Abstract
A total of 220 lactic acid bacteria isolates were screened for antifungal activity using Aspergillus fumigatus and Aspergillus niger as the target strains. Four Lactobacillus strains exhibited strong inhibitory activity on agar surfaces. All four were also identified as having strong inhibitory activity against the human pathogenic fungi Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. One of the four lactobacilli, namely Lb. reuteri ee1p exhibited the most inhibition against dermatophytes. Cell-free culture supernatants of Lb. reuteri ee1p and of the non-antifungal Lb. reuteri M13 were freeze-dried and used to access and compare antifungal activity in agar plate assays and microtiter plate assays. Addition of the Lb. reuteri ee1p freeze-dried cell-free supernatant powder into the agar medium at concentrations greater than 2% inhibited all fungal colony growth. Addition of the powder at 5% to liquid cultures caused complete inhibition of fungal growth on the basis of turbidity. Freeze-dried supernatant of the non-antifungal Lb. reuteri M13 at the same concentrations had a much lesser effect. As Lb. reuteri M13 is very similar to the antifungal strain ee1p in terms of growth rate and final pH in liquid culture, and as it has little antifungal activity, it is clear that other antifungal compounds must be specifically produced (or produced at higher levels) by the anti-dermatophyte strain Lb. reuteri ee1p. Reuterin was undetectable in all four antifungal strains. The cell free supernatant of Lb. reuteri ee1p was analyzed by LC-FTMS using an Accela LC coupled to an LTQ Orbitrap XL mass spectrometer. The high mass accuracy spectrum produced by compounds in the Lb. reuteri ee1p strain was compared with both a multianalyte chromatogram and individual spectra of standard anti-fungal compounds, which are known to be produced by lactic acid bacteria. Ten antifungal metabolites were detected.
Keywords: Epidermophyton floccosum, antifungal, lactic acid bacteria, Microsporum canis, Microsporum gypseum
Introduction
Cutaneous mycoses are among the most common fungal infections and are mostly caused by filamentous keratinophilic fungi that use keratin as a nutrient during skin, scalp and nail infections. Pathogens responsible for skin mycoses are primarily anthropophilic and zoophilic dermatophytes and include the genera Microsporum and Epidermophyton. These fungi attack various parts of the body and the resulting diseases are often called ringworm or tinea. According to the World Health Organization (WHO), dermatophytes affect about 25% of the world population. It is estimated that from 30 to 70% of adults are asymptomatic hosts of these pathogens and that the incidence of the disease increases with age.1,2 Although many antifungal agents have been developed in recent decades and have become available for the treatment of dermatophytosis, they are confined to relatively few chemical groups. In addition, the occurrence of resistance or side effects in clinically isolated strains leads to failure in the treatment of mycoses.3-7 Apart from side-effects like liver-damage or affecting estrogen levels, many medicines can cause allergic reactions. For example, the azole group of drugs is known to cause anaphylaxis.8 There are also many drug interactions, the azole antifungals such as ketoconazole or itraconazole can be both substrates and inhibitors of the P-glycoprotein, which (among other functions) excretes toxins and drugs into the intestines.9 Azole antifungals also are substrates and inhibitors of the cytochrome P450 family CYP3A4, causing increased concentration when administering, for example, with calcium channel blockers, immunosuppressants, chemotherapeutic drugs, benzodiazepines, tricyclic antidepressants, macrolides and SSRIs.10-12 The polyene antimycotics are crucial agents in the management of serious systemic fungal infections. Despite this, their known side effects and toxicity will sometimes require discontinuation of therapy despite a life-threatening systemic fungal infection.13 Polyene antimycotics administration is limited by infusion-related toxicity, an effect postulated to result from proinflammatory cytokine production. The principal acute toxicity of Amphotericin B deoxycholate includes nausea, vomiting, rigors, fever, hypertension or hypotension, and hypoxia. Its principal chronic adverse effect is nephrotoxicity.14,15 Essentially, the majority of the clinically-used antifungals suffer from various drawbacks in terms of toxicity, drug-drug interactions, and lack of fungicidal efficacy, cost and emergence of resistant strains. In spite of the recent introduction of new antifungal drugs, they are still limited in number. Hence, there is a great demand for novel antifungal agents, justifying the intense search for new drugs that are more effective and less toxic than those already in use.16-19
Lactic acid bacteria (LAB) are widely used in food and feed fermentation, contributing to the safety, stability, flavor and structure of food/feed products. Antimicrobial compounds produce by LAB include: organic acids, hydrogen peroxide, diacetyl, CO2 and bacteriocins. There is extensive knowledge about the antibacterial effects by bacteriocins20-23 whereas the number of published studies on the identification of antifungal agents from LAB is rather limited and to date, these are focused on food-associated fungi such as Fusarium, Aspergillus and Penicillium.24-27 Antifungal compounds from LAB involve metabolites derived from organic acids, proteinaceous compounds and other low molecular mass compounds (less than 1,000 Da). Several of these compounds have been isolated and shown to have the ability to retard or eliminate fungal growth or spore outgrowth, either on their own or synergistically.28-32
Given their food grade status, the collections of lactic acid bacteria were screened as a possible source of antifungal activity against dermatophytes. Two relatively safe, fast-growing fungi were used as targets with the aim of finding strains, which might be inhibitory to the infectious fungi Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. The antifungal activity of LAB was demonstrated in bioassays with fungi cultivated in defined medium on agar plates or in liquid cultures.
Results
Screening for antifungal activity of LAB
The sources of LAB isolates included pigs, human infants, mice, cows, sourdough, cheese and cereal samples. Two hundred twenty strains were isolated in total. Their screening for antifungal activity was investigated by streaking out the bacteria in two parallel lines onto mMRS plates inoculated with A. fumigatus and A. niger spores and mycelia. The antifungal activity was evaluated at 30°C as well as 37°C. Incubation at 37°C resulted in a higher number of LAB showing anti-fungal activity against these species. At this temperature, around 40% of the isolates inhibited A. fumigatus, compared with 29% at 30°C; around 60% of the isolates inhibited A. niger, compared with 39% at 30°C. Overall, 77% of the isolates inhibited one fungus, 43% of all isolates showed some degree of antifungal activity against both target fungi. Interestingly, only 12 strains exhibited antifungal activity against two Aspergillus at both temperatures. More LAB isolates from the bovine (83%), human (74%) and porcine (68%) groups showed antifungal activity than isolates from the other sources. Nevertheless, in the majority of cases, the antifungal activity was considered to be weak.
