Abstract
Sialic acids are a diverse family of monosaccharides widely expressed on all cell surfaces of vertebrates and so-called “higher” invertebrates, and on certain bacteria that interact with vertebrates. This overview surveys examples of biological roles of sialic acids in immunity, with emphasis on an evolutionary perspective. Given the breadth of the subject, the treatment of individual topics is brief. Subjects discussed include biophysical effects regulation of factor H; modulation of leukocyte trafficking via selectins; Siglecs in immune cell activation; sialic acids as ligands for microbes; impact of microbial and endogenous sialidases on immune cell responses; pathogen molecular mimicry of host sialic acids; Siglec recognition of sialylated pathogens; bacteriophage recognition of microbial sialic acids; polysialic acid modulation of immune cells; sialic acids as pathogen decoys or biological masks; modulation of immunity by sialic acid O-acetylation, sialic acids as antigens and xeno-autoantigens; anti-sialoglycan antibodies in reproductive incompatibility, and sialic-acid based blood groups.
Keywords: sialic acids, immunity, evolution, selectins, Siglecs, sialidases
Sialic acids (Sias) are unusual sugars with a shared 9-carbon backbone that are widely expressed on the surfaces of all cells in all animals of the deuterostome lineage (vertebrates and so-called “higher” invertebrates), and also in certain pathogenic or symbiotic bacteria that associate with them (Refs. 1–7; Fig. 1A). Given their remarkable diversity in structure, glycosidic linkage, and underlying glycan chains, as well their exposed location, it is not surprising that Sias have numerous roles in many aspects of immunity (by “immunity,” we here mean immunology, as well as aspects of microbiology that are relevant to symbiosis and pathogenesis). Details regarding the occurrence, biosynthesis, structural diversity, cellular expression patterns, rapid evolution, and species variations of sialic acids have been extensively reviewed elsewhere (1–7) and will not be repeated here. This overview surveys the multifarious roles of sialic acids in selected aspects of Immunity. Given the vast breadth of the subject under consideration, the treatment of the selected topics is necessarily brief, and references to the primary literature are not comprehensive. The emphasis is also on topics with which the authors are more familiar.
Figure 1.
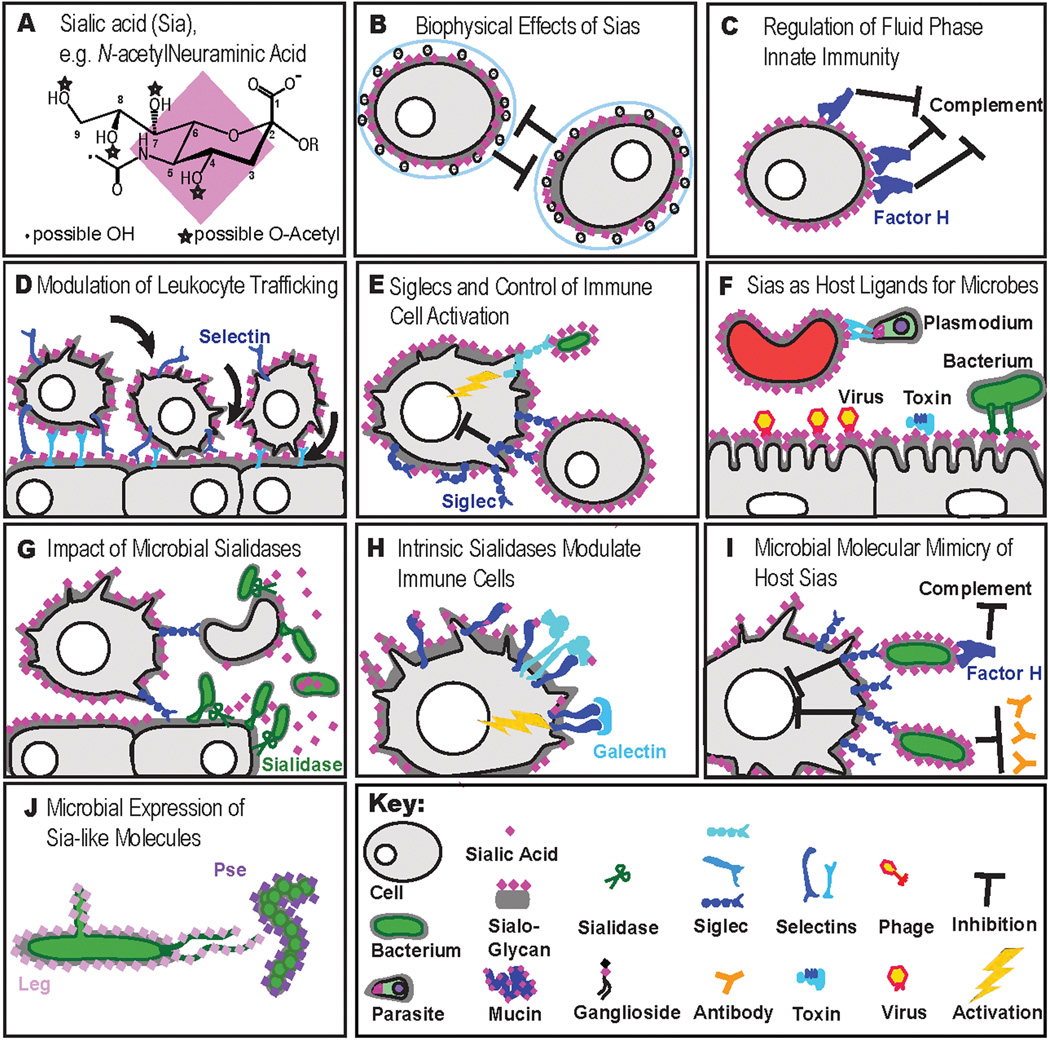
Examples of roles of sialic acids in immunity. Sialic acids are shown as pink diamonds. See the text for details. (A) Neu5Ac, the most common sialic acid in mammals. These acidic sugars share a 9-carbon backbone and can be modified in many ways. (B) The high density of terminal sialic acids on the glycocalyx of vertebrate cells imparts negative charge and hydrophilicity to cell surfaces, altering biophysical properties.(C) Factor H binds cell surface Sias, protecting cell surfaces from the alternative complement pathway.(D) Intrinsic Sia-binding molecules such as selectins on endothelia, leukocytes, and platelets initiate leukocyte rolling on endothelial surfaces, a key initial step for leukocyte extravasation. (E) Intrinsic Sia-binding Siglec molecules on immune cells detect sialylated ligands and can inhibit immune cell activation. There are also activatory Siglecs. (F) Host Sias are frequently exploited as attachment sites (“receptors”) by pathogens including protozoa, viruses, bacteria, and toxins. (G) Microbial sialidases can help pathogens to expose underlying glycan binding sites, to avoid sialylated decoys (see below), and and/or provide Sias as food sources. The loss of SAMPs from cells may then be used by host immune cells to react to pathogens, and/or to clear away desialylated cells or glycoproteins. (H) Endogenous sialidases such as Neu1 can modulate immune cell function by modulating receptor clustering, possibly by exposing underlying galactose residues and facilitating galectin mediated cross-linking of surface molecules. (I) Microbial mimicry of host Sias allows manipulation of host immune response by engaging inhibitory Siglecs, inhibiting complement via factor H binding, and reducing the opportunity of the host to form antibodies. (J) Microbial synthesis of Sia-like molecules, such as legionaminic acid and pseudaminic acid stabilizes fimbriae.
Biophysical effects of sialic acids
Given their ubiquitous presence and abundance at the surface of all cell types (including those of the immune system), Sias have major biophysical effects (8, 9). The typical cell displays tens of millions of Sia molecules, and it is estimated that the local concentrations on the cell surface glycocalyx can approach 100 mM (10). Sialic acids thus provide a large component of negative charge repulsion between cells, which could alter the biophysical properties of cellular interactions (Fig. 1B). Many earlier studies removed Sias from immune cell surfaces using sialidases and showed marked changes in behavior of such cells (11). However, such studies are often confusing, because wholesale removal of cell surface Sias has many potentially pleiotropic effects. First, removal reduces the net charge and hydrophilicity of the cell surface. Second, it can reduce the charge repulsion between adjacent cell surface molecules. Third, it eliminates ligands for endogenous receptors like Siglecs and selectins (see below). Fourth, sialic acid removal exposes underlying glycans (mostly galactose residues), which can be recognized by other endogenous receptors, such as galectins and the galactose-binding proteins of macrophages. Finally, there is potential for sialidase treatment to enhance cell surface interactions and lattices of galectins with the uncapped N-glycans of various surface receptors (see below).
Thus, more subtle alterations in cell surface Sias are needed to investigate their specific functions. In this regard, the use of mild periodate oxidation to eliminate only the C9 and C8 side chain carbon atoms of Sias is a remarkably specific manipulation, which leaves the rest of the sialic acid molecule and its negative charge intact, and is not known to affect other surface structures (12). However, periodate oxidation also generates a C7-aldehyde on the sialic acid side chain, which can potentially react with lysine residues on adjacent proteins, cross-linking cell surface glycoproteins. This may help explain some reported dramatic effects of periodate oxidation on lymphocytes (13). A less risky option would be to modify the type of sialic acid on the surface. One approach is to feed unnatural (bioorthogonal) precursors to generate unnatural Sias (14–16). If high levels of incorporation can be achieved on the cell surface, the altered biophysical properties can then be further studied. However, Sia recognition phenomena could also be altered. In this regard, it would also be worth asking whether the single oxygen atom difference between the common Sia (Neu5Ac) and the non-human one (Neu5Gc) (17) can alter immune cell behavior.
Meanwhile, the high densities of Sias found on surface polysaccharides of various pathogenic bacteria (see below) can also markedly alter the biophysical properties of these organisms (3–7). But again, simply eliminating these Sias by genetic or enzymatic means can have secondary effects, due to exposure of underlying glycans. Overall, while it is clear that the biophysical properties of Sias modulate many cellular and microbial interactions in the immune system, these effects are not easy to study, because the experimental approaches may perturb the very thing that is being explored in a pleiotropic fashion. This is an important and challenging area for future studies.
Sialic acid regulation of fluid phase innate immunity
Classic studies showed a role for Sias in regulating the alternative pathway of the complement activation (18, 19). The mechanism involves the major serum protein factor H, which recognizes Sias as “self," gets recruited to native cell surfaces, and so helps to downregulate the constant “tick-over” of the complement pathway on all surfaces (Refs. 18–21; Fig. 1C). Details of this mechanism have been elucidated, including accelerated dissociation of the C3bBb C3 convertase and acting as a cofactor for factor I-mediated cleavage of C3b (21). While Sias may thus act as “self-associated molecular patterns” (SAMPs) (22) for recognition by factor H, this does not fully explain the relative specificity of factor H for sialoglycan structural variants. There are also complexities involving the type of glycosidic linkage of Sias to the underlying glycan (its presentation in space), which may alter recognition (23, 24). Furthermore, studies have shown that this factor H self-recognition is mediated by certain anion binding sites, which can also recognize sulfated glycosaminoglycans as “self.” The factor H domains involved in these recognition phenomena are largely domains 19–20 (Refs. 25–28), and this mechanism is also hijacked by bacteria that express Sias on their surface polysaccharides (21, 23) (see below). Interestingly, mutations in some of these domains were found by genome-wide association studies to correlate with increased risk of complement-mediated inflammatory processes, such as hemolytic-uremic syndrome, membranoproliferative glomerulonephritis, (29) and age-dependent macular degeneration (27, 30). These experiments of nature provide evidence of the functional significance of Sias as SAMPs for recognition by factor H.
