Abstract
Communication between organisms is crucial for their survival, especially for sessile organisms such as plants that depend upon interactions with mutualistic organisms to maximize their nutrient acquisition. This communication can take the form of the exchange of volatile compounds, metabolites or effectors—small protein signals secreted from the colonizing cell that change the biology of the host cell. We recently characterized the first mutualistic effector protein from an ectomycorrhizal fungus, a small secreted protein named MiSSP7 encoded by Laccaria bicolor. Ectomycorrhizal fungi are soil-borne mutualistic organisms whose hyphae wrap around host roots and grow into the root apoplastic space where the fungus exchanges nutrients such as nitrogen and phosphorus in return for plant derived sugars. The MiSSP7 protein is induced by root exudates and is highly expressed throughout the root colonization process. Its presence was responsible for alterations to the plant transcriptomic profile, a mechanism by which MiSSP7 may aid in the formation of the symbiotic interface. Here we discuss the implications of these findings and, further, we demonstrate that the production of MiSSP7 is induced by two flavonoids, rutin and quercitin, a class of compounds normally found within the exudates of plant roots. We also consider the interesting similarities between the mechanisms of effector induction and action between pathogenic and mutualistic fungi.
Keywords: ectomycorrhizal fungi, effector, Flavonoid, MiSSP, RXLR motif
The large majority of terrestrial organisms, whether directly or indirectly, depend upon the carbon source found within plant tissues. Arguably, the process of carbon extraction is most delicate for organisms dependent on living plant tissues. Pathogenic organisms are well known for their use of small secreted proteins, called effectors, that act to control plant function to the benefit of the colonizing organism.1-4 How mutualistic organisms, and especially mutualistic fungi, are able to live in harmony with their plant hosts has been less clear. Kosuta and colleagues5 found that a diffusible element from arbuscular fungi, later identified as a lipochitooligosaccharide,6 was able to control the expression of plant genes during the colonization process. More recently, for the mutualistic ectomycorrhizal fungus Laccaria bicolor, we were able to characterize a small secreted protein, MiSSP7, and its key role during the colonization process of poplar root tissues.7 Like the lipochitooligosaccharides discovered by Kosuta and colleagues, and like pathogenic effector proteins, MiSSP7 was able to alter the transcriptome of the plant cell which may contribute to its role during the colonization of plant tissues. We hypothesized that the transcriptional regulation was responsible, in part, for the restructuring of plant cell walls to allow entry of fungal hyphae into the root apoplastic space (Fig. 1). MiSSP7 was able to gain access into the plant cell actively through the binding of an RXLR-like motif to membrane phospholipids, an entry mechanism previously only found for pathogenic effectors.8,9 The exact role of RXLR and RXLR-like motifs in mediating cell entry has garnered much debate. Recently, Yaeno and colleagues10 published work demonstrating that it was not these regions that mediated cell entry directly, but rather that mutations to these regions changed the protein conformation such that key charged residues needed for lipid binding were not exposed at the surface of the protein. As it is possible that a similar event is occurring in MiSSP7, we are currently attempting to determine the three-dimensional structure of MiSSP7. Concurrently with our study, Kloppholz and colleagues11 also published work characterizing SP7, a secreted protein from the arbuscular mycorrhizal protein Glomus intraradices, which has similar characteristics to MiSSP7 in its ability to enter host cells and to affect the host transcriptome. These results suggest convergent evolution in the development of colonization strategies between pathogenic and mutualistic organisms.