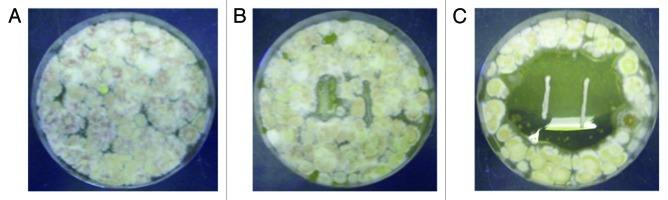
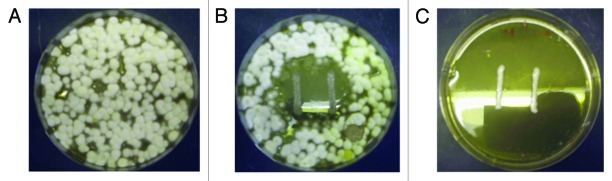
The four strongest antifungal LAB (i.e., showing strong inhibition against both Aspergillus species) were identified and their inhibition profile at both incubation temperatures is summarized in Table 1. Four negative control strains (of the same species) are included. All eight strains were then screened against M. canis DSM10708, M. gypseum DSM3824 and E. floccosum DSM10709. Sizes of clear zones of antifungal activity against the fungi were recorded and these are shown in Table 2. All four antifungal LAB strains also inhibited the three dermatophytes. One, namely Lb. reuteri ee1p, was selected for further study. It was observed to have very strong anti-dermatophyte activity at 30°C in that the distances between the peripheral sides of the bacterial-lines and the starting fungal growth zone were generally large (Table 2). The negative control strain Lb. reuteri M13 does appear to cause a weakened fungal growth adjacent to the LAB streak in some cases. However, on close observation, the fungal mycelium did come in contact with the LAB colonies in these cases. This did not occur with Lb. reuteri ee1p, where there is definite inhibition of M. canis, M. gypseum and E. floccosum mycelial growth typified by large zones (Table 2; Figs. 1 and 2). Lb. reuteri M13 was the most appropriate negative control strain as its growth rate and acid-producing ability in liquid medium were almost identical to that of Lb. reuteri ee1p (Fig. 3).
Table 1. Summary of antifungal activity of isolated lactic acid bacteria against selected Aspergillus species (incubation was performed at 37°C or 30°C).
| Strain |
A. niger |
A.fumigatus |
Strain origin |
||
|---|---|---|---|---|---|
| 37°C | 30°C | 37°C | 30°C | ||
|
Lactobacillus arizonensis (R13) |
+ |
+ |
+ |
+ |
cheese |
|
Lactobacillus arizonensis (R14) |
- |
- |
- |
- |
human |
|
Lactobacillus brevis (JJ2p) |
+ |
- |
+ |
++ |
porcine |
|
Lactobacillus brevis (NL) |
- |
- |
- |
- |
sourdough |
|
Lactobacillus casei (R4) |
++ |
+++ |
+ |
+ |
human |
|
Lactobacillus casei (R21) |
- |
- |
- |
- |
human |
|
Lactobacillus reuteri (ee1p) |
+ |
++ |
+++ |
- |
porcine |
| Lactobacillus reuteri (M13) | - | - | - | - | murine |
The distance between the peripheral sides of the bacterial-lines and the starting growth zone was scored as follows: -, no clear zone; +, distance ≥ 3 mm; ++, distance ≥ 5 mm; +++, distance ≥ 8 mm.
Table 2. Zone sizes (mm) around bacterial streaks indicating antifungal activity of lactic acid bacteria against the dermatophytes Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. The table includes the most inhibitory antifungal LAB strain and a negative control strain for each, indicated by an asterisk. Negative control strains have the same growth rates and exhibit the same final culture pH as their respective antifungal partner.
| Genus and Species |
Microsporum canis |
Microsporum gypseum | Epidermophyton floccosum |
|---|---|---|---|
|
Lactobacillusbrevis JJ2P |
7 |
20 |
20 |
|
Lactobacillusbrevis L1105* |
5 |
15 |
15 |
|
Lactobacillus arizonesis R13 |
7 |
20 |
35 |
|
Lactobacillus arizonesis R14* |
0 |
0 |
0 |
|
Lactobacilluscasei R4 |
4 |
10 |
10 |
|
Lactobacilluscasei R21* |
0 |
4 |
5 |
|
Lactobacillus reuteri ee1p |
5 |
20 |
35 |
| Lactobacillus reuteri M13* | 0 | 2 | 2 |
Figure 1. Antifungal activity of Lb. reuteri ee1p against M. gypseum. (A) M. gypseum grown 15 d at 30°C on mMRS agar plate with no LAB present. (B) M. gypseum grown with negative control Lb. reuteri M13. (C) M. gypseum grown with Lb. reuteri ee1p showing clear zones of fungal inhibition.
Figure 2. Antifungal activity of Lb. reuteri ee1p against Epidermophyton floccosum (A) E. floccosum grown 15 d at 30°C on mMRS agar plate with no LAB present. (B) E. floccosum grown with negative control Lb. reuteri M13. (C) E. floccosum grown with Lb. reuteri ee1p showing complete fungal inhibition.
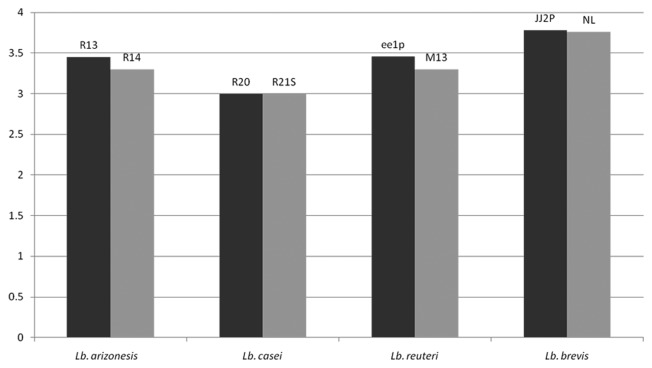
Figure 3. Final pH of the different lactic acid bacterial fully grown cultures in MRS broth after 48 h incubation at 37°C.