Variation in sialic acid side chain O-acetylation can also affect factor H binding. Classic studies suggested that the amount of sialic acid on red blood cells on erythrocytes of different strains of mice might restrict the extent of control of the alternate complement pathway activation (31). It was then shown that the difference was not the amount of sialic acid, but in the extent of sialic acid side-chain O-acetylation, that is, such modified Sias are not good targets for factor H binding (32). These older observations need to be revisited in the light of modern evidence regarding the mechanisms that control Sia O-acetylation (1, 33), as well as genetic and genomic sequences of these strains. Also of note is that ficolins (circulating soluble activators of the lectin pathway of complement activation) can recognize sialic acids, particularly on the surfaces of sialylated bacteria (34–36). This appears to be a host response to molecular mimicry by bacteria (see below).
Modulation of leukocyte trafficking via sialylated selectin ligands
Until the 1980s, factor H was the only known intrinsic vertebrate sialic acid–binding protein. A classic study (37) then noted that pre-treatment of lymph node sections with a sialidase abolished the interaction of lymphocytes with the high-endothelial venules, which normally provide exit sites for lymphocytes. These and other observations eventually led to recognition of the selectin family of cell adhesion molecules and their role in leukocyte trafficking (Refs. 38–42; Fig. 1D). Different isoforms of these endogenous lectins were found expressed on leukocytes (L-selectin), platelets (P-selectin), and endothelium (P- and E-selectin). It then became apparent that Sias in the glycan sequence Siaα2–3Galβ1-3/4(Fucα1-3/4)GlcNAcβ1-R (Sialyl Lewis X/A) are critical components of the natural ligands for these selectins (38, 39, 43–50). In some instances, Sialyl Lewis X/A motifs combine with other features such as sulfation on the Gal or GlcNAc residues (L-selectin ligands), and/or sulfation of adjacent tyrosine residues (P-selectin) and contribute towards specific recognition sites on specific proteins, particularly mucin-like glycoproteins (51). A particularly striking example was the elucidation of a defined amino-terminal sulfoglycopeptide motif on P-selectin glycoprotein ligand-1 (PSGL-1), which serves as a specific high affinity ligand of P-selectin (52).
Whereas Sias form a critical part of most ligands for selectins, recognition does not seem to be affected by other structural details of the Sias themselves, except that the α2–3–linkage to the underlying galactose residue is critical. In keeping with this, some selectin ligands can function with a sulfate ester at the 3 position of galactose, instead of a sialic acid (53). Overall, it appears that Sias are primarily acting as conveyers of a necessary negative charge for selectin interactions, and the details of Sia diversity do not matter. In keeping with this, some 6-O-sulfated glycosaminoglycans, such as heparan sulfate, can act as alternate selectin ligands (54, 55). However, if one oxidizes the side chain of sialic acid with periodate and generates an aldehyde, this reactive group can be cross-linked into the binding pocket of the selectin via a covalent interaction (56). Overall it is evident that α2–3–linkage specific-sialyltransferases play a key role in generating selectin ligands, along with α1-3/4 fucosyltransferases, and GlcNAc sulfotransferases and/or tyrosine sulfotransferases (57).
Siglecs in the control of immune cell activation
In the mid-1980s, some macrophage types were found to form rosettes with sheep erythrocytes in vitro, and that binding could be abolished by sialidase pretreatment of the erythrocytes (58). This sialic acid-dependent receptor was purified and shown to be a very large protein called sialoadhesin, which was then demonstrated to bind sialic acid-containing ligands in vitro (59). However given the size of the sialoadhesin molecule and the era in which this work occurred, cloning proved difficult. Meanwhile, expression cloning of the presumed ligand for a B cell "adhesion molecule" called CD22 had surprisingly yielded a sialyltransferase (60). In fact, it turned out that CD22 was a sialic acid-binding lectin, with recombinant soluble CD22 shown to bind Sias through its extracellular domain, and not to the sialyltransferase identified through expression cloning (the transferase is not at the cell surface but rather generates sialylated ligands of CD22 in the Golgi).(61) Moreover, recognition by CD22 was specific for the α2–6–linkage, with no binding to α2–3–linked Sia (62). Additional studies defined the highly conserved preference of CD22 for this linkage and characterized the interactions further (63). Soon thereafter, the cloning of sialoadhesin revealed that its amino-terminal domains had a homology with CD22 and to similar domains of two other previously known proteins, CD33 and myelin-associated glycoprotein (MAG), suggesting that these molecules might belong to a single family of sialic acid-binding proteins (64). Further studies showed that this was indeed the case, resulting in recognition of a new family of sialic acid-binding proteins (65). It was initially suggested that these molecules be called sialoadhesins (66–68). However, besides the confusing relationship to the first molecule already with this name, some of these proteins did not seem to mediate cell–cell adhesion. The alternate term suggested was Siglec, to stand for sialic acid-recognizing Ig-superfamily lectins, as a subset of I-type lectins (Ig-like lectins) (69). Discussions among those working in the field eventually led to general acceptance of this term, with the founding members sialoadhesin, CD22, CD33, and MAG being designated Siglecs-1–4 (Ref. 70). Studies of expressed sequence tags and mining of genomic sequence data then led to extension of this family of intrinsic vertebrate lectins, which now comprise at least 16 members in primates (71–-79).
Interestingly, a subset of Siglecs seems relatively conserved in mammals (CD22/Siglec-2 and sialoadhesin/Siglec-1), and even among vertebrates (MAG/Siglec-4 and Siglec-15). In contrast, another subset found in one large syntenic cluster (on chromosome 19q in humans) shows the highest amount of variation between species (71, 72). This subfamily was named CD33-related Siglecs (or CD33rSiglecs) and shown to have a variety of Sia- binding properties. CD33rSiglecs can be further subdivided into two categories. Most have cytosolic domains containing ITIM motifs that can be tyrosine phosphorylated, resulting in recruitment of tyrosine phosphates like SHP-1 and -2 (80–84). This in turn results in dephosphorylation of tyrosine residues on various kinases associated with other receptors, effectively downregulating their functions (Fig.1E). Thus, these inhibitory CD33rSiglecs likely serve as innate immune detectors for SAMPs,, thereby downregulating unwanted inflammation, particularly that occurring in response to tissue damage (85). Notably several of these CD33rSiglecs also have a second cytosolic ITIM-like motif whose functions are much less clear. Moreover, there is evidence that some of the inhibitory effects of such Siglecs do not require either of these tyrosine-based motifs (86, 87). This suggests that more attention should be paid to the better-conserved extracellular C2-set domains, as mediators of additional and/or complementary functions.
In contrast to the inhibitory Siglecs, a positively charged amino acid in the transmembrane domain of some activating CD33rSiglecs allows them to engage ITAM-containing adapter molecules like DAP12, which in turn recruits the tyrosine kinase Syk and mediates tyrosine phosphorylation of various receptors and kinases (88–91). In some instances, CD33rSiglecs with inhibitory and activatory properties have undergone gene conversion events that maintain their amino-terminal identity, suggesting that they may be paired receptors, sending opposite signals on binding of the same ligand(s). In this context, it seems likely that the activatory Siglecs represent an evolutionary response to bacteria that are “hijacking” inhibitory Siglecs (see below) (88, 92).
The general subject of Siglecs and their biology and evolution has been extensively discussed elsewhere (73, 74, 76, 77, 79, 84, 93–98) and details will not be repeated here. However, certain features are worthy of special note. First, most Siglecs are typically bound by so-called “cis ligands”, that is, sialylated glycans on the same cell surface (99, 100). However, another cell surface or a soluble ligand with a high enough density of sialylated ligands can compete out the cis ligands and cause engagement (76, 101, 102). Second, the amino terminal V-set Ig-like domain contains the sialic acid recognition site, including a canonical conserved arginine residue that is critical for interaction with the carboxylate of sialylated ligands (103–107). Interestingly, this arginine residue can be naturally mutated, affecting one or more Siglecs unique to a given species or taxon (71, 88, 107). One possibility is that these events occur randomly because the arginine codon (CGN) is highly mutable. However, there are instances where the arginine appears to mutate and then reappear in one phylogenetic branch, for example, for Siglecs-5 and -14 in humans versus great apes.(88) Taken together with the high frequency of such events, it is more likely that these mutations are an evolutionary mechanism for rapidly "retiring" a Siglec, that is, curtailing its interactions with sialic acid–containing ligands without losing the entire molecule, leaving the option to “resurrect” it later. By convention, such Siglecs are referred to by a Roman numeral (e.g., Siglec-XII in humans and Siglec-V in chimpanzees). Both the arginine mutations and the paired receptors mentioned above are likely to be evolutionarily related to the interactions of sialylated microbes with Siglecs (see below).
Sialic acids as host ligands (receptors) for microbes
Given the location and abundance of Sia on cell surfaces, it is not surprising that numerous viruses and some bacteria use host-sialylated structures as targets for binding and recognition (Refs.108–112; Fig. 1F). The same is also true of several important bacterial toxins (112). In the case of viruses that bind Sia via a hemagglutinin, most also often express a sialidase (neuraminidase) that cleaves the same receptor (113). This dualistic recognition and removal of sialic acid is best studied for influenza viruses (114). The traditional term neuraminidase is being replaced by sialidase, since neuraminic acid (with a free amino group) is not only vanishingly rare in nature but is also actually resistant to the neuraminidases studied to date.
Unfortunately, given the history of virology, where viruses were originally characterized by their hemagglutinin (H) or neuraminidase (N), by antigenicity/serology, and now by their RNA genotypes, it would be difficult to ask this particular field to change nomenclature, for example from H1N1 to H1S1.
When it comes to natural sialic acid modifications as pathogen ligands, further subtleties abound. For example, some viruses recognize O-acetyl-Sias and have a receptor-destroying enzyme that removes the O-acetyl group (115–125). Several eukaryotic pathogens also employ sialic acid recognition as part of interactions with hosts (the falciparum malarial merozoite) (126–129). Meanwhile, a bacterial SubAb toxin selectively recognizes ligands bearing the Neu5Gc sialic acid (130). Examples of such binding phenomena are numerous and have been reported in detail elsewhere (108, 109, 112).
Impact of microbial sialidases on the immune system
We have already mentioned the striking effects on immune cell function of adding exogenous sialidases. Given the marked instability of vertebrate sialidases in extracellular fluids, the only sialidases that could have been used for such studies have been of microbial origin, particularly soluble bacterial sialidases, which are easily found in nature (113, 131). Why would so many bacteria express sialidases? The most obvious answer is that the first structure encountered by them on and around most cell surfaces is likely to be a sialic acid. Thus, without a mechanism to bind to sialic acid (as is the case with a majority of bacteria), it is useful to bacteria remove this negatively charged sugar. This may help in the breakdown of both soluble mucins (sialic acid–rich glycoproteins secreted by epithelia) and cell surface glycoconjugates on the way to cellular entry or interactions (Fig. 1G). Some bacteria, for example, Haemophilus influenzae also utilize the free Sia as a source of energy by “browsing” on host Sia (132, 133). Such free Sia can be broken down to the useful energy sources pyruvate and ManNAc (the latter after it is converted into GlcNAc) (132, 133).
Other functions for bacterial sialidases are now becoming apparent. For example, released Sia may be taken up by some bacteria and used to decorate their surfaces (see section below on the expression of Sia by certain bacteria using exogenous sources). There is also evidence that free Sia can act as a signal to certain bacteria, for example, Pneumococcus (134), directing them towards biofilm formation and/or colonization. . Perhaps free sialic acid is a way for the bacterium to recognize that it has arrived in a vertebrate environment suitable for colonization. In most of the situations above, the roles of different sialic acid types and glycosidic linkage types have not been considered. However, in some instances it is clear that modifications of Sias, such as the N-glycolyl group at the 5 position or O-acetyl groups on the side chain, can limit the action of bacterial sialidases (by “masking the mask”) (135). Further studies are needed to understand the significance of this inhibitory effect. Finally, given the role of Sias as a SAMP recognized by molecules such as Siglecs (22), bacterial desialylation could also perturb natural self-recognition phenomena, perhaps increasing inflammatory responses by exposing desialylated danger associated molecular patterns (DAMPS) (136, 137). This concept requires further study.