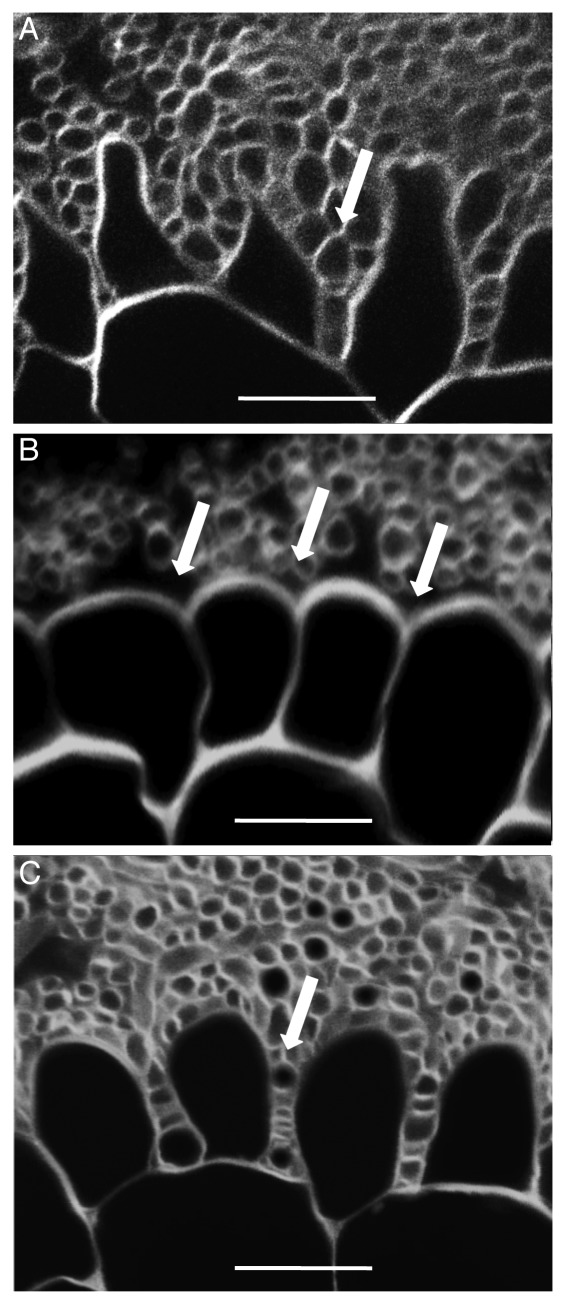
Figure 1. MiSSP7 is required for root cell remodeling and hyphal penetration into the root apoplastic space. In the interaction between the ectomycorrhizal fungus Laccaria bicolor and poplar roots, the fungus surrounds the root and induces morphological changes in the epidermal cells and loosens the connections between the root cells such that fungal hyphae are able to penetrate in between the cells as seen in (A; arrow). When the production of the small secreted effector protein MiSSP7 of L. bicolor is reduced the same alterations to root epidermal cells are not observed nor are the connections between root cells loosened such that the fungal hyphae are not able to grow into the apoplastic space (B; arrows). Alterations to plant cell morphology and hyphal ingrowth are restored when MiSSP7 is expressed heterologously in poplar roots (C; arrow). All experiments were performed in triplicate to ensure reproducibility. Scale bar = 20 µm.
MiSSP7 and a group of other similarly sized secreted proteins from L. bicolor were first annotated as putative signaling agents during the colonization of plant tissues based on their transcriptional regulation in mycorrhizal root tissues.12 We were therefore surprised in our early work characterizing the MiSSP7 protein to find that it was expressed very early in the interaction with plant roots, potentially even before root contact as root exudates were able to induce its expression.7 In retrospect, based on the proposed action of MiSSP7 in preparing the plant tissues to accept colonization by the fungus, this early expression is very important for the proper functioning of MiSSP7. This leads to questions concerning the specific mechanisms and signals in place that are responsible for initiating the expression of MiSSP7—an area that we have begun investigating. We were able to show in our initial characterization that MiSSP7 was induced by root excretions from both host (poplar) and non-host (Arabidopsis thaliana) roots, suggesting that there are both specific and non-specific signals within the rhizosphere that are responsible for the induction of MiSSP7.7 Although there are thousands of proteins and metabolites produced by plant roots, a number of signals are commonly associated with signaling within the rhizosphere.13,14 Of these, we have initially considered the role of nutrients and flavonoids in their ability to induce the expression of MiSSP7. We found that the flavonoids rutin and quercetin were able to induce the expression of MiSSP7 in the absence of a tree host (Fig. 2). Similarly, it has been found that the flavonoid luteolin was able to induce the expression of Nod factors in mutualistic bacteria Rhizobia spp.15 and the flavonoid pisatin was able to induce expression of PDA1 in the soil pathogen Nectria hematococca MPVI.16 Further, rutin alters the growth characteristics of many fungi, inducing hyphal growth in different strains of Pisolithus, another ectomycorrhizal fungus,17 as well as in the phytopathogenic fungi Alternaria alternata, Botrytis cinerea and Fusarium solani.18 Given the wide range of organisms in which flavonoids are able to induce transcription, it is likely that flavonoids act as non-specific signals within the rhizosphere, and here during the induction of MiSSP7. Based on the demonstration that several root exudates have inductive potential for any one mutualistic or pathogenic effector during inter-organism interactions,19,20 these studies would suggest that multiple compounds within the rhizosphere, both specific and non-specific, are responsible for the induction of MiSSPs, and MiSSP7 in particular, in a natural ecosystem. Therefore further work must be done to identify and differentiate host specific and non-specific signals that induce the expression of MiSSP7 and the other MiSSP proteins.