To account for the influence of acidic conditions on fungal mycelia’s growth, M. canis, M. gypseum and E. floccosum were independently grown for 15 d on SD agar surface at pHs ranging from 2.0 to 9.0. Colony diameters were shown to be same at pHs 3.5 to 8.0 for all the selected fungi. At pH 3.0, the growth of all fungi was reduced, while at pH 2.5 or less, no fungal growth occurred. The data are summarized in Table 3. It indicated that while low pH did have a slight effect on fungal inhibition, other factor(s) related to Lb. reuteri ee1p was contributing to the inhibition. In addition, the acid-producing ability during growth of the four antifungal LABs and the four negative control strains (of the same species) were also assessed (Fig. 3). The acid producing ability of both bacterial strains in each pair of positive and negative LAB is similar.
Table 3. Effect of pH on fungal growth. Fungal colony diameter (cm) after inoculation of Microsporum canis, Microsporum gypseum and Epidermophyton floccosum on mMRS agar adjusted to pHs ranging from 2.0 to 9.0.
| pH of agar | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 |
|---|---|---|---|---|---|---|---|---|---|---|
|
Microsporum canis |
0 |
0 |
3.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
|
Microsporum gypseum |
0 |
0 |
3.0 |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
3.5 |
| Epidermophyton floccosum | 0 | 0 | 1.8 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 2.5 |
Analysis for presence of reuterin by colorimetric assay
3-hydroxypropionaldehyde (3-HPA), also known as reuterin, is an antimicrobial compound produced by Lb. reuteri. The presence or absence of reuterin was evaluated using a colorimetric assay. Acrolein at 0.05 mM 0.5 mM, and 50 mM were used as the positive controls (3-HPA is a precursor to acrolein, 1 mol of 3-HPA dehydrates to 1 mol of acrolein). Optical densities of acrolein solutions at 605 nm were observed to be 0.090 (0.05 mM), 0.451 (0.5 mM) and 0.870 (50 mM). Lb. reuteri ee1p did not yield any colorimetric change, indicating a lack of production of reuterin at levels greater than 0.05 mM.
Identification of antifungal compounds in Lb. reuteri ee1p using LC-FTMS
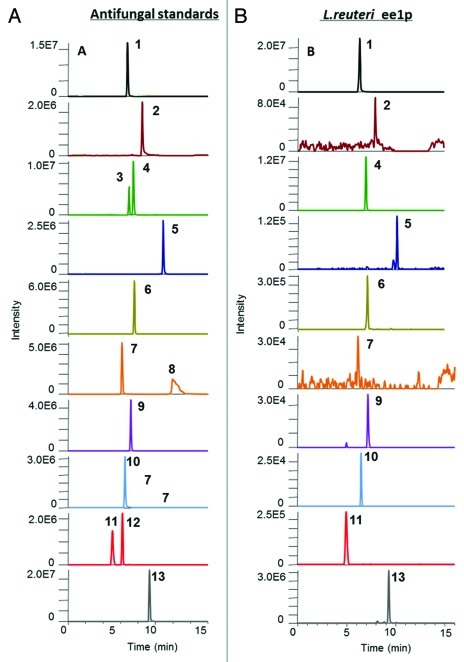
The cell free supernatant of Lb. reuteri ee1p was analyzed by liquid chromatography Fourier transform mass spectrometry (LC-FT-MS) using an Accela LC instrument coupled to a LTQ Orbitrap XL mass spectrometer. The high mass accuracy spectrum produced by compounds in the Lb. reuteri ee1p strain was compared with both a multianalyte chromatogram and individual characteristic spectra of 13 standard anti-fungal compounds (Fig. 4A). Ten anti-fungal compounds, (S)–(-)-2–hydroxyisocapric acid (m/z 131.07082), hydrocinnamic acid (m/z 149.06025), phenyllactic acid (m/z 165.05517), decanoic acid (m/z 171.1385), azelaic acid (m/z 187.09703), 4-hydroxybenzoic acid (m/z 137.02387), p-coumaric acid (m/z 163.03952), vanillic acid (m/z 167.03443), DL-Þ-hydroxyphenyllactic acid (m/z 181.05008) and 3-hydroxydecanoic acid (m/z 187.13342) were identified in the Lb. reuteri ee1p strain (Fig. 4B). All identified anti-fungal compounds were matched against their equivalent standard peak retention times and spectra. Each identified negative ion, [M-H]-, mass was compared with its theoretical mass and a PPM error value was calculated. PPM errors below 3 ppm tolerance ensures that there is only one possible molecular formula for that identified compound. All identified anti-fungal compounds in the Lb. reuteri ee1p strain had PPM errors between 0.5–2 PPM when compared with their equivalent standard [M-H]-ions. Therefore we can unequivalently conclude that these ten anti-fungal compounds are present in the Lb. reuteri ee1p strain.
Figure 4. Chromatograms of anti-fungal standards and anti-fungal compounds detected in the supernatant from Lb. reuteri ee1p following SPE clean up.54 (A) Antifungal standards: (1), (S)–(-)-2–hydroxyisocapric acid (6.80 min, m/z 131.07); (2), hydrocinnamic acid (8.48 min, m/z 149.06); (3), 3-(4-hydroxyphenyl)–propionic acid (6.98 min, m/z 165.05); (4), phenyllactic acid (7.47 min, m/z 165.05); (5), decanoic acid (10.88 min, m/z 171.13); (6), azelaic acid (7.57 min, m/z 187.09); (7), 4-hydroxybenzoic acid (6.18 min, m/z 137.02); (8), salicylic acid (11.97 min, m/z 137.02); (9), p-coumaric acid (7.17 min, m/z 163.03); (10), vanillic acid (6.50 min, m/z 167.03); (11), DL-Þ-hydroxyphenyllactic acid (5.00 min, m/z 181.05); (12), 3,4-dihydroxyhydrocinnamic acid (6.23 min, m/z 181.05); (13), 3-hydroxydecanoic acid (9.32 min, m/z 187.13). (B) Antifungal compounds identified in broth (1), (S)–(-)-2–hydroxyisocapric acid (6.77 min, m/z 131.07); (2), hydrocinnamic acid (8.49 min, m/z 149.06); (4), phenyllactic acid (7.44 min, m/z 165.05); (5), decanoic acid (10.87 min, m/z 171.13); (6), azelaic acid (7.58 min, m/z 187.09); (7), 4-hydroxybenzoic acid (6.16 min, m/z 137.02); (9), p-coumaric acid (7.17 min, m/z 163.03); (10), vanillic acid (6.51 min, m/z 167.03); (11), DL-Þ-hydroxyphenyllactic acid (4.98 min, m/z 181.05); (13), 3-hydroxydecanoic acid (9.29 min, m/z 187.13).