Modulation of immune cell responses by intrinsic sialidases
As mentioned earlier, active vertebrate sialidases are not reported in extracellular fluids, as they are unstable. While there are four sialidases in vertebrate cells (Neu1–4), the major one in most cells is Neu-1 (131, 138–140). The fact that the Neu1 gene is located within the major histocompatibility locus, and that it has altered activity in some mouse strains, is of great interest from the immunological perspective (141, 142). Although this enzyme is primarily in the lysosome, it is now known to also exist at the cell surface. In both instances, Neu-1 is very unstable unless it is in a complex with two other proteins, beta galactosidase and protective protein/cathepsin A (PPCA) (143). A selective advantage for this instability can be considered. It is reasonable to suggest that a vertebrate organism with Sias covering all its cell surfaces and terminating the glycans on extracellular glycoproteins would not benefit from having constitutive extracellular sialidase activity, risking damage to its SAMPs, and exposing underlying glycans. Following this logic, it may be that maintaining the extracellular fluid in a sialidase-free state also allows exploitation of the sudden appearance of a sialidase as a “danger signal,” indicating the presence of a bacterial or viral organism––that is, giving a potential higher fidelity to the “desialylation signal.” Regardless of these speculations, it appears that endogenous Neu-1 is capable of being translocated to the cell surface and desialylating certain surface molecules, such as TLRs, TCRs, and integrins (Refs 144–146; Fig.1H), and modifying signaling (147, 148) and phagocytosis (149). The resulting alteration of receptor functions is poorly understood, and candidate mechanisms include the loss of charge repulsion and/or altered galectin-mediated clustering (150). Meanwhile, Neu3 is also found on the cell membrane, but has been shown to act specifically on the sialic acids of gangliosides (151, 152).
Microbial molecular mimicry of host sialic acids
As mentioned earlier and extensively documented elsewhere, several bacterial pathogens express Sias on their surfaces (Figs. 3, 5, 153; Fig. 1I). Every possible way in which Sias might be expressed has been exploited, indicating a strong evolutionary selection pressure to achieve this state. Given the apparent restriction of Sias to multicellular animals of the deuterostome linage, these examples were once assumed to be due to co-opting of vertebrate genes. However, in every case examined, bacterial sialic acid biosynthesis appears to represent convergent (parallel) evolution, recruiting and modifying ancient pathways for synthesis of bacterial nonulosonic acids (3, 154, 155) to instead produce Sias (see below). In combination with independently evolved sialyltransferases that catalyze addition of Sias to the tips of glycan chains, this has enabled remarkable levels of molecular mimcry, involving multiple novel genes that can recreate sialylated glycans essentially identical to those found on host cell surfaces. Based on the discussion above about the role of Sias and SAMPs, (22) one can see the benefit to the microbe of synthesizing vertebrate host-like sialylated glycans. They could provide the pathogen with suppression of the alternate pathway via factor H (156), hijack host Siglecs, and inhibit the formation of antibodies against underlying glycan structures. Finally, Sias also serve to block recognition of underlying (non-mammalian) glycans by naturally occurring antibodies circulating in most vertebrates (157).
Mechanism of microbial expression of sialic acids and sialic acid-like molecules
With regard to the repeated convergent evolution of sialic acid biosynthesis pathways, a picture is now emerging that can explain this remarkable phenomenon. In turns out that ~20% of the first thousand prokaryotic genomes that were sequenced have clusters of genes similar to those involved in the biosynthesis of Sias (3). In most cases, these organisms are synthesizing a more ancient family of nine-carbon backbone acidic sugars called nonulosonic acids, such as pseudaminic acid (Pse) and legionaminic acid (Leg) (Ref. 155; Fig. 1J). As discussed in detail elsewhere, the homology of the genes, metabolic intermediates and steps in the pathway make it very likely that bacteria have co-opted this ancient pathway for nonulosonic acid biosynthesis and simply remodeled it for the production of vertebrate-like Sias (3). In this regard, we have suggested that the term Sias be reserved for the molecules based on Neu or Kdn backbone originally found in deuterostomes and some of their pathogens, and that the term bacterial Sias be replaced by the family name nonulosonic acid (NulO), which includes the Sias (3). It remains to be seen whether NulOs, such as pseudaminic acid (Pse) or legionaminic acid (Leg), are also recognized by Siglecs.
Sialoadhesin recognition of sialylated pathogens
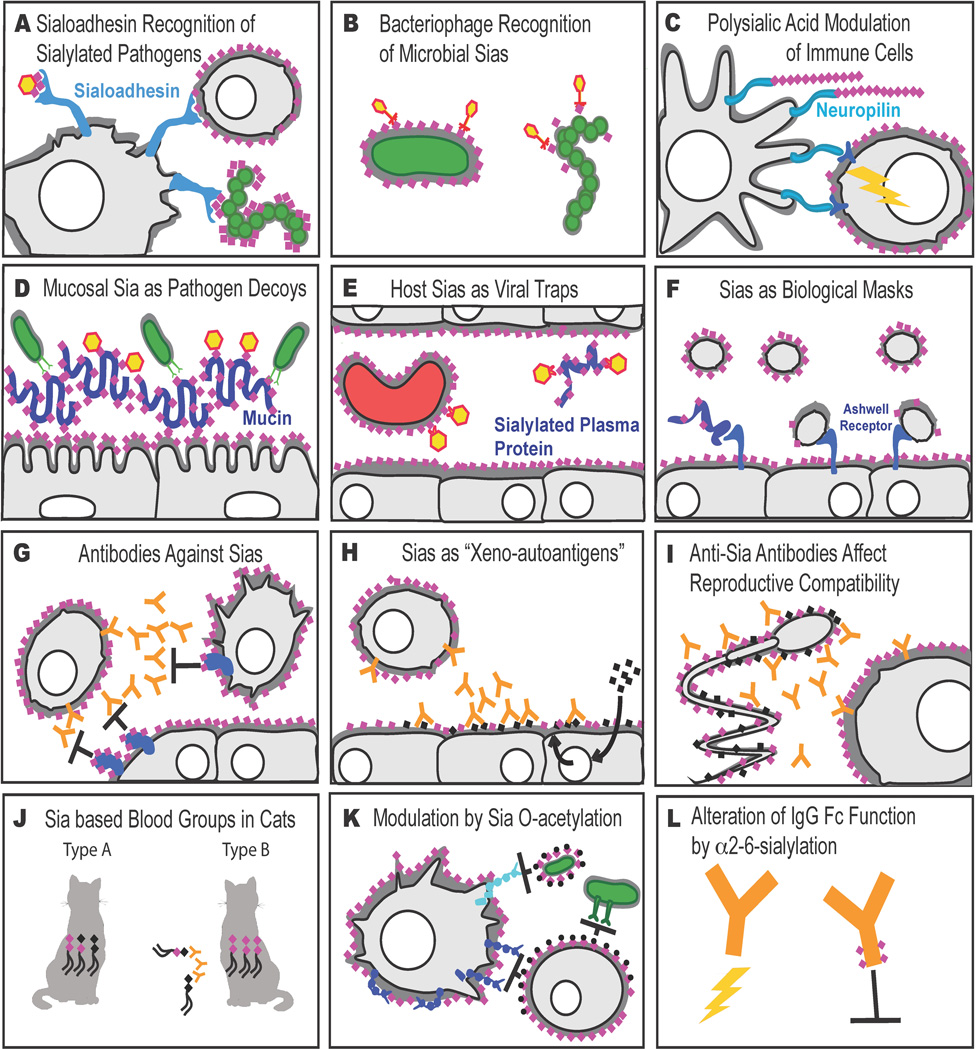
If bacteria mimic vertebrate cells by expressing Sias, the immune system must find a way to distinguish sialylated pathogens (and perhaps other pathogens that express nonulosonic acids), even while maintaining tolerance towards self sialic acid structures. While several of the previously mentioned CD33rSiglecs can recognize sialylated pathogens and mediate endocytosis (158–161), it is unclear whether this is an in vitro artifact, related to the unexplained tendency of CD33rSiglecs to undergo endocytosis when cross-linked. However, Siglec-1 (sialoadhesin) has no signaling properties, and instead has the size, length, and structure to carry out this protective phagocytic function (Fig. 2A). The highly conserved specificity of Siglec-1 for α2–3– or α2–8–linked Neu5Ac (not Neu5Gc) supports this idea—as these are exactly the types of structures that bacteria express (no microorganism has ever been shown to synthesize Neu5Gc, and α2–6–linked Neu5Ac is rare in bacteria). Sialoadhesin is also expressed on the right cell types (macrophages) and in the right locations (the marginal zones on lymph nodes and filtering areas of the spleen) to carry out the functions described. Conversely, certain viruses that emerge from host cells with a coating of sialic acids can “hijack” sialoadhesin for use as a mode of entry into macrophages (162, 163). Sialoadhesin also contributes to intrinsic immune functions and immune changes found in sialoadhesin-deficient mice indicate a role in regulation of the adaptive immune system (164–167).
Figure 2.
More examples of roles of sialic acids in immunity. Sialic acids are shown as pink diamonds. See key in Figure 1, and the text for details. (A) Siglec-1 (sialoadhesin) expressed on macrophages recognizes Sias in patterns commonly found on microbial pathogens and facilitates phagocytosis. Siglec-1 may also mediate immune cell interactions with one another. Some viruses exploit Siglec-1 binding to gain access to host cells. (B) Certain bacteriophages use Sias on their microbial hosts as “receptors” for invasion. (C) Polysialic acid on immune molecules such as neuropilin on dendritic cells modulates interactions with T cells. (D) Sia-rich secretions on host epithelia can act as decoys for Sia-binding microbes. (E) Sia-covered erythrocytes and Sia-rich plasma proteins can act as “viral traps.” (F) Sias act as biological masks by blocking interactions between intrinsic receptors and underlying glycan structures. (G) Sias on potentially antigenic glycoconjugates prevent the formation of antibodies to “cryptoantigens.” Less commonly, Sias can be autoantigens. (H) Non-self Sias can be metabolically incorporated from dietary sources and become “xeno-autoantigens,” targeted by intrinsic anti-Sia antibodies. (I) Female genital tract reactions to non-self Sia on sperm can lead to reproductive incompatibility. (J) Some mammals, such as cats, have blood groups defined by Sia-containing glycolipids. (K) O-acetylation of Sias can block Sia recognition by intrinsic lectins like Siglecs, and modulate microbial lectin interactions, in a positive or negative fashion. (L) Alpha-2–6 sialylation of IgG-Fc region N-glycans can change the effects of IgG antibodies from activating to inhibitory.
Bacteriophage recognition of microbial sialic acids
When bacteria mimic vertebrate cells by expressing similar sialoglycans, they are also likely using variations of this mechanism to escape recognition by bacteriophages that normally recognize underlying bacterial glycans. However, phages evolve even faster than bacteria, and some have evolved to recognize these sialylated bacterial capsules (Ref. 168, 169; Fig. 2B). Many more such phages probably exist and remain to be discovered. For example, this may help explain why Group B Streptococcus has evolved so many different polysaccharide variants, each terminating with the same human-like sialylated trisaccharide (170). The possibility of using such phages as alternates to antibiotic therapy has also been considered (171). It is also interesting to note that the Cholera toxin ganglioside-binding subunit B is encoded by non-lysogenic phage that was recruited by V. cholerae to mediate its own pathogenicity (172).
Polysialic acid modulation of immune cells
Sias are usually found as single monosaccharide unit at the end of glycan chains. However they can sometimes be linked to each other, generating short or long homopolymers. In the typical form, the polysialic acid consists of α2–8–linked Neu5Ac units. This structure is found on certain proteins in the brain and is known to have major functions in the development, morphogenesis, and function of various neural systems (173, 174). However, polysialic acid has also since been discovered on a few immune cells. These include dendritic cells (175, 176), and some stages of T cell development (177, 178). In these instances, the polysialic acid is attached to specific proteins such as neuropilins, modulating cellular interactions in meaningful ways (Fig. 2C).
