654:59–75 [DOI] [PubMed] [Google Scholar]
- 2.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev 2005;85:1255–1270 [DOI] [PubMed] [Google Scholar]
- 3.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006;27:356–370 [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-Cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JD, Luciani DS. Mechanisms of pancreatic beta-cell apoptosis in diabetes and its therapies. Adv Exp Med Biol 2010;654:447–462 [DOI] [PubMed] [Google Scholar]
- 6.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 7.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 8.Meier JJ, Butler AE, Saisho Y, et al. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology 2008;149:2251–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 2010;95:E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin MM, Kushner JA. Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manesso E, Toffolo GM, Saisho Y, et al. Dynamics of beta-cell turnover: evidence for beta-cell turnover and regeneration from sources of beta-cells other than beta-cell replication in the HIP rat. Am J Physiol Endocrinol Metab 2009;297:E323–E330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab 2010;11:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku HT. Minireview: pancreatic progenitor cells—recent studies. Endocrinology 2008;149:4312–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 16.Deltour L, Leduque P, Blume N, et al. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci USA 1993;90:527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology 2009;150:1627–1635 [DOI] [PubMed] [Google Scholar]
- 18.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis 2000;26:99–109 [PubMed] [Google Scholar]
- 19.Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 2011;8:281–293 [DOI] [PubMed] [Google Scholar]
- 20.Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development 2007;134:427–438 [DOI] [PubMed] [Google Scholar]
- 21.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 2009;17:849–860 [DOI] [PubMed] [Google Scholar]
- 23.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 25.Dror V, Nguyen V, Walia P, Kalynyak TB, Hill JA, Johnson JD. Notch signalling suppresses apoptosis in adult human and mouse pancreatic islet cells. Diabetologia 2007;50:2504–2515 [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Jensen JN, Seymour PA, et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci USA 2009;106:9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimajiri Y, Kosaka Y, Scheel DW, et al. A mouse model for monitoring islet cell genesis and developing therapies for diabetes. Dis Model Mech 2011;4:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabat M, Kalynyak TB, Lim GE, et al. Musashi expression in beta-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes. Cell Death Dis 2011;2:e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Herrera PL, Carreira C, et al. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology 2010;138:1954–1965 [DOI] [PubMed] [Google Scholar]
- 31.Pipeleers DG. Heterogeneity in pancreatic β-cell population. Diabetes 1992;41:777–781 [DOI] [PubMed] [Google Scholar]
- 32.Soria B, Chanson M, Giordano E, Bosco D, Meda P. Ion channels of glucose-responsive and -unresponsive β-cells. Diabetes 1991;40:1069–1078 [DOI] [PubMed] [Google Scholar]
- 33.Osterbye T, Funda DP, Fundová P, Månsson JE, Tlaskalová-Hogenová H, Buschard K. A subset of human pancreatic beta cells express functional CD14 receptors: a signaling pathway for beta cell-related glycolipids, sulfatide and β-galactosylceramide. Diabetes Metab Res Rev 2010;26:656–667 [DOI] [PubMed] [Google Scholar]
- 34.Hodish I, Absood A, Liu L, et al. In vivo misfolding of proinsulin below the threshold of frank diabetes. Diabetes 2011;60:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabat M, Pourghaderi P, Soukhatcheva G, et al. Kinetics and genomic profiling of adult human and mouse β-cell maturation. Islets 2011;3:175–187 [DOI] [PubMed] [Google Scholar]
- 36.Jermendy A, Toschi E, Aye T, et al. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia 2011;54:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 38.Blau HM, Baltimore D. Differentiation requires continuous regulation. J Cell Biol 1991;112:781–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger SL. The complex language of chromatin regulation during transcription. Nature 2007;447:407–412 [DOI] [PubMed] [Google Scholar]
- 40.Beisel C, Paro R. Silencing chromatin: comparing modes and mechanisms. Nat Rev Genet 2011;12:123–135 [DOI] [PubMed] [Google Scholar]
- 41.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007;128:735–745 [DOI] [PubMed] [Google Scholar]
- 42.Kouzarides T. Chromatin modifications and their function. Cell 2007;128:693–705 [DOI] [PubMed] [Google Scholar]
- 43.Ptashne M. On the use of the word ‘epigenetic’. Curr Biol 2007;17:R233–R236 [DOI] [PubMed] [Google Scholar]
- 44.Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell 2011;20:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collombat P, Mansouri A, Hecksher-Sorensen J, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003;17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci 2010;13:1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu C, Stein GH, Pan N, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab 2010;11:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt M, Lin S, Pape M, et al. The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev Biol 2009;329:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadkhodaei B, Ito T, Joodmardi E, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci 2009;29:15923–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 2007;27:49–63 [DOI] [PubMed] [Google Scholar]