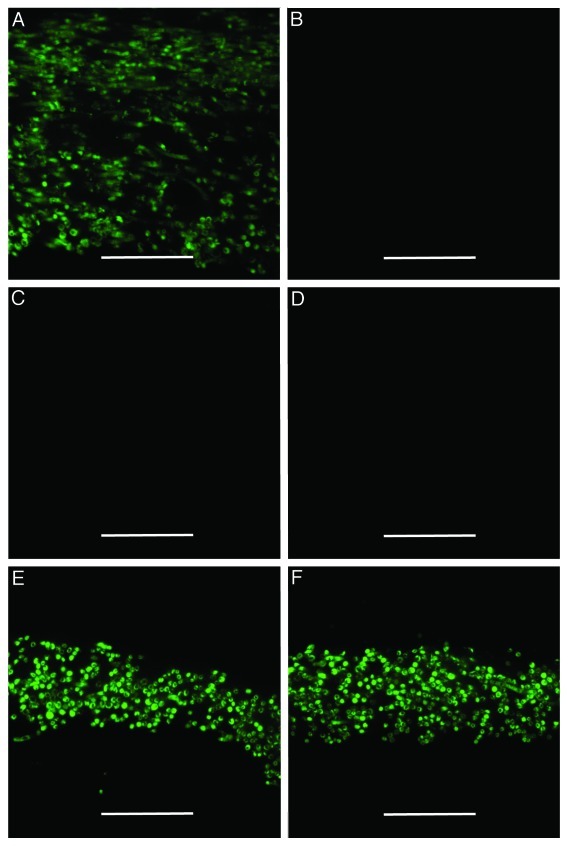
Figure 2. Root flavonoids act as a signal to induce the production of MiSSP7. Indirect immunolocalizations of MiSSP7 performed as described by Plett and colleagues (2011) on L. bicolor grown on cellophane in indirect contact with poplar roots (A) or dosed with water (B; control treatment) or with 100 µM of glucose (C), rhamnose (D), rutin (E) or quercitin (F) every 2 d for 1 week. MiSSP7 is only produced when in indirect contact with root tissue or when treated with the flavonoids rutin and quercitin. All experiments were performed in triplicate to ensure reproducibility. Scale bar = 70 µm.
One final thought that we brought out in our discussion of the results characterizing MiSSP7,7 and that is further reinforced here, is the intriguing similarity between the mechanisms of colonization used by mutualistic and pathogenic organisms. In our first paper on MiSSP7 we demonstrated that this protein gained access to the plant cell via recognition of an entry motif normally found in pathogenic effectors, and that MiSSP7 was able to alter the plant transcriptome as do pathogenic effector proteins. Here we demonstrated that flavonoids are a root-derived signal that induce the production of MiSSP7, a group of compounds also known for their role in the induction of other mutualistic and pathogenic colonization factors. Together these results continue to highlight the fact that the boundary that separates pathogens and mutualists is a gray area. It will be interesting in the coming years to try and dissect the checks and balances that make one interaction mutually beneficial for both partners while in other relationships one partner is able to exploit the other.
Acknowledgments
We would like to thank the European Commission within the Project Energypoplar (FP7-211917), the Network of Excellence EVOLTREE (FP6-016322), the ANR project FungEffector as well as the Genomic Science Program, US Department of Energy Office of Science, Biological and Environmental Research (contract DE-AC05-00OR22725) for the funding that made this work possible.