Host sialic acids as pathogen decoys
We have already discussed how host Sias can be the binding target (often called “receptor”) for a variety of viruses, toxins, and some bacteria. One ubiquitous and simple function of Sias is likely to act as a decoy against such organisms. Thus, for example, a virus or other sialic acid-binding organism that reaches a mucosal surface will first encounter mucins, which are heavily sialylated but mostly secreted glycoproteins. Even membrane-anchored mucins can be shed. These soluble molecules can act as decoys, preventing the organism from reaching its intended target on the cell surface (Refs. 179, 180; Fig. 2D). This is a testable hypothesis that has yet to be carefully evaluated, though loss of O-linked glycans on mucins has been linked to increased frequency and severity of colitis (181). It is also possible that the destruction or bypassing of these decoys represents a function of the sialidases (neuraminidases) found on numerous viruses. However, the only study to date on this subject was done in cell culture (182), in the absence of the mucins that could be the dominant decoys in the natural state.
Another decoy function might be mediated by the heavily sialylated glycoproteins found in plasma and extracellular fluids (179, 180). Again, sialic acid-binding pathogens would first encounter these heavily sialylated glycoproteins and have to escape from them before approaching the intended target. In the case of viruses, the target cells typically need a nucleus, since the virus needs to take advantage of the cellular machinery for synthesis and replication of its own nucleic acid. Another decoy example might be non-nuclear cells such as erythrocyte, which represents about 50% of the total volume of blood, and could act as “viral traps” (Fig. 2E). A sialic acid–binding virus such as influenza virus that manages to make its way into the bloodstream would immediately encounter this extensive cell surface that it can bind to, but lacks appropriate mechanisms to allow invasion and replication. Ironically then, the hemagglutination reaction that helps define the binding preferences and specificities of a variety of viruses may actually represent the host’s attempt to evade the very same virus. Over large-scales of evolutionary time one might thus expect the sialome of erythrocytes to evolve to keep up with the rapidly evolving sialic acid–binding specificities of pathogens and those of new pathogens that arrive at various times (179, 180). Meanwhile there is a propensity of malarial parasites to use Sias to invade erythrocytes, inside of which they asexually replicate (183, 184). Taken together, all of the above considerations may explain why there is such extreme inter-species variation in sialomes of erythrocytes, mucins, and plasma glycoproteins. It might also explain the sudden changes in sialylation patterns and levels occurring during inflammation within a given species (e.g., the acute phase reaction) (185).
Sialic acids as biological masks
Schauer originally emphasized the dualistic roles of Sias as binding sites and as biological masks (186). The first discovered vertebrate glycan binding protein was the asialo-glycoprotein receptor on hepatocytes (the so-called “Ashwell receptor”) (187). As the name suggests, binding to this receptor occurs when one removes Sias from a glycoprotein and exposes the underlying beta-linked galactose residues (Fig. 2F). Since this discovery, additional beta-galactose-binding receptors in macrophages have been discovered. However, in most instances gene knockouts of these proteins failed to uncover a clear-cut natural function in intrinsic systems.(188) On the other hand, when a sialidase of bacterial origin enters the circulation, there can be extensive desialylation of cells and proteins, and these receptors become relevant. This was recently shown as a host mechanism to clear away the excess of platelets that might result in increased coagulopathy that is associated with microbial sepsis.(189) The removal of Sias could also generate "eat me" signals that allow macrophages to recognize and eliminate dying or apoptotic cells.(190) It is important to recognize and differentiate the galactose-binding receptors involved in such phenomena from soluble galectins, which can bind terminal or sub-terminal galactose residues on cell surfaces.(191, 192) Galectins may actually function in the opposite direction, acting to reduce endocytosis of the cell surface proteins by forming lattices, and may thus be more important in regulation of signaling, as shown by others.(193–195) The numerous other functions of galectins in the immune system (192, 195) will not be discussed here, except to say that they can be modulated by the presence or absence of terminal Sias, particularly α2–6-linked ones (196). Also, unlike most galectins that prefer N-acetyllactosamine ligands with non-sialylated terminal beta-Gal residues, galectin-8 and -9 have domains that preferentially recognize α2–3-sialylated N-acetyllactosamines (197, 198).
Antibodies against intrinsic sialic acids
Not surprisingly, it is uncommon to find antibodies against sialic acid-containing glycans, if the sialic acid in question is already intrinsic within the host (Fig. 2G). Presumably, this is because B cells that happen to express a B cell receptor (sIgM) that can recognize sialylated glycans are tolerized and eliminated before they leave the bone marrow. Indeed, as discussed earlier, this might be one of the selective advantages to pathogens that express Sias. In mammals, most of these comments reflect upon the common Neu5Ac sialic acid. On the other hand, it is possible to induce mice to generate monoclonal antibodies that detect Neu5Ac-containing glycans (199, 200), and the addition of an O-acetyl group to the sialic acid can increase the probability of getting such an antibody (201). Overall, while host-intrinsic Sias can be generally considered “immunosuppressive” for the host organism, exceptions can be found.
Sialic acids as xenoautoantigens
The above comments do not apply if a particular sialic acid is missing in a species. This appears to be the case both in humans (17) and in the sauropsid lineage of animals (birds and reptiles) (202), which appear unable to synthesize the common mammalian sialic acid Neu5Gc from its precursor Neu5Ac. In the case of humans, the basis for this phenotype is a fixed loss-of function mutation of the cytidine monophosphate N-acetylneuraminic acid hydroxylase (CMAH) gene (203, 204), which remains intact in our closest evolutionary relatives the chimpanzees (204). The significance of the independent loss in the sauropsid lineage is unclear, though it does make for convenient source of anti-Neu5Gc antibodies by immunizing chickens, which generate a robust response (205). Unexplained also is the fact that similar antibodies appear when chickens that become infected with the Marek’s disease lymphoma virus (206).
In the case of humans, more information is available. It appears that Neu5Gc from dietary sources can be metabolically incorporated either into our tissues (207) or into commensal bacteria such as Haemophilus influenzae, which specialize in taking up low quantities of Sias present in the upper oropharynx (208). One or both mechanisms appear to be the cause of moderate to high levels of anti-Neu5Gc antibodies in humans (Fig. 2H). Current studies suggest that these antibodies may be interacting with the metabolically incorporated Neu5Gc of dietary origin to generate chronic inflammation (17). This may help explain the propensity of red meat (beef, pork and lamb, the richest sources of dietary Neu5Gc) to increase the risk of inflammation-associated diseases such as carcinomas, cardiovascular disease, and macular degeneration. These findings also relate to the classic reports of the Hanganutziu-Deicher “heterophile” antibodies, which reacted with animal red blood cells (209, 210).
Anti-sialyl antibodies can affect reproductive incompatibility
The female reproductive tract has levels of IgG antibodies and complement levels similar to that found in the serum (211). Thus a sperm that enters the uterus must negotiate this immunological gauntlet before it can reach the ovum and fertilize it, further up in the fallopian tube. There may well be multiple anti-glycan systems that can affect sperm, but the one so far documented involves antibodies against Neu5Gc, which can enter the uterine fluid and affect both sperm and embryos that happen to have Neu5Gc on them (Ref. 211; Fig. 2I). In this regard, it is suggested that this could even be a mechanism of speciation in the genus Homo, due to the loss of Neu5Gc in ancestors, after it initially became polymorphic.
Sialic-acid based blood groups in mammals
The above considerations about the lack of immunogenicity of Neu5Ac do not apply if the Neu5Ac is attached to a specific polypeptide that is foreign to the host. An example is the MN blood groups in humans, where individual variations in the amino acid sequence of the red cell protein glycophorin result in differential presentation of small O-linked sialylated chains at the aminoterminus of the protein (212). While dictated by the underlying polypeptide, these antigenic variations also require the sialylated glycans, generating the antibodies that interact between humans and affect blood transfusion occasionally. Similar considerations apply to some other blood group antibodies (213).
Neu5Gc-based blood groups in cats
As with humans, there is one major antibody system that appears to restrict blood transfusion within cats. However, the two major blood groups in cats (A and B) were shown to be due to antibodies in B cats against a sialylated glycolipid on the red blood cells of A cats (Fig. 2J). The difference appears to be the presence or absence of Neu5Gc in the ganglioside GD3 (214). We do not as yet know if the differential expression of Neu5Gc in the red blood cells of these cats extends to other tissues of the animal. There is evidence that changes in the promoter region of the CMAH gene might explain the differential expression in red blood cells (215). There are similar erythrocyte Neu5Gc polymorphisms in dogs (216), but evidence of anti-Neu5Gc antibodies has not been reported. In both instances, strain differences would be worth studying further. Thus, it is possible that Sias can exist as alloantigens within populations of the same species as well as xenoantigens within different species.
Modulation of immunity by sialic acid O-acetylation
Given variable expression of O-acetyl groups on Sias and their diverse effects in immunity, a separate section on this modification seems justified. We have already mentioned the impact on factor H recognition, the relative resistance to bacterial sialidases to Sias with this modification, the blockade of binding of some virus hemagglutinins, and the facilitation of binding of others. Sias on certain bacterial polysaccharides can be O-acetylated. Surprisingly, this modification is actually detrimental to the bacterium in the host–pathogen interaction, either reducing recognition by CD33rSiglecs and/or enhancing immunogenicity (217). The logical explanation is that these modifications assist the bacteria in surviving in other situations, such as protection from other microbial sialidases, and/or bacteriophage-binding proteins. The exception is O-acetyl blockade of recognition by sialoadhesin (218), which could be beneficial to the bacterium, avoiding phagocytosis (32, 217).
Unlike the case with the selectins, sialic acid-binding by Siglecs almost invariably requires recognition of the C7–C9 side-chain of the molecule. This is exemplified by the loss of recognition upon mild periodate oxidation of this side-chain (62, 63, 99). In view of this, it is not surprising that addition of an O-acetyl group to the side chain blocks the binding of all Siglecs studied to date (218, 219). A Siglec selectively recognizing O-acetylated Sias has yet to be found. Thus sialic acid O-acetylation seemed a logical candidate for regulation of Siglec function (Fig. 2K). This was indeed shown to be the case in mice with a defect in a sialic acid specific esterase (SIAE), which normally downregulates sialic acid O-acetylation on B cells (220). The mutant mice thus have overreactive B cells, apparently due to lack of proper SAMP ligands for CD22 and Siglec-G (221). In keeping with this, humans with autoimmune diseases have a higher frequency of harboring mutations in the SIAE gene (79, 222).
In another setting, O-acetylation of the outer sialic acid of the ganglioside GD3 was first reported as a melanoma-specific antigen not found in other normal tissues (201). However, it later turned out that normal T cells can also express this structure (223–225). Indeed, this is the basis of the CD60 group of antigens (see Table 1). Interestingly, while GD3 is pro-apoptotic, O-acetyl-GD3 has opposite effects (226, 227). The claimed mechanisms for these effects are fascinating, involving mitochondrial and other apoptotic pathways. However, there are topological issues that remain unresolved (228).
Table 1.