Dermatophyte morphology in the presence of LAB supernatants
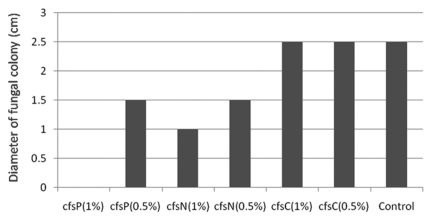
Lb. reuteri ee1p was used to generate a freeze-dried cell-free supernatant (cfsP). This was added to agars to determine its effect of fungal colony morphology for M. canis, M. gypseum and E. floccosum. As a negative control, cfsN, a freeze-dried cell-free supernatant of the non-antifungal strain M13 was used. As an additional control to exclude the effect of acid, cfsC was used which was essentially a freeze-dried uninoculated mMRS broth which had been adjusted to pH 4.0 with lactic acid. Using E. floccosum as an example, addition of cfsP and cfsN at a concentration of 0.5% did affect morphology of the fungus: fungal colony diameter decreased from 2.5 cm (seen for cfsC and also for the negative control plate with no additive) to 1.5 cm (with cfsN and cfsP). At a concentration of 1%, complete inhibition of colony growth was observed for the cfsP plate; the fungus grew and colony diameter was 1.0 cm on the cfsN plate (negative control). On acidified control plates to which cfsC (freeze-dried mMRS) was added, no differences were observed when compared with the diameter on the control plate (no additives) (Fig. 5). This showed that cfsP contained a distinct antifungal factor or factors. Addition of the Lb. reuteri ee1p freeze-dried cell-free supernatant powder (cfsP) into the agar medium at concentrations greater than 1% completely inhibited M. canis colony growth; concentrations greater than 2% were necessary to inhibit M. gypseum colony growth (data not shown).
Figure 5. Diameter (cm) of Epidermophyton floccosum colonies incubated for 15 d on Sabouraud dextrose agar plates containing freeze-dried cell-free supernatant of Lb. reuteri ee1p at pH 4 (cfsP), freeze-dried cell-free supernatant of Lb. reuteri M13 at pH 4 (cfsN), freeze-dried mMRS at pH 4 (cfsC) or no freeze-dried additives (control).
Inhibition of fungal growth in liquid culture
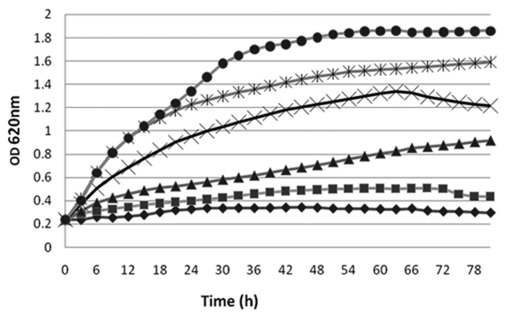
Microtiter assays were used to examine the effect of different concentrations of cfs on M. canis, M. gypseum and E. floccosum in liquid culture. Using M. canis as an example, when ≥ 5% cfsP was used, no change in OD620 was observed over 120 h. When 1.25% or 2.5% cfsP was added, M. canis growth was inhibited during incubation over the same time range. Reducing the concentration of cfsP in SDB to 0.6% resulted in the loss of antifungal activity. Profiles are shown in Figure 6. Concentrations of cfsP lower than 0.6% did not affect the growth of M. canis (data not shown). Addition of cfsP at 5% was also necessary to inhibit the other two fungi, M. gypseum and E. floccosum (data not shown).
Figure 6. Growth of M. canis in Sabouraud dextrose broth containing 10% (◆), 5% (■), 2.5% (▾), 1.25% (╳), 0.6% (•) or 0% (*) of freeze-dried cell-free supernatant of Lb. reuteri ee1p (cfsP).
Discussion
Fungal infection of the skin is a common global problem. Currently, 20–25% of the world’s population suffers from skin mycosis, making these one of the most frequent forms of infection.2 Microsporum and Epidermophyton are two fungal dermatophytes responsible for dermatophytosis (commonly called tinea or ringworm) of the scalp, glabrous skin, and nails. These fungi are frequently resistant to traditional fungicides.34 Most antifungal agents, even those newly developed, still remain within the two main antifungal drug families, the azoles and the allylamines, particularly itraconazole and terbinafine. These antifungal medications have be associated with potential hepatic toxicity and possible drug-drug interactions.35 Therefore it is necessary to search for more effective and less toxic novel antifungal agents that would overcome these disadvantages.
Lactic acid bacteria are widely used for the production of fermented foods and are also part of the intestinal microflora. A total of 220 different isolates of LAB from a variety of environments were screened for antifungal activity by spraying fungal spore suspensions of A. fumigatus and A. niger strains onto agar surfaces, which were then streaked with all LAB isolates. Overall, 77% of the isolates inhibited at least one fungus, 43% of all isolates showed some degree of antifungal activity against both target fungi, which is a very high incidence and indicates the high sensitivity of Aspergillus to LAB. Previous studies in our laboratory using other food fungi such as Penecillium showed lower sensitivity in general. The reason for using Aspergillus strains in the initial screening was that they were considered easier and safer to work with for routine screening in our laboratory, by comparison with many dermatophytes. The four isolates, which showed strong antifungal activity against Aspergillus were evaluated against M. canis, M. gypseum and E. floccosum. All selected LAB were observed to be capable of inhibiting the three dermatophytes. One of these strains was Lb. reuteri ee1p, was selected for further study. Among the non-antifungal strains identified, one namely Lb. reuteri M13 was chosen as a negative control strain because it is very similar to Lb. reuteri ee1p in terms of growth rate and the final pH generated in liquid culture and thus was comparable to the antifungal strain ee1p.