Glossary
Abbreviation:
- MiSSP
MYCORRHIZA iNDUCED SMALL SECRETED PROTEIN
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18357
References
- 1.Torto TA, Li S, Styer A, Huitema E, Testa A, Gow NA, et al. EST mining and functional expression assays identify extracellular effector proteins from Phytophthora. Genome Res. 2003;13:1675–85. doi: 10.1101/gr.910003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan W, Cao M, Leung D, Tyler BM. The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol Plant Microbe Interact. 2004;17:394–403. doi: 10.1094/MPMI.2004.17.4.394. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong MR, Whisson SC, Pritchard L, Bos JI, Venter E, et al. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc Natl Acad Sci USA. 2005;102:7766–71. doi: 10.1073/pnas.0500113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehmany AP, Gordon A, Rose LE, Allen RL, Armstrong MR, et al. Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell. 2005;17:1839–50. doi: 10.1105/tpc.105.031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosuta S, Chabaud M, Lougnon G, Gough C, De´narie´ J, Barker DG, et al. A Diffusible Factor from Arbuscular Mycorrhizal Fungi Induces Symbiosis-Specific MtENOD11 Expression in Roots of Medicago truncatula. Plant Physiol. 2003;131:952–62. doi: 10.1104/pp.011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maillet F, Poinsot V, Andre´ O, Puech-Pages V, Haouy A, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63. doi: 10.1038/nature09622. [DOI] [PubMed] [Google Scholar]
- 7.Plett JM, Kemppainen M, Kale SD, Kohler A, Legue´ V, Brun A, et al. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr Biol. 2011;21:1197–203. doi: 10.1016/j.cub.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Dou D, Kale SD, Wang X, Jiang RHY, Bruce NA, Arredondo PD, et al. RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell. 2008;20:1930–47. doi: 10.1105/tpc.107.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kale SD, Gu B, Capelluto DGS, Dou D, Feldman E, Rumore A, et al. External phosphatidylinositol-3-phosphate mediates host cell entry by eukaryotic pathogen effectors. Cell. 2010;142:284–95. doi: 10.1016/j.cell.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Yaenoa T, Lib H, Chaparro-Garciac A, Schornackc S, Koshibab S, Watanabeb S, et al. Phosphatidylinositol monophosphate-binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. Proc Nat Acad Sci USA. 2011;108:14682–7. doi: 10.1073/pnas.1106002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloppholz S, Kuhn H, Requena N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol. 2011;21:1204–9. doi: 10.1016/j.cub.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Martin F, Aerts A, Ahrn D, Brun A, Danchin EGJ, Duchaussoy F, et al. The genome sequence of the basidiomycete fungus Laccaria bicolor provides insights into the mycorrhizal symbiosis. Nature. 2008;452:88–92. doi: 10.1038/nature06556. [DOI] [PubMed] [Google Scholar]
- 13.Walker TS, Bais HP, Grotewold E, Vivanco JM. Root exudation and rhizosphere biology. Plant Physiol. 2003;132:44–51. doi: 10.1104/pp.102.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–66. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 15.Peters NK, Frost JW, Long SR. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986;233:977–80. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- 16.Khan R, Straney DC. Regulatory signals influencing expression of the PDA1 gene of Nectria haematococca MPVI in culture and during pathogenesis of pea. Mol Plant Microbe Interact. 1999;12:733–42. doi: 10.1094/MPMI.1999.12.8.733. [DOI] [Google Scholar]
- 17.Lagrange H, Jay-Allgmand C, Lapeyrie F. Rutin, the phenolglycoside from eucalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations. New Phytol. 2001;149:349–55. doi: 10.1046/j.1469-8137.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalinova J, Radova S. Effect of rutin on the growth of Botrytis cinerea Alternaria alternata and Fusarium solani. Acta Phytopathol Entomol Hung. 2009;44:39–47. doi: 10.1556/APhyt.44.2009.1.5. [DOI] [Google Scholar]
- 19.Phillips DA, Joseph CM, Maxwell CA. Trigonelline and stachydrine released from alfalfa seeds activate NodD2 protein in Rhizobium meliloti. Plant Physiol. 1992;99:1526–31. doi: 10.1104/pp.99.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winans SC. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev. 1992;56:12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]