CD (cluster of differentiation) numbers related to sialic acid biology
| CD number | Common name(s) | Roles in immunity |
|---|---|---|
| CD15s | Sialyl Lewis (sLeX) | Key component of sialylated selectin ligands and of preferred ligands for some Siglecs. |
| CD22 | Siglec-2 (sialic acid-binding Ig-like lectin 2) | Dampens B cell reactivity via selective recognition of α2-6-linked Sias. |
| CD24 | Heat stable antigen | Heavily glycosylated, sialylated GPI-anchored molecule; some glycoforms may be ligands for Siglec-10 or P-selectin. |
| CD33 | Siglec-3 (sialic acid-binding Ig-like lectin 3) | Myeloid lineage marker; can downregulate reactivity of innate immune cells. |
| CD34 | Hematopoietic progenitor cell antigen | Heavily glycosylated and sialylated cell surface mucin-like protein; stem cell marker; some glycoforms can be L-selectin ligands. |
| CD43 | Leukosialin (leucocyte sialoglycoprotein) (sialophorin) | Heavily sialylated major cell surface mucin-like protein; modulates immune cell responses. |
| CD45 | CD45 leukocyte common antigen | Differing glycoforms in different immune cell types due to alternate splicing; tyrosine phosphatase; possible CD22 ligand. |
| CD52 | CAMPATH-1 | Heavily glycosylated and sialylated GPI-anchored cell surface molecule. |
| CD56 | Neural cell adhesion molecule 1 | NK cell marker. Can carry polysialic acid, which can be recognized by Siglec-7 and Siglec-11. |
| CD60a | GD3 ganglioside | Glycolipid with two sialic acids; human T cell marker; promotes apoptosis?; Siglec-7 ligand? |
| CD60b | 9-O-acetyl-GD3 ganglioside | Presence of 9-O-acetyl group on outer sialic acid of GD3; protects from apoptosis?. |
| CD60c | 7-O-acetyl-GD3 ganglioside | Converted to 9-O-acetyl-GD3 over time due to non-enzymatic migration of the O-acetyl group. |
| CD62E | E-selectin (endothelial leukocyte adhesion molecule 1) | Mediates leukocyte adhesion and rolling on endothelium; expression upregulated by inflammatory cytokines. |
| CD62L | L-selectin (lymph node homing receptor) | Mediates leukocyte adhesion and rolling on endothelium, including lymphocyte trafficking. |
| CD62P | P-selectin (granule membrane protein 140, GMP-140) | Mediates platelet and endothelial interactions with leukocytes, via correctly modified PSGL-1. |
| CD68 | Macrosialin (Gp110) | Marker of macrophages and few other cells; receptor for oxidized low density lipoprotein? |
| CD75s | α2-6-sialylated lactosamines | Cluster of Siaα2-6Galβ1-4GlcNAcβ1-R units produced by ST6Gal-I, mainly on N-glycans. |
| CD169 | Sialoadhesin (sialic acid-binding Ig-like lectin 1) (Siglec-1) | Macrophage subset marker; recognizes sialic acids on endogenous ligands and on microbes. |
| CD170 | Sialic acid-binding Ig-like lectin 5 (Siglec-5) | Inhibitory Siglecs on human innate immune cells, and also on lymphocytes in “great apes.” |
| CD175s | Sialyl-Tn | Siaα2-6GalNAcα1-Ser/Thr; truncated O-glycan mostly found on malignant cells. |
| CD176s | Sialylated form of Thomsen-Friedenreich (T) antigen | Siaα2-3Gal1-3GalNAcα1-Ser/Thr; common O-glycan on many cell types. |
| CD227 | MUC-1, polymorphic epithelial mucin, episialin | Major cell surface mucin on epithelial cells; on activated T cells, can send inhibitory signals. |
| CD235a | Glycophorin-A (PAS-2) (MN sialoglycoprotein) | Major sialic acid carrier on RBCs; target for binding by malarial merozoite via EBA-175. |
| CD235b | Glycophorin-B (PAS-3) (sialoglycoprotein delta) | Major sialic acid carrier on RBCs; target for binding by malarial merozoite via EBL-1. |
| CD236 | Glycophorin-C (PAS-2') (glycoprotein beta) | Major sialic acid carrier on RBCs. Target for binding by malarial merozoite via EBA-140. |
| CD327 | Sialic acid-binding Ig-like lectin 6 (Siglec-6) | Inhibitory Siglecs on B cells in primates, and also on placental trophoblast (in humans only). |
| CD328 | Sialic acid-binding Ig-like lectin 7 (Siglec-7); AIRM1 | Inhibitory Siglecs on human NK cells; lower levels on monocytes and macrophages. |
| CD329 | Sialic acid-binding Ig-like lectin 9 (Siglec-9); | Inhibitory Siglecs found on human neutrophils; monocytes and macrophages |
For more information, see http://www.hcdm.org/MoleculeInformation/tabid/54/Default.aspx
Alteration of IgG Fc function by alpha2–6–sialylation
Recent studies have shown that the minor subset of circulating IgG that has α2–6–linked Sias terminating its N-glycan has a inhibitory potential, working through the human DC-SIGN receptor on a regulatory macrophage population to upregulate FcRγ-IIB on other macrophages, and thereby dampen immune responses (Refs. 229–231; Fig. 2L). This is also suggested to be the mechanism of action of intravenous pooled human IgG (IVIG) that is used for immune suppression in the clinic. While the data are consistent and compelling, the work mostly involves a single model system for autoimmune disease. It also assumes that the other suggested mechanisms of IVIG action (e.g., scavenging of activated complement (232), and anti-Siglec antibodies (233)) do not contribute significantly. This is a very interesting avenue for future research, especially given than the relevant sialyltransferase (ST6Gal-I) is highly regulated in response to inflammation (185). and alters cellular activation and proliferation (196, 234)
Sialylated molecules or sialic acid-binding proteins as cluster of differentiation markers
It would be incomplete to discuss the immune system without mentioning cluster of differentiation (CD) markers. There is a long list of CD molecules that are either sialylated or are involved in sialic acid recognition. Space does not allow a full discuss of each of these antigens, but a brief summary can be found in Table 1.
Conclusions and perspectives
As the outermost “onion layer” on all vertebrate cells types, Sias were predestined to play roles as the “molecular frontier” in ongoing evolutionary arms races, both as targets for attack and as SAMPs. Sialome patterns evolve rapidly, likely because they are prone to being exploited by rapidly evolving pathogens and parasites. Meanwhile, changes in the intrinsic “landscape” of self-Sias have to be closely tracked by the intrinsic lectins such as Siglecs in order to maintain homeostasis, even while they themselves are being exploited by pathogens expressing Sias. On the background of such ongoing “molecular dialectics” (revolution and counterrevolution), major sialome changes at the level of a species, such as the wholesale loss of Neu5Gc in humans likely required major system-wide accommodations. Meanwhile, vertebrate hosts are “locked in” to maintaining the numerous intrinsic roles of Sias in reproduction, development and normal physiology. Vertebrate host species have evolved a precarious compromise of using the presence of self Sias as “self associated patterns,” even while discriminating against close mimics, as well as against molecular surfaces lacking self Sias. Several other cell surface glycans such as galactose, fucose, and glycosaminoglycans also mediate immunity-related functions, including some of the ones discussed here. However, given their shear abundance, structural diversity, and vertebrate lineage-defining nature, Sias have been recruited for multifarious roles in immunity, only some of which we have addressed here.
Acknowledgments
We thank Shiv Pillai and Takashi Angata for critical comments and suggestions. Supported by grants from the NIH and by the Mathers Foundation of New York.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Schauer R, Srinivasan GV, Wipfler D, Kniep B, Schwartz-Albiez R. O-Acetylated sialic acids and their role in immune defense. Adv. Exp. Med. Biol. 2011;705:525–548. doi: 10.1007/978-1-4419-7877-6_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis AL, Desa N, Hansen EE, Knirel YA, Gordon JI, Gagneux P, Nizet V, Varki A. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc. Natl. Acad. Sci. U S A. 2009;106:13552–13557. doi: 10.1073/pnas.0902431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A, Schauer R. Sialic Acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 199–218. [PubMed] [Google Scholar]
- 5.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 6.Troy FA. Polysialylation: From bacteria to brains. Glycobiology. 1992;2:5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne B, Donohoe GG, O'Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Rutishauser U. Polysialic acid at the cell surface: Biophysics in service of cell interactions and tissue plasticity. J. Cell. Biochem. 1998;70:304–312. doi: 10.1002/(sici)1097-4644(19980901)70:3<304::aid-jcb3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. U S A. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilatte Y, Bignon J, Lambré CR. Sialic acids as important molecules in the regulation of the immune system: Pathophysiological implications of sialidases in immunity. Glycobiology. 1993;3:201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- 12.Gahmberg CG, Andersson LC. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J. Biol. Chem. 1977;252:5888–5894. [PubMed] [Google Scholar]
- 13.Dehoux-zenou SM, Guenounou M, Zinbi H, Ougen P, Couderc R, Agneray JC. Behavior of aldehyde moieties involved in the activation of suppressor cells by sodium periodate. J Immunol. 1987;138:1157–1163. [PubMed] [Google Scholar]
- 14.Jacobs CL, Yarema KJ, Mahal LK, Nauman DA, Charters NW, Bertozzi CR. Metabolic labeling of glycoproteins with chemical tags through unnatural sialic acid biosynthesis. Methods Enzymol. 2000;327:260–275. doi: 10.1016/s0076-6879(00)27282-0. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Meledeo MA, Wang Z, Khanna HS, Paruchuri VD, Yarema KJ. Metabolic glycoengineering: sialic acid and beyond. Glycobiology. 2009;19:1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U S A. 2010;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc. Natl. Acad. Sci. U S A. 1978;75:1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pangburn MK, Muller-Eberhard HJ. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc. Natl. Acad. Sci. U S A. 1978;75:2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: Regulation via a sialic acid /polyanion binding site on factor H. Proc Natl Acad Sci USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid-binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan "self-associated molecular patterns" dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21:1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston JW, Coussens NP, Allen S, Houtman JC, Turner KH, Zaleski A, Ramaswamy S, Gibson BW, Apicella MA. Characterization of the N-Acetyl-5-neuraminic Acid-binding Site of the Extracytoplasmic Solute Receptor (SiaP) of Nontypeable Haemophilus influenzae Strain 2008. J. Biol. Chem. 2019;283:855–865. doi: 10.1074/jbc.M706603200. [DOI] [PubMed] [Google Scholar]
- 24.Ram S, Lewis LA, Agarwal S. Meningococcal group w-135 and y capsular polysaccharides paradoxically enhance activation of the alternative pathway of complement. J. Biol. Chem. 2011;286:8297–8307. doi: 10.1074/jbc.M110.184838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, Kraiczy P, Noris M, Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- 26.Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc. Natl. Acad. Sci. U S A. 2011;108:2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan HP, Jiang J, Herbert AP, Kavanagh D, Uhrin D, Barlow PN, Hannan JP. Crystallographic determination of the disease-associated T1184R variant of complement regulator factor H. Acta Crystallogr D Biol Crystallogr. 2011;67:593–600. doi: 10.1107/S0907444911015423. [DOI] [PubMed] [Google Scholar]
- 28.Shaughnessy J, Ram S, Bhattacharjee A, Pedrosa J, Tran C, Horvath G, Monks B, Visintin A, Jokiranta TS, Rice PA. Molecular Characterization of the Interaction between Sialylated Neisseria gonorrhoeae and Factor H. J. Biol. Chem. 2011;286:22235–22242. doi: 10.1074/jbc.M111.225516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J. Exp. Med. 2007;204:1245–1248. doi: 10.1084/jem.20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donoso LA, Vrabec T, Kuivaniemi H. The role of complement Factor H in age-related macular degeneration: a review. Surv Ophthalmol. 2010;55:227–246. doi: 10.1016/j.survophthal.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Nydegger UE, Fearon DT, Austen KF. Autosomal locus regulates inverse relationship between sialic acid content and capacity of mouse erythrocytes to activate human alternative complement pathway. Proc. Natl. Acad. Sci. U S A. 1978;75:6078–6082. doi: 10.1073/pnas.75.12.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J. Biol. Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 33.Arming S, Wipfler D, Mayr J, Merling A, Vilas U, Schauer R, Schwartz-Albiez R, Vlasak R. The human Cas1 protein: a sialic acid-specific O-acetyltransferase? Glycobiology. 2011;21:553–564. doi: 10.1093/glycob/cwq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaer TR, Hansen AG, Sorensen UB, Nielsen O, Thiel S, Jensenius JC. Investigations on the pattern recognition molecule M-ficolin: quantitative aspects of bacterial binding and leukocyte association. J. Leukoc. Biol. 2011;90:425–437. doi: 10.1189/jlb.0411201. [DOI] [PubMed] [Google Scholar]
