Abstract
Somatic embryogenesis (SE) in Cichorium involves dedifferentiation and redifferentiation of single cells and can be induced by specific in vitro culture conditions. We have tested the effect of various treatments on the incidence of SE (ISE) of an interspecific embryogenic hybrid (C. endivia x C. intybus) and of different commercial chicories (C. endivia and C. intybus) that are typically recalcitrant to SE in standard culture conditions. We found that the ISE of the hybrid is significantly increased by pretreatment of tissues by submersion in solutions of glycerol, abscisic acid, spermine, putrescine or of combinations of these compounds. Interestingly, the most efficient of these pretreatments also had an unexpectedly high effect on the ISE of the C. intybus cultivars. The ISE of the hybrid and of the commercial chicories were increased when explants were co-cultured with highly embryogenic chicory explants or when they were cultured in conditioned medium. These observations established that unidentified SE-promoting factors are released in the culture medium. HPLC analyses of secreted Arabino-Galactan Proteins (AGPs), which are known to stimulate SE, did not allow identifying a fraction containing differentially abundant AGP candidates. However, pointing to their role in promoting SE, we found that the hybrid had a drastically higher ISE when amino sugars and L-Proline, the putative precursors of secreted AGPs, were both added to the medium.
Keywords: ABA, AGP, co-culture, polyamine, totipotency
Introduction
Besides sexual reproduction, plants can propagate clonally owing to the unique ability of their cells to dedifferentiate and re-differentiate into different cell types. Among the different morphogenetic pathways, the totipotent cells related to stem cell-like cells in plants, can generate fully functional bipolar embryos following a complex process referred to as somatic embryogenesis (SE).1 In vitro cultures were also convenient to study the acquisition of pluripotency that could occur in a few differentiated-somatic cells; pluripotent cells belong to another class of stem cell-like cells only able to give rise to organ or callus (organogenesis or callogenesis patterns).1 So far, the mechanisms and the regulations underlying the acquisition of totipotency and the subsequent differentiation into somatic embryos are largely unknown. SE can be triggered by axenic in vitro culture of plant tissues using appropriate conditions gathering both culture medium and abiotic stresses.1 Indeed, the incidence of SE (ISE) varies depending on the conditions, like (1) the pre-treatment of the donor pants, (2) the exogenous addition of promoting factors or (3) the use of conditioned medium.
Unlike in many other plant species, SE in Cichorium is direct and arises from single cells.2 Previous experiments using the embryogenic interspecific hybrid (Cichorium endivia x C. intybus) designed as ‘hybrid 474’ showed that the content of endogenous polyamines (PAs), which are involved in development, senescence and stress responses was increased during the development of somatic embryos.3-6 Also, the exogenous addition of spermine (spm) and putrescine (put) had a positive effect on improving the yield of somatic embryos in chicory but also in other SE models.5-7 In various models of SE, the exogenous supply of abscisic acid (ABA), a well-known stress hormone of plants, also led to increase the ISE.8-12 Also, the report of an extracellular matrix connecting cell wall-plasmalemma-cytoskeleton led to the hypothesis that the cell wall could be sensitive to osmotic stresses.13-16 Supporting this possibility, several groups reported that treatments with plasmolysing agents led to increase the ISE of gymnosperms and Eleutherococcus.17,18
Numerous studies have reported that soluble molecules are released in the medium during the acquisition of totipotency. These enriched medium, called conditioned medium (CM), contain factors able to promote the SE of primary explants.19-23
In SE models involving cell suspensions, the co-culture of embryogenic and non-embryogenic lines led to increase SE competencies.24,25 However, the effect of co-culturing tissue explants with different ISE has not yet been investigated.
In several SE models, the soluble ArabinoGalactan Proteins (AGPs) were proposed to carry the SE-promoting activity.26,28 However, the exact nature of the AGPs responsible for promoting SE has not yet been determined.
We report here the effect (1) of pretreatment of plantlets by immersion before induction of SE, (2) of addition of compounds to the culture media, (3) of conditioned medium and (4) of co-culture of different cultivars on the incidence of SE of the embryogenic chicory hybrid ‘474’ and of commercial chicories which are typically recalcitrant to standard SE conditions. The signaling roles of soluble molecules, including AGPs secreted/released in the culture media is assayed and discussed.