The antifungal agents produced by Lb. reuteri ee1p affected growth of both mycelia and conidia of the dermatophytes. Addition of Lb. reuteri ee1p freeze-dried cell-free supernatant (cfsP) into agar medium at 2% or greater had an inhibitory effect on all fungal growth by comparison with the negative control Lb. reuteri M13 freeze-dried cell-free supernatant (cfsN) and freeze-dried mMRS broth (cfsC) at the same concentrations and pH. Addition of cfsP at 5% was necessary to inhibit M. canis, M. gypseum and E. floccosum growth in broth cultures in micro-titer assays. When Lb. reuteri M13, a very similar strain to Lb. reuteri ee1p in terms of growth rate and final culture pH in liquid medium, was used as a negative control strain, much less antifungal activity was identified. This indicated that antifungal compounds must be specifically produced (or produced at higher levels) by the anti-dermatophyte strain Lb. reuteri ee1p. Several compounds with a strong antifungal activity have been isolated from lactic acid bacterial cultures and the majority of those identified to-date are of low molecular weight and include organic acids,36,37 reuterin,38,39 hydrogen peroxide,29 proteinaceous compounds,27,32,40 hydroxy fatty acids41 and phenolic compounds.42 Additional compounds identified in this work, which according to the scientific literature exhibit antifungal activity43-45 are (S)–(-)-2–Hydroxyisocapric acid (m/z 131.07082), hydrocinnamic acid (m/z 149.06025), phenyllactic acid (m/z 165.05517), decanoic acid (m/z 171.1385), azelaic acid (m/z 187.09703), 4-hydroxybenzoic acid (m/z 137.02387), p-coumaric acid (m/z 163.03952), vanillic acid (m/z 167.03443), DL-Þ-Hydroxyphenyllactic acid (m/z 181.05008) and 3-Hydroxydecanoic acid (m/z 187.13342). Other natural agents, which are being assessed against dermatophytes in other laboratories, are a variety of plant-derived agents such as the Ageratina pichinchensis var bustamenta,46 the essential oil of Calea clematidea,16 the plant oil of Leptospermum petersonii and Syzygium aromaticum,47 the clove essential oil from Syzygium,48 and various organic extracts of Nandina domestica Thunb.19 The aerial parts of Ageratina pichinchensis were active against T. rubrum and T. mentagrophytes.46 The Calea clematidea oil of the leaves showed a moderate antifungal activity against a number of Trichophyton species, with the compound clemateol shown to be of importance in this observation.16 The essential oils of Leptospermum petersonii and Syzygium aromaticum showed antifungal activity against the dermatophytes M. canis, M. gypseum, E. floccosum, T. mentagrophytes and T. rubrum.47 Within the group of lactic acid bacteria examined for antifungal activity in our study, there was considerable variation, possibly also connected with environmental and genetic factors. In the context of plant-derived antifungals, some compounds have been reported to induce side-effects in humans. Lactic acid bacteria are widely used in food and feed fermentation, contributing to the safety, stability, flavour and structure of the productsit, therefore it is possible that their antifungal agents do not induce side-effects in humans, although this requires further research. Nevertheless, the use of lactic acid bacteria and their products may well provide alternative or complimentary approaches for inhibition of dermatophytes. Previous work from our labortory reported inhibition of the human pathgenic fungus Trichophyton tonsurans by another Lactobacillus strain but no compounds was identified at that point.49 This research has identified ten antifungal compounds in strain Lb. reuteri ee1p, which are likely to play a role in the inhibition of M. gypseum, M. canis and E. floccosum. Future research will look for additional compounds as well as testing the antifungal efficacy of purified compounds and also synergy between compounds.
Materials and Methods
Bacterial cultures identification
Lactic acid bacteria were identified upon sequencing of the first 900 bp of the 16S rDNA.50 To determine the closest relatives of the partial 16S rDNA sequences, a GeneBank DNA database search was conducted. A similarity of > 98% to 16S rDNA sequences of type strains was used as the criterion for identification. LAB were routinely grown on MRS agar plates (FlukaChemie AG) under microaerophilic conditions for 48 h at 30 or 37°C. Long-term storage was done in 40% glycerol at -80°C.
Fungal culture preparation
Initially antifungal screening was performed by using Aspergillus fumigates J9 and Aspergillus niger A1. Molds were cultivated on 10 ml of potato dextrose agar (PDA) slants at 25°C for 7 d (or until sporulation occurred). Spores were harvested by vigorously shaking slants with 20 ml Ringer solution, providing a fungal cell and conidial suspension of approximately 105 spores per ml. M. canis DSM10708, M. gypseum DSM3824 and E. floccosum DSM10709 were obtained from (DSMZ) German Collection of Microorganisms and Cell Cultures, Germany (www.dsmz.de). The fungal pathogen was grown on Sabouraud dextrose agar (SDA) (Sigma-Aldrich) plates at 30°C for 15 d and then stored at 4°C until further use. Small piece from SDA plate inoculated with sporulating colonies was transferred into 500 ml of synthetic-nutrient-poor bouillon (SNB).33 The suspensions were incubated at 25°C (120 rpm) for 7–15 d to induce conidia formation. Concentrations of 1.0 × 105 to 3.0 × 105 CFU/ml were measured by plating out serial dilutions on SDA-plates.
Antifungal LAB screening using plate assays
Antifungal activity of LAB against A. fumigatus J9 and A. niger A1 was tested by nebulising 100 μl of fungal spore-mycelia suspension (approx. 104 CFU) onto the surface of Petri-dishes containing 20 ml of MRS agar modified as follows (mMRS): pH was adjusted to 6.0, sodium acetate and potassium dihydrogenphosphate were omitted. After 30 min, bacteria were inoculated as two parallel lines of 3 cm length; keeping a distance between the lines of approx. 2 cm. Plates were incubated under microaerophilic conditions at 30 as well as 37°C for 48 h followed by an additional incubation for 48 h under aerobic conditions at 25°C to promote fungal growth. The antifungal activity of each LAB was ascertained by measuring the size of the halo surrounding the bacterial streaks. Antifungal activity against M. canis, M. gypseum and E. floccosum was tested by mixing 2 ml of fungal spore-mycelia suspension into 18 ml of MRS agar modified as follows (mMRS): pH adjusted to 6.0, sodium acetate as well as potassium dihydrogenphosphate were omitted. After 30 min, lactic acid bacteria were inoculated as two parallel lines of 3 cm length; keeping a distance between the lines of approx. 2 cm. Plates were incubated under microaerophilic conditions at 30 or 37°C for 48 h followed by an additional incubation for 15 d under aerobic conditions at 30°C to promote fungal growth. The antifungal activity of each LAB was ascertained by measuring the size of the halo surrounding the bacterial streaks. The overall growth of the fungi was compared with that in control plates (i.e., with no LAB present).