- 35.Honore C, Rorvig S, Hummelshoj T, Skjoedt MO, Borregaard N, Garred P. Tethering of Ficolin-1 to cell surfaces through recognition of sialic acid by the fibrinogen-like domain. J. Leukoc. Biol. 2010;88:145–158. doi: 10.1189/jlb.1209802. [DOI] [PubMed] [Google Scholar]
- 36.Gout E, Garlatti V, Smith DF, Lacroix M, Dumestre-Perard C, Lunardi T, Martin L, Cesbron JY, Arlaud GJ, Gaboriaud C, Thielens NM. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid-binding specificity of M-ficolin. J. Biol. Chem. 2010;285:6612–6622. doi: 10.1074/jbc.M109.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen SD, Singer MS, Yednock TA, Stoolman LM. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985;228:1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- 38.McEver RP. Selectins: Novel receptors that mediate leukocyte adhesion during inflammation. Thromb. Haemost. 1991;65:223–228. [PubMed] [Google Scholar]
- 39.Cummings RD, Smith DF. The selectin family of carbohydrate-binding proteins: Structure and importance of carbohydrate ligands for cell adhesion. BioEssays. 1992;14:849–856. doi: 10.1002/bies.950141210. [DOI] [PubMed] [Google Scholar]
- 40.Rosen SD. L-selectin and its biological ligands. Histochemistry. 1993;100:185–191. doi: 10.1007/BF00269091. [DOI] [PubMed] [Google Scholar]
- 41.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 43.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Moore KL, Smith DF, Varki A, McEver RP, Cummings RD. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J. Cell Biol. 1991;115:557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyrrell D, James P, Rao N, Foxall C, Abbas S, Dasgupta F, Nashed M, Hasegawa A, Kiso M, Asa D, Kidd J, Brandley BK. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci USA. 1991;88:10372–10376. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handa K, Nudelman ED, Stroud MR, Shiozawa T, Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Lea and sialosyl-Lex, and sulfated glycans modulate this binding. Biochem. Biophys. Res. Commun. 1991;181:1223–1230. doi: 10.1016/0006-291x(91)92069-v. [DOI] [PubMed] [Google Scholar]
- 47.Berg EL, Magnani J, Warnock RA, Robinson MK, Butcher EC. Comparison of L-selectin and E-selectin ligand specificities: The L-selectin can bind the E-selectin ligands sialyl Lex and sialyl Lea. Biochem. Biophys. Res. Commun. 1992;184:1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- 48.Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, Hasegawa A, Asa D, Brandley BK. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewisx oligosaccharide. J. Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen GR, Sako D, Ahern TJ, Shaffer M, Erban J, Sajer SA, Gibson RM, Wagner DD, Furie BC, Furie B. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J. Biol. Chem. 1992;267:11104–11110. [PubMed] [Google Scholar]
- 50.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J. Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a core1 extension beta1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 52.Leppänen A, Mehta P, Ouyang YB, Ju TZ, Helin J, Moore KL, Van Die I, Canfield WM, McEver RP, Cummings RD. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J. Biol. Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- 53.Larkin M, Ahern TJ, Stoll MS, Shaffer M, Sako D, O'Brien J, Yuen C-T, Lawson AM, Childs RA, Barone KM, Langer-Safer PR, Hasegawa A, Kiso M, Larsen GR, Feizi T. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J. Biol. Chem. 1992;267:13661–13668. [PubMed] [Google Scholar]
- 54.Norgard-Sumnicht KE, Varki NM, Varki A. Calcium-dependent heparin-like ligands for L-selectin in nonlymphoid endothelial cells. Science. 1993;261:480–483. doi: 10.1126/science.7687382. [DOI] [PubMed] [Google Scholar]
- 55.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- 56.Norgard KE, Han H, Powell L, Kriegler M, Varki A, Varki NM. Enhanced interaction of L-selectin with the high endothelial venule ligand via selectively oxidized sialic acids. Proc Natl Acad Sci USA. 1993;90:1068–1072. doi: 10.1073/pnas.90.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 58.Crocker PR, Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 1986;164:1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crocker PR, Kelm S, Dubois C, Martin B, McWilliam AS, Shotton DM, Paulson JC, Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991;10:1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamenkovic I, Sgroi D, Aruffo A, Sy MS, Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2–6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 61.Sgroi D, Varki A, Braesch-Andersen S, Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J. Biol. Chem. 1993;268:7011–7018. [PubMed] [Google Scholar]
- 62.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J. Biol. Chem. 1993;268:7019–7027. [PubMed] [Google Scholar]
- 63.Powell LD, Varki A. The oligosaccharide binding specificities of CD22beta, a sialic acid-specific lectin of B cells. J. Biol. Chem. 1994;269:10628–10636. [PubMed] [Google Scholar]
- 64.Crocker PR, Mucklow S, Bouckson V, McWilliam A, Willis AC, Gordon S, Milon G, Kelm S, Bradfield P. Sialoadhesin, a macrophage sialic acid-binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994;13:4490–4503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, De Bellard M-E, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, Crocker PR. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 66.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–2012. [PubMed] [Google Scholar]
- 67.Kelm S, Schauer R, Crocker PR. The sialoadhesins - A family of sialic acid-dependent cellular recognition molecules within the immunoglobulin superfamily. Glycoconjugate J. 1996;13:913–926. doi: 10.1007/BF01053186. [DOI] [PubMed] [Google Scholar]
- 68.Collins BE, Kiso M, Hasegawa A, Tropak MB, Roder JC, Crocker PR, Schnaar RL. Binding specificities of the sialoadhesin family of I-type lectins - Sialic acid linkage and substructure requirements for binding of myelin-associated glycoprotein, Schwann cell myelin protein, and sialoadhesin. J. Biol. Chem. 1997;272:16889–16895. doi: 10.1074/jbc.272.27.16889. [DOI] [PubMed] [Google Scholar]
- 69.Powell LD, Varki A. I-type lectins. J. Biol. Chem. 1995;270:14243–14246. doi: 10.1074/jbc.270.24.14243. [DOI] [PubMed] [Google Scholar]
- 70.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, Kelm S, Le Douarin N, Powell L, Roder J, Schnaar RL, Sgroi DC, Stamenkovic K, Schauer R, Schachner M, Van den Berg TK, Van der Merwe PA, Watt SM, Varki A. Siglecs: a family of sialic-acid binding lectins [letter] Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 71.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. U S A. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angata T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol Divers. 2006;10:555–566. doi: 10.1007/s11030-006-9029-1. [DOI] [PubMed] [Google Scholar]
- 73.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 74.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann. N. Y. Acad. Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–417. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 76.O'Reilly MK, Paulson JC. Multivalent ligands for Siglecs. Methods Enzymol. 2010;478:343–363. doi: 10.1016/S0076-6879(10)78017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao H, Crocker PR. Evolution of CD33-related Siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132:18–26. doi: 10.1111/j.1365-2567.2010.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park CS, Bochner BS. Potential targeting of Siglecs, mast cell inhibitory receptors, in interstitial cystitis. Int Neurourol J. 2011;15:61–63. doi: 10.5213/inj.2011.15.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and Immune regulation. Annu. Rev. Immunol. 2012;30 doi: 10.1146/annurev-immunol-020711-075018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu ZB, Maoui M, Wu LT, Banville D, Shen SH. mSiglec-E, a novel mouse CD33-related Siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. J. 2001;353:483–492. doi: 10.1042/0264-6021:3530483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitney G, Wang SL, Chang H, Cheng KY, Lu P, Zhou XD, Yang WP, McKinnon M, Longphre M. A new Siglec family member, Siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur. J. Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 82.Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J. Biol. Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 83.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The Membrane-Proximal Immunoreceptor Tyrosine-Based Inhibitory Motif Is Critical for the Inhibitory Signaling Mediated by Siglecs-7 and -9, CD33-Related Siglecs Expressed on Human Monocytes and NK Cells. J. Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 84.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol. Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signalling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 2005;280:19843–19851. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 87.Mitsuki M, Nara K, Yamaji T, Enomoto A, Kanno M, Yamaguchi Y, Yamada A, Waguri S, Hashimoto Y. Siglec-7 mediates nonapoptotic cell death independently of its immunoreceptor tyrosine-based inhibitory motifs in monocytic cell line U937. Glycobiology. 2010;20:395–402. doi: 10.1093/glycob/cwp195. [DOI] [PubMed] [Google Scholar]
- 88.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 89.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 90.Yasui K, Angata T, Matsuyama N, Furuta RA, Kimura T, Okazaki H, Tani Y, Nakano S, Narimatsu H, Hirayama F. Detection of anti-Siglec-14 alloantibodies in blood components implicated in nonhaemolytic transfusion reactions. Br. J. Haematol. 2011;153(6):794–796. doi: 10.1111/j.1365-2141.2010.08488.x. [DOI] [PubMed] [Google Scholar]
- 91.Hiruma Y, Hirai T, Tsuda E. Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem. Biophys. Res. Commun. 2011;409:424–429. doi: 10.1016/j.bbrc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur. J. Immunol. 2008;38:2303–2315. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 93.Varki A, Angata T. Siglecs--the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 94.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36:1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 95.O'Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varki A. Natural ligands for CD33-related Siglecs? Glycobiology. 2009;19:810–812. doi: 10.1093/glycob/cwp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jandus C, Simon HU, von Gunten S. Targeting Siglecs--a novel pharmacological strategy for immuno- and glycotherapy. Biochem. Pharmacol. 2011;82:323–332. doi: 10.1016/j.bcp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 98.Magesh S, Ando H, Tsubata T, Ishida H, Kiso M. High-Affinity Ligands of Siglec Receptors and their Therapeutic Potentials. Curr. Med. Chem. 2011;18:3537–3550. doi: 10.2174/092986711796642580. [DOI] [PubMed] [Google Scholar]
- 99.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 1998;95:7469–7474. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawasaki Y, Ito A, Withers DA, Taima T, Kakoi N, Saito S, Arai Y. Ganglioside DSGb5, preferred ligand for Siglec-7, inhibits NK cell cytotoxicity against renal cell carcinoma cells. Glycobiology. 2010;20:1373–1379. doi: 10.1093/glycob/cwq116. [DOI] [PubMed] [Google Scholar]
- 101.Blixt O, Han S, Liao L, Zeng Y, Hoffmann J, Futakawa S, Paulson JC. Sialoside analogue arrays for rapid identification of high affinity Siglec ligands. J. Am. Chem. Soc. 2008;130:6680–6681. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cui L, Kitov PI, Completo GC, Paulson JC, Bundle DR. Supramolecular complexing of membane Siglec CD22 mediated by a polyvalent heterobifunctional ligand that templates on IgM. Bioconjug Chem. 2011;22:546–550. doi: 10.1021/bc100579d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vinson M, Van der Merwe PA, Kelm S, May A, Jones EY, Crocker PR. Characterization of the sialic acid-binding site in sialoadhesin by site-directed mutagenesis. J. Biol. Chem. 1996;271:9267–9272. doi: 10.1074/jbc.271.16.9267. [DOI] [PubMed] [Google Scholar]