Results
Effect of pretreatments on the incidence of SE
Pretreatment of the embryogenic hybrid ‘474’
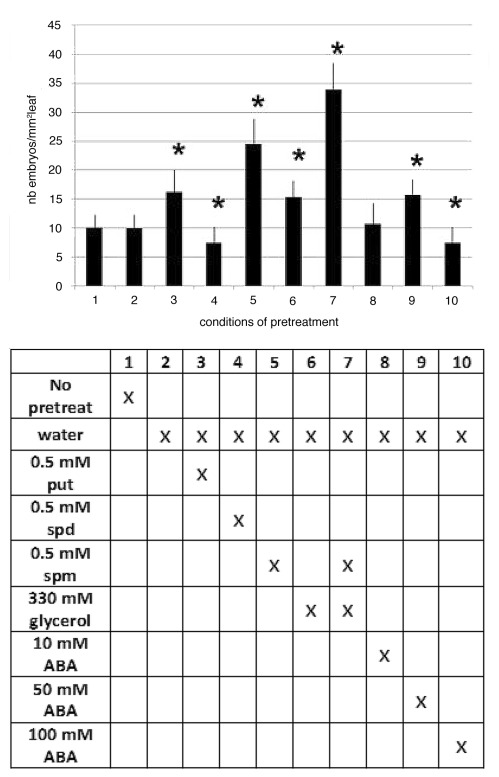
Unlike pretreatment with water which had no effect on the ISE of the ‘474’ hybrid, pretreatments with 330 mM glycerol, which serves as osmoticum and carbon source, with 50 μM ABA, or with the polyamines putrescine (put) or spermine (spm) significantly increased the number of somatic embryos (Fig. 1). Pretreatment with spm, which was the most efficient of the individual pretreatments tested, had a synergistic effect, leading to differentiation of three times more SE, when used in combination with water and 330 mM glycerol. In contrast, pretreatments with 100 mM ABA or 0.5 mM spermidine (spd) had a slightly negative effect on the ISE. These experiments show that the ISE of the embryogenic hybrid ‘474’ can be potentiated by pretreatments of leaves before SE induction.
Figure 1.
Effects of pretreatment of leaves of the embryogenic hybrid of Cichorium, with 0.5 mM polyamines (putrescine, spermidine, or spermine), of 330 mM glycerol,or of several concentrations of (± ) cis, trans abscisic acid (ABA), on the number of somatic embryos (mean ± SEM for n = 9 independent experiments). Conditions in abscissa (1 to 10) correspond to the pretreatment described just below in the column from the associated table. * indicates the values that are significantly different from that obtained without pretreatment (p < 0.05; Student-Fischer law).
Pretreatment of commercial cultivars
The results we obtained with the embryogenic hybrid ‘474’ prompted us to assay the effect of these combined pretreatments on 15 commercial chicories considered “low embryogenic” or “recalcitrant to SE” in our standard conditions. Whatever the pretreatments used, C. endivia var crispa and var latifolia were as recalcitrant to SE as without pretreatment (Table 1). In contrast, C. intybus cultivars and wild chicories exhibited a significant increase of their ISE when the different combined pretreatments were used. As in the case of leaves of the embryogenic hybrid, pretreatment with 330 mM glycerol, 0.5 mM spm and 50 μM ABA had a synergistic effect on the ISE of roots but not on that of leaves. These experiments showed that combined pretreatments with different molecules that mimic different abiotic stresses lead to promote the ISE of certain but not all Cichorium species in a tissue specific manner.
Table 1. Effect of different pretreatements on the incidence of SE of 15 commercial chicories.
|
Cichorium genotypes |
Names |
Commercial origin (France) |
Pretreated organs |
Pretreatments |
|||
|---|---|---|---|---|---|---|---|
| a | a+b | a+b+c | a+b+c+d | ||||
|
1. C. intybus var sativum cv Magdebourg = roasted chicory |
Cassel Orchies Pe´vèle |
Desprez Desprez Desprez |
mm−1 root mm−2 leaf mm−1 root mm−2 leaf mm−1 root mm−2 leaf |
0.00 (0) 0.03 (10) 0.04 (15) 0.00 (0) 0.02 (10) 0.00 (0) |
0.14 (40) 0.01 (2) 0.10 (10) 0.00 (0) 0.04 (15) 0.00 (0) |
0.60 (80) 0.12 (35) 0.06 (20) 0.00 (0) 0.08 (40) 0.04 (8) |
0.46 (100) 0.08 (10) 0.22 (75) 0.02 (5) 0.19 (65) 0.01 (2) |
|
C. endivia var crispa |
Elodie |
Caillard |
mm−1 root |
0.00 (0) |
0.00 (0) |
0.00 (0) |
0.00 (0) |
|
C. endivia var latifolia = Scarole salad |
Ge´ante maraîchère Grosse boucle´e 2 Margot Samoa Sole´ra Traviata |
Caillard Vilmorin Brumaux Caillard Caillard Vilmorin |
mm−2 leaf |
0.00 (0) |
0.00 (0) |
0.00 (0) |
0.00 (0) |
| |
Sauvage ame´liore´e |
Caillard |
mm−1 root mm−2 leaf |
0.00 (0) 0.00 (0) |
0.00 (0) 0.00 (0) |
0.10 (40) 0.00 (0) |
0.10 (40) 0.00 (0) |
| Wild Chicories | Cornet d’Anjou Cre´sola rouge Pain de sucre Rouge de Ve´rone |
Caillard Caillard Caillard Abondance |
mm−1 root mm−2 leaf mm−1 root mm−2 leaf mm−1 root mm−2 leaf mm−1 root mm−2 leaf |
0.00 (0) 0.00 (0) 0.08 (30) 0.00 (0) 0.10 (35) 0.10 (2) 0.02 (10) 0.00 (0) |
- 0.10 (35) 0.00 (0) 0.30 (65) 0.10 (9) 0.56 (80) 0.00 (0) |
0.20 (65) 0.00 (0) 0.40 (60) 0.10 (10) 0.15 (50) 0.02 (4) |
- 0.59 (95) 0.00 (0) 0.30 (75) 0.01 (3) 0.39 (70) 0.10 (20) |
The number of embryos was scored per mm of root and per mm2 of leaf. Plantlets grown in vitro were submerged in the indicated solutions for 4 d before transfer to the culture medium. Water (a), 330 mM glycerol (b), 0.5 mM spermine (c) and 50 µM ABA (d) were used as indicated. The results obtained with leaves and roots are from independent experiments (n = 10 for leaves and n = 5 for roots). The percentage of explants exhibiting somatic embryos after 12 d (roots) or 20 d (leaf) of culture is indicated in brackets.