- 51.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med 2005;202:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etchberger JF, Lorch A, Sleumer MC, et al. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev 2007;21:1653–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendricks TJ, Fyodorov DV, Wegman LJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 2003;37:233–247 [DOI] [PubMed] [Google Scholar]
- 54.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson NC, Dillard ME, Baluk P, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 2008;22:3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eade KT, Fancher HA, Ridyard MS, Allan DW. Developmental transcriptional networks are required to maintain neuronal subtype identity in the mature nervous system. PLoS Genet 2012;8:e1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arntfield ME, van der Kooy D. β-Cell evolution: how the pancreas borrowed from the brain: the shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. BioEssays 2011;33:582–587 [DOI] [PubMed] [Google Scholar]
- 58.Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell 2004;6:757–770 [DOI] [PubMed] [Google Scholar]
- 59.Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature 2009;458:885–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshak S, Benshushan E, Shoshkes M, Havin L, Cerasi E, Melloul D. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3beta transcription factors mediate beta-cell-specific expression. Mol Cell Biol 2000;20:7583–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alejandro EU, Lim GE, Mehran AE, et al. Pancreatic β-cell Raf-1 is required for glucose tolerance, insulin secretion, and insulin 2 transcription. FASEB J 2011;25:3884–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson JD. Pancreatic beta-cell apoptosis in maturity onset diabetes of the young. Can J Diabetes 2007;31:001–008 [Google Scholar]
- 63.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia 2001;44:1203–1214 [DOI] [PubMed] [Google Scholar]
- 64.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 1998;12:1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lottmann H, Vanselow J, Hessabi B, Walther R. The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med (Berl) 2001;79:321–328 [DOI] [PubMed] [Google Scholar]
- 66.Thomas MK, Devon ON, Lee JH, et al. Development of diabetes mellitus in aging transgenic mice following suppression of pancreatic homeoprotein IDX-1. J Clin Invest 2001;108:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holland AM, Góñez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of β-cells in the adult pancreas. Diabetes 2005;54:2586–2595 [DOI] [PubMed] [Google Scholar]
- 68.Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev 2011;25:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson JD, Bernal-Mizrachi E, Alejandro EU, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA 2006;103:19575–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 2005;54:482–491 [DOI] [PubMed] [Google Scholar]
- 71.Meur G, Qian Q, da Silva Xavier G, et al. Nucleo-cytosolic shuttling of FoxO1 directly regulates mouse Ins2 but not Ins1 gene expression in pancreatic beta cells (MIN6). J Biol Chem 2011;286:13647–13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shih DQ, Heimesaat M, Kuwajima S, Stein R, Wright CV, Stoffel M. Profound defects in pancreatic beta-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1alpha, and Hnf-3beta. Proc Natl Acad Sci USA 2002;99:3818–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boj SF, Petrov D, Ferrer J. Epistasis of transcriptomes reveals synergism between transcriptional activators Hnf1alpha and Hnf4alpha. PLoS Genet 2010;6:e1000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 2009;136:3567–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson KA, Dursun U, Jordan N, et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465 [DOI] [PubMed] [Google Scholar]
- 76.Apelqvist A, Li H, Sommer L, et al. Notch signalling controls pancreatic cell differentiation. Nature 1999;400:877–881 [DOI] [PubMed] [Google Scholar]
- 77.Dror V, Kalynyak TB, Bychkivska Y, et al. Glucose and endoplasmic reticulum calcium channels regulate HIF-1beta via presenilin in pancreatic beta-cells. J Biol Chem 2008;283:9909–9916 [DOI] [PubMed] [Google Scholar]
- 78.Szabat M, Johnson JD, Piret JM. Reciprocal modulation of adult beta cell maturity by activin A and follistatin. Diabetologia 2010;53:1680–1689 [DOI] [PubMed] [Google Scholar]
- 79.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab 2007;5:207–219 [DOI] [PubMed] [Google Scholar]
- 80.Eade KT, Allan DW. Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling. J Neurosci 2009;29:3852–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 2011;9:17–23 [DOI] [PubMed] [Google Scholar]
- 82.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elghazi L, Weiss AJ, Barker DJ, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology 2009;136:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katsuta H, Akashi T, Katsuta R, et al. Single pancreatic beta cells co-express multiple islet hormone genes in mice. Diabetologia 2009;53:128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J Endocrinol 2000;165:93–99 [DOI] [PubMed] [Google Scholar]
- 86.Guz Y, Nasir I, Teitelman G. Regeneration of pancreatic beta cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology 2001;142:4956–4968 [DOI] [PubMed] [Google Scholar]
- 87.Johnson JD, Ahmed NT, Luciani DS, et al. Increased islet apoptosis in Pdx1+/- mice. J Clin Invest 2003;111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009;138:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Habener JF. Alpha cells beget beta cells. Cell 2009;138:424–426 [DOI] [PubMed] [Google Scholar]
- 90.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000;127:2317–2322 [DOI] [PubMed] [Google Scholar]