- 104.Van der Merwe PA, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J. Biol. Chem. 1996;271:9273–9280. [PubMed] [Google Scholar]
- 105.Crocker PR, Vinson M, Kelm S, Drickamer K. Molecular analysis of sialoside binding to sialoadhesin by NMR and site-directed mutagenesis. Biochem. J. 1999;341:355–361. [PMC free article] [PubMed] [Google Scholar]
- 106.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs - evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 107.Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J. Biol. Chem. 2001;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- 108.Mandal C. Sialic acid-binding lectins. Experientia. 1990;46:433–441. doi: 10.1007/BF01954221. [DOI] [PubMed] [Google Scholar]
- 109.Traving C, Schauer R. Structure, function and metabolism of sialic acids. Cell Mol Life Sci. 1998;54:1330–1349. doi: 10.1007/s000180050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isa P, Arias CF, Lopez S. Role of sialic acids in rotavirus infection. Glycoconj. J. 2006;23:27–37. doi: 10.1007/s10719-006-5435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gee GV, Dugan AS, Tsomaia N, Mierke DF, Atwood WJ. The role of sialic acid in human polyomavirus infections. Glycoconj. J. 2006;23:19–26. doi: 10.1007/s10719-006-5434-z. [DOI] [PubMed] [Google Scholar]
- 112.Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taylor G. Sialidases: Structures, biological significance and therapeutic potential. Curr. Opin. Struct. Biol. 1996;6:830–837. doi: 10.1016/s0959-440x(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 114.von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov. 2007;6:967–974. doi: 10.1038/nrd2400. [DOI] [PubMed] [Google Scholar]
- 115.Herrler G, Rott R, Klenk HD, Muller HP, Shukla AK, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rogers GN, Herrler G, Paulson JC, Klenk HD. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986;261:5947–5951. [PubMed] [Google Scholar]
- 117.Vlasak R, Krystal M, Nacht M, Palese P. The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology. 1987;160:419–425. doi: 10.1016/0042-6822(87)90013-4. [DOI] [PubMed] [Google Scholar]
- 118.Vlasak R, Luytjes W, Leider J, Spaan W, Palese P. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J Virol. 1988;62:4686–4690. doi: 10.1128/jvi.62.12.4686-4690.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herrler G, Klenk HD. Structure and function of the HEF glycoprotein of influenza C virus. Adv Virus Res. 1991;40:213–234. doi: 10.1016/S0065-3527(08)60280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pleschka S, Klenk HD, Herrler G. The catalytic triad of the influenza C virus glycoprotein HEF esterase: characterization by site-directed mutagenesis and functional analysis. J. Gen. Virol. 1995;76:2529–2537. doi: 10.1099/0022-1317-76-10-2529. [DOI] [PubMed] [Google Scholar]
- 121.Cornelissen LAHM, Wierda CMH, van dMFJ, Herrewegh AAPM, Horzinek MC, Egberink HF, De GRJ. Hemagglutinin-esterase: A novel structural protein of torovirus. J. Virol. 1997;71:5277–5286. doi: 10.1128/jvi.71.7.5277-5286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wurzer WJ, Obojes K, Vlasak R. The sialate-4-O-acetylesterases of coronaviruses related to mouse hepatitis virus: a proposal to reorganize group 2 Coronaviridae. J. Gen. Virol. 2002;83:395–402. doi: 10.1099/0022-1317-83-2-395. [DOI] [PubMed] [Google Scholar]
- 123.de Groot RJ. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 2006;23:59–72. doi: 10.1007/s10719-006-5438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwegmann-Wessels C, Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj. J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Langereis MA, van Vliet AL, Boot W, de Groot RJ. Attachment of mouse hepatitis virus to O-acetylated sialic acid is mediated by hemagglutinin-esterase and not by the spike protein. J. Virol. 2010;84:8970–8974. doi: 10.1128/JVI.00566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orlandi PA, Klotz FW, Haynes JD. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2–3)Gal- sequences of glycophorin A. J. Cell Biol. 1992;116:901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dolan SA, Proctor JL, Alling DW, Okubo Y, Wellems TE, Miller LH. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 128.DeLuca GM, Donnell ME, Carrigan DJ, Blackall DP. Plasmodium falciparum merozoite adhesion is mediated by sialic acid. Biochem. Biophys. Res. Commun. 1996;225:726–732. doi: 10.1006/bbrc.1996.1242. [DOI] [PubMed] [Google Scholar]
- 129.Baum J, Ward RH, Conway DJ. Natural selection on the erythrocyte surface. Mol. Biol. Evol. 2002;19:223–229. doi: 10.1093/oxfordjournals.molbev.a004075. [DOI] [PubMed] [Google Scholar]
- 130.Byres E, Paton AW, Paton JC, Lofling JC, Smith DF, Wilce MC, Talbot UM, Chong DC, Yu H, Huang S, Chen X, Varki NM, Varki A, Rossjohn J, Beddoe T. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456:648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roggentin P, Schauer R, Hoyer LL, Vimr ER. The sialidase superfamily and its spread by horizontal gene transfer. Mol. Microbiol. 1993;9:915–921. doi: 10.1111/j.1365-2958.1993.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 132.Vimr E, Lichtensteiger C, Steenbergen S. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 2000;36:1113–1123. doi: 10.1046/j.1365-2958.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 133.Johnston JW, Zaleski A, Allen S, Mootz JM, Armbruster D, Gibson BW, Apicella MA, Munson RSJ. Regulation of sialic acid transport and catabolism in Haemophilus influenzae. Mol. Microbiol. 2007;66:26–39. doi: 10.1111/j.1365-2958.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- 134.Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, Pozzi G, Andrew PW, Oggioni MR. Sialic Acid: A Preventable Signal for Pneumococcal Biofilm Formation, Colonization, and Invasion of the Host. J. Infect. Dis. 2009;199:1497–1505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 135.Corfield AP, Sander WM, Veh RW, Wember M, Schauer R. The action of sialidases on substrates containing O-acetylsialic acids. Biol Chem Hoppe Seyler. 1986;367:433–439. doi: 10.1515/bchm3.1986.367.1.433. [DOI] [PubMed] [Google Scholar]
- 136.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 137.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: a minireview. Glycoconj. J. 2004;20:189–198. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- 139.Magesh S, Savita V, Moriya S, Suzuki T, Miyagi T, Ishida H, Kiso M. Human sialidase inhibitors: design, synthesis, and biological evaluation of 4-acetamido-5-acylamido-2-fluoro benzoic acids. Bioorg Med Chem. 2009;17:4595–4603. doi: 10.1016/j.bmc.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 140.Monti E, Bonten E, D'Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, Tettamanti G. Sialidases in vertebrates a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403–479. doi: 10.1016/S0065-2318(10)64007-3. [DOI] [PubMed] [Google Scholar]
- 141.Carrillo MB, Milner CM, Ball ST, Snoek M, Campbell RD. Cloning and characterization of a sialidase from the murine histocompatibility-2 complex: low levels of mRNA and a single amino acid mutation are responsible for reduced sialidase activity in mice carrying the Neu1a allele. Glycobiology. 1997;7:975–986. doi: 10.1093/glycob/7.7.975. [DOI] [PubMed] [Google Scholar]
- 142.Rottier RJ, Bonten E, D'Azzo A. A point mutation in the neu-1 locus causes the neuraminidase defect in the SM/J mouse. Hum. Mol. Genet. 1998;7:313–321. doi: 10.1093/hmg/7.2.313. [DOI] [PubMed] [Google Scholar]
- 143.Van dSA, Bonten E, D'Azzo A. Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein cathepsin A. EMBO J. 1998;17:1588–1597. doi: 10.1093/emboj/17.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amith SR, Jayanth P, Franchuk S, Siddiqui S, Seyrantepe V, Gee K, Basta S, Beyaert R, Pshezhetsky AV, Szewczuk MR. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. 2009;26:1197–1212. doi: 10.1007/s10719-009-9239-8. [DOI] [PubMed] [Google Scholar]
- 145.Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am. J. Pathol. 2008;173:1042–1056. doi: 10.2353/ajpath.2008.071081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feng C, Zhang L, Almulki L, Faez S, Whitford M, Hafezi-Moghadam A, Cross AS. Endogenous PMN sialidase activity exposes activation epitope on CD11b/CD18 which enhances its binding interaction with ICAM-1. J. Leukoc. Biol. 2011;90:313–321. doi: 10.1189/jlb.1210708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR. Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell. Signal. 2010;22:314–324. doi: 10.1016/j.cellsig.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 148.Nan X, Carubelli I, Stamatos NM. Sialidase expression in activated human T lymphocytes influences production of IFN-{gamma} J. Leukoc. Biol. 2007;81:284–296. doi: 10.1189/jlb.1105692. [DOI] [PubMed] [Google Scholar]
- 149.Seyrantepe V, Iannello A, Liang F, Kanshin E, Jayanth P, Samarani S, Szewczuk MR, Ahmad A, Pshezhetsky AV. Regulation of phagocytosis in macrophages by neuraminidase 1. J. Biol. Chem. 2010;285:206–215. doi: 10.1074/jbc.M109.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 151.Monti E, Bassi MT, Papini N, Riboni M, Manzoni M, Venerando B, Croci G, Preti A, Ballabio A, Tettamanti G, Borsani G. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 2000;349:343–351. doi: 10.1042/0264-6021:3490343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Yamaguchi K, Wada T, Hata K, Zhao XJ, Fujimoto T, Miyagi T. A close association of the ganglioside-specific sialidase Neu3 with caveolin in membrane microdomains. J. Biol. Chem. 2002;277:26252–26259. doi: 10.1074/jbc.M110515200. [DOI] [PubMed] [Google Scholar]
- 153.Vimr E, Lichtensteiger C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 2002;10:254–257. doi: 10.1016/s0966-842x(02)02361-2. [DOI] [PubMed] [Google Scholar]
- 154.Schoenhofen IC, McNally DJ, Brisson JR, Logan SM. Elucidation of the CMP-pseudaminic acid pathway in Helicobacter pylori: synthesis from UDP-N-acetylglucosamine by a single enzymatic reaction. Glycobiology. 2006;16:8C–14C. doi: 10.1093/glycob/cwl010. [DOI] [PubMed] [Google Scholar]
- 155.Schoenhofen IC, Vinogradov E, Whitfield DM, Brisson JR, Logan SM. The CMP-legionaminic acid pathway in Campylobacter: biosynthesis involving novel GDP-linked precursors. Glycobiology. 2009;19:715–725. doi: 10.1093/glycob/cwp039. [DOI] [PubMed] [Google Scholar]
- 156.Marques MB, Kasper DL, Pangburn MK, Wessels MR. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campagnari AA, Gupta MR, Dudas KC, Murphy TF, Apicella MA. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect. Immun. 1987;55:882–887. doi: 10.1128/iai.55.4.882-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by Siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 159.Avril T, Wagner ER, Willison HJ, Crocker PR. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 2006;74:4133–4141. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khatua B, Ghoshal A, Bhattacharya K, Mandal C, Saha B, Crocker PR, Mandal C. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and Siglec mediated host-cell recognition. FEBS Lett. 2010;584:555–561. doi: 10.1016/j.febslet.2009.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vanderheijden N, Delputte PL, Favoreel HW, Vandekerckhove J, Van Damme J, Van Woensel PA, Nauwynck HJ. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS ONE. 2008;3:e1967. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jiang HR, Hwenda L, Makinen K, Oetke C, Crocker PR, Forrester JV. Sialoadhesin promotes the inflammatory response in experimental autoimmune uveoretinitis. J. Immunol. 2006;177:2258–2264. doi: 10.4049/jimmunol.177.4.2258. [DOI] [PubMed] [Google Scholar]
- 165.Oetke C, Vinson MC, Jones C, Crocker PR. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin m levels. Mol. Cell. Biol. 2006;26:1549–1557. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ip CW, Kroner A, Crocker PR, Nave KA, Martini R. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol Dis. 2007;25:105–111. doi: 10.1016/j.nbd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 167.Wu C, Rauch U, Korpos E, Song J, Loser K, Crocker PR, Sorokin LM. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J. Immunol. 2009;182:6508–6516. doi: 10.4049/jimmunol.0804247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hallenbeck PC, Vimr ER, Yu F, Bassler B, Troy FA. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987;262:3553–3561. [PubMed] [Google Scholar]