Analysis for reuterin production using colorimetric assay
Reuterin [3-hydroxypropionaldehyde (3-HPA)] was tested using the chemical method of Cohen and Altshuller,51 and Cadieux et al.39 Briefly, cells were harvested from liquid cultures by centrifugation and washed twice with 50 mM potassium phosphate buffer (pH 7.5). Approximately 100 mg cells (wet weight) were resuspended in 14 mL 250 mM glycerol, and the cells in glycerol were incubated at 37°C for 2 h. The supernatant was passed through a 0.45-μm-pore-size syringe filter and stored at 4°C. The assay for 3-HPA content was based on Cohen and Altshuller49 colorimetric method developed for acrolein. 3-HPA was first dehydrated to acrolein, which in turn reacts with 4-hexylresorcinol, in the presence of HgCl2 as catalyst, to form a colored complex that absorbs light at 605 nm. Briefly, 0.5 ml saturated trichloroacetic acid (TCA), 0.012 ml of a 4-hexylresorcinol solution (50% w/v inethanol) and 0.02 ml of an HgCl2 solution (3% w/v in ethanol) were mixed with 0.5 ml of sample. The mixture was incubated at 60°C for15 min, allowed to cool down at 20°C for an additional 15 min and the absorbance immediately recorded at 605 nm. Sample was diluted to assure absorbance readings below 0.85. Since 3-HPA is not commercially available, acrolein, a derivative of 3-HPA was used at 0.05mM, 0.5 mM and 50 mM as positive controls (1 mol of 3-HPA dehydrates to 1 mol of acrolein) as previously described by Bauer et al.52
Production of cell-free supernatant (cfs) powders of lactic acid bacteria
Freeze-dried supernatant powders of the most inhibiting (positive) strain, cfsP, and of a non-inhibiting strain belonging to the same species (cfsN) were produced to serve as base material for the experiments describing the nature of the antifungal compounds. Briefly, overnight cultures of bacteria were inoculated in 500 ml of mMRS broth to reach an initial concentration of 104 CFU/ml. The bacteria were grown for 5 d at 37°C (temperature at which the antifungal strain showed its highest activity). Cells were separated from the supernatant by centrifuging twice at 3,000 g for 15 min. The cell-free supernatant was freeze-dried and the powder stored at 4°C. Freeze-dried mMRS broth (cfsC) powder, which was used as a control for some experiments, was obtained using same procedure. Typically, 500 ml of supernatant gave rise to 25 g of lyophilized powder. Powders were routinely reconstituted in sterile distilled water.
pH tolerance testing of M. canis, M. gypseum and E. floccosum
The pH tolerance of M. canis, M. gypseum and E. floccosum were evaluated by adjusting the pH of mMRS to 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0 and 9.0 using either HCl or NaOH. 10 μL of spore-mycelia suspension were inoculated as a spot in the center of the mMRS plates after which they were incubated for 15 d at 30°C under aerobic conditions. pH susceptibility was monitored by measuring the diameter of fungal colonies at the different pHs.
Identification of antifungal compounds from Lb. reuteri ee1p using LC-FTMS
Anti-fungal standard compounds: (1) (S)-(-)-2-hydroxyisocapric acid; (2) hydrocinnamic acid; (3) 3-(4-hydroxyphenyl)-propionic acid; (5) decanoic acid; (6) azelaic acid; (7) 4-hydroxybenzoic acid; (8) salicylic acid; (9) p-coumaric acid; (10) vanillic acid; (11) DL-Þ-hydroxyphenyllactic acid; (12) 3,4-dihydroxyhydrocinnamic acid; (13) 3-hydroxydecanoic acid) and the mobile phase additive acetic acid were purchased from Sigma Aldrich. (4) Phenyllactic acid was obtained by Bachem (Weil am Rhein). LC-MS grade solvents were sourced from Thermo Fisher Scientific. Solid phase extraction (SPE) cartridges (Isolate C18-EC) were purchased from Biotage AB.
Separation of the 13 compounds was obtained on an Accela LC system (Thermo Fisher Scientific) using a Gemini C18 (150 × 2 mm, 5 µm; Phenomenex) column equipped with a Security Guard cartridge (C18, 4 × 2 mm; Phenomenex). The column was maintained at a temperature of 30oC and a flow rate of 300 µl/min. A stepped gradient elution was used (A-water with 0.1% acetic acid; B-acetonitrile with 0.1% acetic acid). Initial conditions were 10% B held for 3 min increasing to 95% B over 10 min, this was held for 3 min, before returning to the initial starting conditions to equilibrate.53 Sample preparation involved applying the crude Lb. reuteri ee1p extract, following centrifugation, to an Isolate C18-EC SPE cartridge as outlined in the method by Strom et al.54
The LTQ Orbitrap XL hybrid mass spectrometer (Thermo Fisher Scientific) was connected to the Accela LC system. It was operated in negative ion mode with an electrospray interface (ESI). A universal ion source tune method was developed optimising the capillary temperature at 300°C, capillary voltage at -50 V, tube lens at -110 V, sheath gas at 45 arb and the auxiliary gas at 15 arb. The instrument was calibrated as per the manufacture’s instructions and applied at a resolution of 30,000 FWHM giving sufficient data points (n = 15) under each chromatographic peak.53
Fungal morphology in the presence of LAB supernatant
A 50% (w/w) cfsP, cfsN and cfsC working-solution were prepared by dissolving the powder in distilled water, adjusted pH to 4 using commercial D/L-lactic acid (Sigma-Aldrich) and variable amounts of 4 M NaOH, and then filter sterilized using a 0.45 μm MINISART®-plus filter (Sartorius). SDA plates were prepared containing 0 (control), 0.5, 1, 2% (m/v) cfsP. For each concentration, negative control plates were prepared containing same amount of cfsN, acidified control plates were prepared containing same amount of cfsC. After cooling, 10 uL of spore-mycelia suspension were inoculated as a spot in the center of the SDA-plates. The plates were incubated for 15 d at 30°C under aerobic conditions. The fungal growth was monitored by measuring the growth area of fungal colonies.