- 169.Gerardy-Schahn R, Bethe A, Brennecke T, Mühlenhoff M, Eckhardt M, Ziesing S, Lottspeich F, Frosch M. Molecular cloning and functional expression of bacteriophage PK1E-encoded endoneuraminidase Endo NE. Mol. Microbiol. 1995;16:441–450. doi: 10.1111/j.1365-2958.1995.tb02409.x. [DOI] [PubMed] [Google Scholar]
- 170.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 2005;73:3096–3103. doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob. Agents Chemother. 2004;48:1503–1508. doi: 10.1128/AAC.48.5.1503-1508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Waldor MK, Friedman DI. Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol. 2005;8:459–465. doi: 10.1016/j.mib.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 173.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 174.Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic Ablation of Polysialic Acid Causes Severe Neurodevelopmental Defects Rescued by Deletion of the Neural Cell Adhesion Molecule. J. Biol. Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 175.Bax M, van Vliet SJ, Litjens M, Garcia-Vallejo JJ, van Kooyk Y. Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLoS ONE. 2009;4:e6987. doi: 10.1371/journal.pone.0006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rey-Gallardo A, Delgado-Martin C, Gerardy-Schahn R, Rodriguez-Fernandez JL, Vega MA. Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology. 2011;21:655–662. doi: 10.1093/glycob/cwq216. [DOI] [PubMed] [Google Scholar]
- 177.Drake PM, Nathan JK, Stock CM, Chang PV, Muench MO, Nakata D, Reader JR, Gip P, Golden KP, Weinhold B, Gerardy-Schahn R, Troy FAn, Bertozzi CR. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J. Immunol. 2008;181:6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Drake PM, Stock CM, Nathan JK, Gip P, Golden KP, Weinhold B, Gerardy-Schahn R, Bertozzi CR. Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc. Natl. Acad. Sci. U S A. 2009;106:11995–12000. doi: 10.1073/pnas.0905188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 180.Bishop JR, Gagneux P. Evolution of carbohydrate antigens--microbial forces shaping host glycomes? Glycobiology. 2007;17:23R–34R. doi: 10.1093/glycob/cwm005. [DOI] [PubMed] [Google Scholar]
- 181.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ghate AA, Air GM. Influenza type B neuraminidase can replace the function of type A neuraminidase. Virology. 1999;264:265–277. doi: 10.1006/viro.1999.9936. [DOI] [PubMed] [Google Scholar]
- 183.Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. U S A. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Persson KE, McCallum FJ, Reiling L, Lister NA, Stubbs J, Cowman AF, Marsh K, Beeson JG. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 2008;118:342–351. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dalziel M, Lemaire S, Ewing J, Kobayashi L, Lau JTY. Hepatic acute phase induction of murine beta-galactoside:alpha2,6 sialyltransferase (ST6Gal I) is IL-6 dependent and mediated by elevation of Exon H-containing class of transcripts. Glycobiology. 1999;9:1003–1008. doi: 10.1093/glycob/9.10.1003. [DOI] [PubMed] [Google Scholar]
- 186.Schauer R. Sialic acids and their role as biological masks. Trends Biochem Sci. 1985;10:357–360. [Google Scholar]
- 187.Weiss P, Ashwell G. The asialoglycoprotein receptor: properties and modulation by ligand. Prog Clin Biol Res. 1989;300:169–184. [PubMed] [Google Scholar]
- 188.Braun JR, Willnow TE, Ishibashi S, Ashwell G, Herz J. The major subunit of the asialoglycoprotein receptor is expressed on the hepatocellular surface in mice lacking the minor receptor subunit. J. Biol. Chem. 1996;271:21160–21166. doi: 10.1074/jbc.271.35.21160. [DOI] [PubMed] [Google Scholar]
- 189.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elward K, Gasque P. "Eat me" and "don't eat me" signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol. Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 191.Liu FT. Galectins: A new family of regulators of inflammation. Clin Immunol. 2000;97:79–88. doi: 10.1006/clim.2000.4912. [DOI] [PubMed] [Google Scholar]
- 192.Rabinovich GA, Rubinstein N, Toscano MA. Role of galectins in inflammatory and immunomodulatory processes. Biochim Biophys Acta Gen Subj. 2002;1572:274–284. doi: 10.1016/s0304-4165(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 193.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 194.Mendelsohn R, Cheung P, Berger L, Partridge E, Lau K, Datti A, Pawling J, Dennis JW. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 2007;67:9771–9780. doi: 10.1158/0008-5472.CAN-06-4580. [DOI] [PubMed] [Google Scholar]
- 195.Bi S, Baum LG. Sialic acids in T cell development and function. Biochim. Biophys. Acta. 2009;1790:1599–1610. doi: 10.1016/j.bbagen.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 196.Zhuo Y, Bellis SL. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 2011;286:5935–5941. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ideo H, Matsuzaka T, Nonaka T, Seko A, Yamashita K. Galectin-8-N-domain Recognition Mechanism for Sialylated and Sulfated Glycans. J. Biol. Chem. 2011;286:11346–11355. doi: 10.1074/jbc.M110.195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoshida H, Teraoka M, Nishi N, Nakakita S, Nakamura T, Hirashima M, Kamitori S. X-ray structures of human galectin-9 C-terminal domain in complexes with a biantennary oligosaccharide and sialyllactose. J. Biol. Chem. 2010;285:36969–36976. doi: 10.1074/jbc.M110.163402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fukushi Y, Nudelman E, Levery SB, Higuchi T, Hakomori S. A novel disialoganglioside (IV3NeuAcIII6NeuAcLc4) of human adenocarcinoma and the monoclonal antibody (FH9) defining this disialosyl structure. Biochemistry. 1986;25:2859–2866. doi: 10.1021/bi00358a018. [DOI] [PubMed] [Google Scholar]
- 200.Song Y, Kitajima K, Inoue Y. Monoclonal antibody specific to alpha-2-->3-linked deaminated neuraminyl beta-galactosyl sequence. Glycobiology. 1993;3:31–36. doi: 10.1093/glycob/3.1.31. [DOI] [PubMed] [Google Scholar]
- 201.Cheresh DA, Reisfeld RA, Varki A. O-Acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984;225:844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- 202.Schauer R, Srinivasan GV, Coddeville B, Zanetta JP, Guerardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr. Res. 2009;344:1494–1500. doi: 10.1016/j.carres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 203.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 204.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fujii Y, Higashi H, Ikuta K, Kato S, Naiki M. Specificities of human heterophilic Hanganutziu and Deicher (H-D) antibodies and avian antisera against H-D antigen-active glycosphingolipids. Mol. Immunol. 1982;19:87–94. doi: 10.1016/0161-5890(82)90250-4. [DOI] [PubMed] [Google Scholar]
- 206.Naiki M, Fujii Y, Ikuta K, Higashi H, Kato S. Expression of Hanganutziu and Deicher type heterophile antigen on the cell surface of Marek's disease lymphoma. Adv Exp Med Biol. 1982;152:445–456. [PubMed] [Google Scholar]
- 207.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, Varki A. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J. Exp. Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Merrick JM, Zadarlik K, Milgrom F. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing N-glycolylneuraminic acid. Int Arch Allergy Appl Immunol. 1978;57:477–480. doi: 10.1159/000232140. [DOI] [PubMed] [Google Scholar]
- 210.Higashi H, Naiki M, Matuo S, Okouchi K. Antigen of "serum sickness" type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem. Biophys. Res. Commun. 1977;79:388–395. doi: 10.1016/0006-291x(77)90169-3. [DOI] [PubMed] [Google Scholar]
- 211.Ghaderi D, Springer SA, Ma F, Cohen M, Secrest P, Taylor RE, Varki A, Gagneux P. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc. Natl. Acad. Sci. U S A. 2011 doi: 10.1073/pnas.1102302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sadler JE, Paulson JC, Hill RL. The role of sialic acid in the expression of human MN blood group antigens. J Biol Chem. 1979;254:2112–2119. [PubMed] [Google Scholar]
- 213.Uemura K, Roelcke D, Nagai Y, Feizi T. The reactivities of human erythrocyte autoantibodies anti-Pr2, anti-Gd, Fl and Sa with gangliosides in a chromatogram binding assay. Biochem. J. 1984;219:865–874. doi: 10.1042/bj2190865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Andrews GA, Chavey PS, Smith JE, Rich L. N-Glycolylneuraminic acid and N-acetylneuraminic acid define feline blood group A and B antigens. Blood. 1992;79:2485–2491. [PubMed] [Google Scholar]
- 215.Bighignoli B, Niini T, Grahn RA, Pedersen NC, Millon LV, Polli M, Longeri M, Lyons LA. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007;8:27. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yamakawa T, Suzuki A, Hashimoto Y. Genetic control of glycolipid expression. Chem Phys Lipids. 1986;42:75–90. doi: 10.1016/0009-3084(86)90044-7. [DOI] [PubMed] [Google Scholar]
- 217.Weiman S, Uchiyama S, Lin FY, Chaffin D, Varki A, Nizet V, Lewis AL. O-Acetylation of sialic acid on Group B Streptococcus inhibits neutrophil suppression and virulence. Biochem. J. 2010;428:163–168. doi: 10.1042/BJ20100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kelm S, Schauer R, Manuguerra J-C, Gross H-J, Crocker PR. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconjugate J. 1994;11:576–585. doi: 10.1007/BF00731309. [DOI] [PubMed] [Google Scholar]
- 219.Sjoberg ER, Powell LD, Klein A, Varki A. Natural ligands of the B cell adhesion molecule CD22beta can be masked by 9-O-acetylation of sialic acids. J. Cell Biol. 1994;126:549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, Kalloo G, Connole M, Shi HN, Varki N, Varki A, Pillai S. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J. Exp. Med. 2009;206:125–138. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends Immunol. 2009;30:488–493. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, Haider K, Netravali I, Le S, Elia R, Dow E, Lee A, Freudenberg J, De Jager PL, Chretien Y, Varki A, Macdonald ME, Gillis T, Behrens TW, Bloch D, Collier D, Korzenik J, Podolsky DK, Hafler D, Murali M, Sands B, Stone JH, Gregersen PK, Pillai S. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kniep B, Flegel WA, Northoff H, Rieber EP. CDw60 glycolipid antigens of human leukocytes: Structural characterization and cellular distribution. Blood. 1993;82:1776–1786. [PubMed] [Google Scholar]
- 224.Rieber EP, Rank G. CDw60: a marker for human CD8+ T helper cells. J. Exp. Med. 1994;179:1385–1390. doi: 10.1084/jem.179.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fox DA, He X, Abe A, Hollander T, Li LL, Kan L, Friedman AW, Shimizu Y, Shayman JA, Kozarsky K. The T lymphocyte structure CD60 contains a sialylated carbohydrate epitope that is expressed on both gangliosides and glycoproteins. Immunol Invest. 2001;30:67–85. doi: 10.1081/imm-100104017. [DOI] [PubMed] [Google Scholar]
- 226.Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 2002;196:1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Birks SM, Danquah JO, King L, Vlasak R, Gorecki DC, Pilkington GJ. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro Oncol. 2011;13:950–960. doi: 10.1093/neuonc/nor108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen HY, Varki A. O-acetylation of GD3: an enigmatic modification regulating apoptosis? J. Exp. Med. 2002;196:1529–1533. doi: 10.1084/jem.20021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 230.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol. 2010;30(Suppl 1):S9–S14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 231.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Durandy A, Kaveri SV, Kuijpers TW, Basta M, Miescher S, Ravetch JV, Rieben R. Intravenous immunoglobulins--understanding properties and mechanisms. Clin Exp Immunol. 2009;158(Suppl 1):2–13. doi: 10.1111/j.1365-2249.2009.04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.von Gunten S, Simon HU. Cell death modulation by intravenous immunoglobulin. J. Clin. Immunol. 2010;30(Suppl 1):S24–S30. doi: 10.1007/s10875-010-9411-8. [DOI] [PubMed] [Google Scholar]
- 234.Jones MB, Nasirikenari M, Feng L, Migliore MT, Choi KS, Kazim L, Lau JT. Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J. Biol. Chem. 2010;285:25009–25017. doi: 10.1074/jbc.M110.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]