Inhibition of fungal growth in liquid culture
The effect of different concentration of cfsP on the growth of M. canis, M. gypseum and E. floccosum were examined by using a microplate assay. The spore suspension was adjusted to 1.0 × 105 ml−1. Aliquots of 50 ml were centrifuged at 3000 g for 10 min and the supernatant was discarded. The conidia pellets were resuspended in 5 ml Sabouraud dextrose broth (SDB), and then 100ul of conidia solution were adding to the wells of a sterile 96-well microplate (Sarstedt AG and Co.). 100 µl of cfsP dilutions were adding to the wells, and the final concentrations of cfsP were 0, 0.075, 0.15, 0.3, 0.6, 1.25, 2.5, 5.0, 10 or 20.0(%). The microplate was sealed with optically clear seal for QPCR (Thermo Scientific). The microplates were incubated for 120 h at 30°C inside a Multiskan FC microplate-reader (Thermo Scientific). The optical density at 620 nm (OD620) was automatically recorded for each well every 3 h. The changes in OD620 over time were used to generate dermatophytes’ growth curves at each cfsP concentration. The experiment was performed in duplicate.
Acknowledgments
This work was supported by grants CRS/07/CR03, 08RDCIT600 and 08RDC607.
Glossary
Abbreviations:
- WHO
World Health Organization
- LAB
lactic acid bacteria
- LC-FTMS
liquid chromatography fourier transform mass Spectrometry
- PDA
potato dextrose agar
- SDA
sabouraud dextrose agar
- SNB
synthetic-nutrient-poor bouillon
- mMRS
modified MRS agar
- cfs
freeze-dried cell free supernatant
- cfsP
antifungal strain Lb. reuteri ee1p freeze-dried cell-free supernatant
- cfsN
negative control strain Lb. reuteri M13 freeze-dried cell-free supernatant
- cfsC
freeze-dried mMRS broth
- SDB
Sabouraud dextrose broth
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/19624
References
- 1.Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–52. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 2.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 3.Me´ndez-Tovar LJ, Manzano-Gayosso P, Velásquez-Hern´ndez V, Millan-Chiu B, Hern´ndez-Hern´ndez F, Mondrago´n-González R, et al. [Resistance to azolic compounds in clinical Trichophyton spp. strains] Rev Iberoam Micol. 2007;24:320–2. doi: 10.1016/s1130-1406(07)70065-7. [DOI] [PubMed] [Google Scholar]
- 4.Manzano-Gayosso P, Me´ndez-Tovar LJ, Hern´ndez-Hern´ndez F, Lo´pez-Marti´nez R. [Antifungal resistance: an emerging problem in Mexico] Gac Med Mex. 2008;144:23–6. [PubMed] [Google Scholar]
- 5.Coelho LM, Aquino-Ferreira R, Maffei CM, Martinez-Rossi NM. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J Antimicrob Chemother. 2008;62:758–61. doi: 10.1093/jac/dkn245. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Rossi NM, Peres NT, Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia. 2008;166:369–83. doi: 10.1007/s11046-008-9110-7. [DOI] [PubMed] [Google Scholar]
- 7.Peres NT, Maranhão FC, Rossi A, Martinez-Rossi NM. Dermatophytes: host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–67. doi: 10.1590/S0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Song X, Yang P, Wang J. Appearance of anaphylactic shock after long-term intravenous itraconazole treatment. Ann Pharmacother. 2009;43:537–41. doi: 10.1345/aph.1L343. [DOI] [PubMed] [Google Scholar]
- 9.Tapaninen T, Backman JT, Kurkinen KJ, Neuvonen PJ, Niemi M. Itraconazole, a P-glycoprotein and CYP3A4 inhibitor, markedly raises the plasma concentrations and enhances the renin-inhibiting effect of aliskiren. J Clin Pharmacol. 2011;51:359–67. doi: 10.1177/0091270010365885. [DOI] [PubMed] [Google Scholar]
- 10.Back DJ, Tjia JF. Comparative effects of the antimycotic drugs ketoconazole, fluconazole, itraconazole and terbinafine on the metabolism of cyclosporin by human liver microsomes. Br J Clin Pharmacol. 1991;32:624–6. doi: 10.1111/j.1365-2125.1991.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–80. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ohno T, Nakayama K, Nakade S, Kitagawa J, Ueda S, Miyabe H, et al. Effect of itraconazole on the pharmacokinetics of imidafenacin in healthy subjects. J Clin Pharmacol. 2008;48:330–4. doi: 10.1177/0091270007310386. [DOI] [PubMed] [Google Scholar]
- 13.Klepser M. The value of amphotericin B in the treatment of invasive fungal infections. J Crit Care. 2011;26:225–, e1-10. doi: 10.1016/j.jcrc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Laniado-Labori´n R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26:223–7. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Baginski M, Czub J. Amphotericin B and its new derivatives—mode of action. Curr Drug Metab. 2009;10:459–69. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 16.Flach A, Gregel B, Simionatto E, da Silva UF, Zanatta N, Morel AF, et al. Chemical analysis and antifungal activity of the essential oil of Calea clematidea. Planta Med. 2002;68:836–8. doi: 10.1055/s-2002-34414. [DOI] [PubMed] [Google Scholar]
- 17.Mimica-Dukicá N, Bozin B, Sokovicá M, Mihajlovicá B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003;69:413–9. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 18.Rapp RP. Changing strategies for the management of invasive fungal infections. Pharmacotherapy. 2004;24:4S–28S, quiz 29S-32S. doi: 10.1592/phco.24.3.4S.33151. [DOI] [PubMed] [Google Scholar]
- 19.Bajpai VK, Yoon JI, Kang SC. Antifungal potential of essential oil and various organic extracts of Nandina domestica Thunb. against skin infectious fungal pathogens. Appl Microbiol Biotechnol. 2009;83:1127–33. doi: 10.1007/s00253-009-2017-5. [DOI] [PubMed] [Google Scholar]
- 20.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–88. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 21.De Vuyst L, Leroy F. Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol. 2007;13:194–9. doi: 10.1159/000104752. [DOI] [PubMed] [Google Scholar]
- 22.Sit CS, Vederas JC. Approaches to the discovery of new antibacterial agents based on bacteriocins. Biochem Cell Biol. 2008;86:116–23. doi: 10.1139/O07-153. [DOI] [PubMed] [Google Scholar]
- 23.Piper C, Cotter PD, Ross RP, Hill C. Discovery of medically significant lantibiotics. Curr Drug Discov Technol. 2009;6:1–18. doi: 10.2174/157016309787581075. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett. 2003;219:129–35. doi: 10.1016/S0378-1097(02)01207-7. [DOI] [PubMed] [Google Scholar]
- 25.Ström K, Schnürer J, Melin P. Co-cultivation of antifungal Lactobacillus plantarum MiLAB 393 and Aspergillus nidulans, evaluation of effects on fungal growth and protein expression. FEMS Microbiol Lett. 2005;246:119–24. doi: 10.1016/j.femsle.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Hassan YI, Bullerman LB. Antifungal activity of Lactobacillus paracasei ssp. tolerans isolated from a sourdough bread culture. Int J Food Microbiol. 2008;121:112–5. doi: 10.1016/j.ijfoodmicro.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Mauch A, Dal Bello F, Coffey A, Arendt EK. The use of Lactobacillus brevis PS1 to in vitro inhibit the outgrowth of Fusarium culmorum and other common Fusarium species found on barley. Int J Food Microbiol. 2010;141:116–21. doi: 10.1016/j.ijfoodmicro.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Lavermicocca P, Valerio F, Visconti A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl Environ Microbiol. 2003;69:634–40. doi: 10.1128/AEM.69.1.634-640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnürer J, Magnusson J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol. 2005;16:70–8. doi: 10.1016/j.tifs.2004.02.014. [DOI] [Google Scholar]
- 30.Dal Bello F, Clarke CI, Ryan LAM, Ulmer H, Schober TJ, Strom K, et al. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J Cereal Sci. 2007;45:309–18. doi: 10.1016/j.jcs.2006.09.004. [DOI] [Google Scholar]
- 31.Lind H, Sjögren J, Gohil S, Kenne L, Schnürer J, Broberg A. Antifungal compounds from cultures of dairy propionibacteria type strains. FEMS Microbiol Lett. 2007;271:310–5. doi: 10.1111/j.1574-6968.2007.00730.x. [DOI] [PubMed] [Google Scholar]
- 32.Ryan LAM, Dal Bello F, Arendt EK. The use of sourdough fermented by antifungal LAB to reduce the amount of calcium propionate in bread. Int J Food Microbiol. 2008;125:274–8. doi: 10.1016/j.ijfoodmicro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Nirenberg H. Untersuchungen über die morphologische Differenzierung in der Fusarium- SektionLiseola. Mitt. Biol Bundesanst. Land–Forstwirtsch. 1976;169:1–117. [Google Scholar]
- 34.Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166:353–67. doi: 10.1007/s11046-008-9109-0. [DOI] [PubMed] [Google Scholar]
- 36.Kuwaki S, Ohhira I, Takahata M, Murata Y, Tada M. Antifungal activity of the fermentation product of herbs by lactic acid bacteria against tinea. J Biosci Bioeng. 2002;94:401–5. [PubMed] [Google Scholar]
- 37.Martirosyan AO, Mndzhoyan SL, Charyan LM, Akopyan LG, Nikishchenko MN. Antimicrobial activity of lactic acid bacteria from sour milk products Narine, Karine, and Matsun. Appl Biochem Microbiol. 2004;40:178–80. doi: 10.1023/B:ABIM.0000018922.03283.b9. [DOI] [PubMed] [Google Scholar]
- 38.Talarico TL, Casas IA, Chung TC, Dobrogosz WJ. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother. 1988;32:1854–8. doi: 10.1128/aac.32.12.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cadieux P, Wind A, Sommer P, Schaefer L, Crowley K, Britton RA, et al. Evaluation of reuterin production in urogenital probiotic Lactobacillus reuteri RC-14. Appl Environ Microbiol. 2008;74:4645–9. doi: 10.1128/AEM.00139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouse S, Harnett D, Vaughan A, van Sinderen D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J Appl Microbiol. 2008;104:915–23. doi: 10.1111/j.1365-2672.2007.03619.x. [DOI] [PubMed] [Google Scholar]
- 41.Sjögren J, Magnusson J, Broberg A, Schnürer J, Kenne L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol. 2003;69:7554–7. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal V, Sen SK, Mandal NC. Detection, isolation and partial characterization of antifungal compound(s) produced by Pediococcus acidilactici LAB 5. Nat Prod Commun. 2007;2:671–4. [Google Scholar]
- 43.Broberg A, Jacobsson K, Ström K, Schnürer J. Metabolite profiles of lactic acid bacteria in grass silage. Appl Environ Microbiol. 2007;73:5547–52. doi: 10.1128/AEM.02939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan LA, Zannini E, Dal Bello F, Pawlowska A, Koehler P, Arendt EK. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int J Food Microbiol. 2011;146:276–83. doi: 10.1016/j.ijfoodmicro.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Sjögren J, Magnusson J, Broberg A, Schnürer J, Kenne L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol. 2003;69:7554–7. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilar-Guadarrama B, Navarro V, Leo´n-Rivera I, Rios MY. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat Prod Res. 2009;23:1559–65. doi: 10.1080/14786410902843301. [DOI] [PubMed] [Google Scholar]
- 47.Park MJ, Gwak KS, Yang I, Choi WS, Jo HJ, Chang JW, et al. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J Microbiol. 2007;45:460–5. [PubMed] [Google Scholar]
- 48.Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2009;58:1454–62. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Mauch A, Galle S, Murphy P, Arendt EK, Coffey A. Inhibition of growth of Trichophyton tonsurans by Lactobacillus reuteri. J Appl Microbiol. 2011;111:474–83. doi: 10.1111/j.1365-2672.2011.05032.x. [DOI] [PubMed] [Google Scholar]
- 50.Meroth CB, Walter J, Hertel C, Brandt MJ, Hammes WP. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2003;69:475–82. doi: 10.1128/AEM.69.1.475-482.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen RI, Altshuller AP. A new spectrophotometric method for the determination of acrolein in combustion gases and in the atmosphere. Anal Chem. 1961;33:726–33. doi: 10.1021/ac60174a020. [DOI] [Google Scholar]
- 52.Bauer R, du Toit M, Kossmann J. Influence of environmental parameters on production of the acrolein precursor 3-hydroxypropionaldehyde by Lactobacillus reuteri DSMZ 20016 and its accumulation by wine lactobacilli. Int J Food Microbiol. 2010;137:28–31. doi: 10.1016/j.ijfoodmicro.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Brosnan B, Coffey A, Arendt E, Furey A. Rapid Identification of Anti-Fungal Compounds Produced by Lactic Acid Bacteria using the LTQ—Orbitrap XL Hybrid Mass Spectrometer. Anal Bioanal Chem. 2011 doi: 10.1007/s00216-012-5955-1. In press. [DOI] [PubMed] [Google Scholar]
- 54.Ström K, Sjögren J, Broberg A, Schnürer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol. 2002;68:4322–7. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]