Effect of co-culturing the embryogenic hybrid and a recalcitrant chicory
Co-culture of different organs of the embryogenic hybrid
One possible explanation for the naturally high ISE of the embryogenic hybrid is that it releases SE-promoting molecules in the culture medium. We tested this hypothesis by using co-culture experiments and we anticipated that the embryogenic hybrid ‘474’ would promote the ISE of the commercial C. intybus cv Pe´vèle. These co-culture experiments were done in Magenta boxes in which each of the two compartments is used for culture of one genotype and is physically separated from the other compartment by a 25 µm polypropylene membrane. Different conditions were tested using either leaf or root explants of the embryogenic hybrid and of the recalcitrant cv Pe´vèle alone (Fig. 2). Whatever the compartment considered, no significant difference in the ISE of the hybrid was recorded when the same organ was used in both compartments of the box (Fig. 2, conditions A, B). Interestingly, the ISE of leaf explants was slightly enhanced when co-cultured with root explants (Fig. 2, condition C).
Figure 2. Effects of co-culturing different explants from different chicories on the incidence of SE. Co-cultures involving only the embryonic hybrid (A–C), only the recalcitrant C. intybus cv Pe´vèle (H), or both plants (D–G). Root explants (shown in black for the embryogenic hybrid and in white for cv Pe´vèle) and/or leaves (shown with black hatchings for embryogenic hybrid and with small dots for cv Pe´vèle) were cultured for 12 d in Magenta boxes where the upper compartment (I) and the lower compartment (II) are physically isolated by a polypropylene membrane. A khi2 test was applied with Yates correction (α = 0.05). Co-culture of cv Pe´vèle leaves and hybrid roots condition (D) show significative differences and is indicated by *. Mean number of somatic embryo ± standard error of the mean is given for mm2 leaf or for mm linear root.
Co-culture of embryogenic and commercial cultivar explants
Co-cultures of root or leaf explants of C. intybus cv Pe´vèle did not alter the ISE of the hybrid ‘474’ (Fig. 2, conditions D, E, F). However conversely, the ISE of the recalcitrant cv Pe´vèle was strongly enhanced by co-culture with explants of the embryogenic hybrid, especially when leaf explants of both chicories were co-cultured (Fig. 2, condition D). The ISE of cv Pe´vèle root explants was also stimulated by co-culture with embryogenic hybrid root explants (Fig. 2, condition G). The co-culture of leaf and root explants of the cv Pe´vèle did not increase the ISE of leaf explants, but few SE developed on 18% of the root explants (Fig. 2, condition H). Thus, these experiments show that certain SE-promoting molecules are released in the culture medium and have stronger effects on root than on leaf explants. Also, our data suggest that the SE-promoting molecules act in an organ-specific manner.
Effect of conditioned medium on the incidence of SE
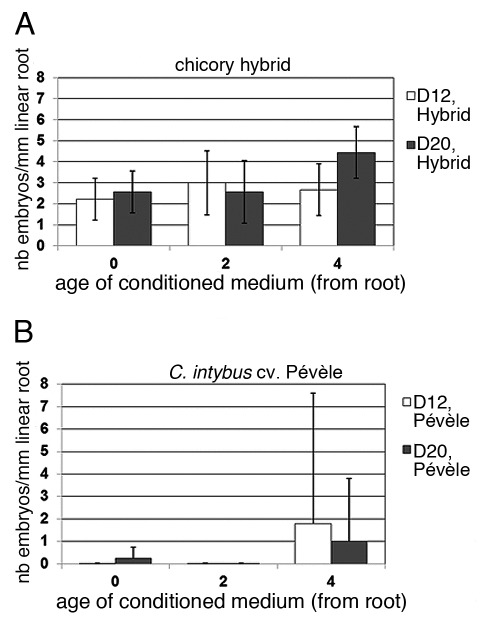
To further strengthen our conclusions that SE-promoting molecules are released in the culture media, we used conditioned medium (CM) obtained after 0, 2, or 4 d of culture of root explants of the embryogenic hybrid. Naïve root explants of the embryogenic hybrid (Fig. 3A) or of the cv Pe´vèle (Fig. 3B) were then cultured in these CM for 12 or 20 d.
Figure 3. Effects of conditioned medium on the incidence of SE. The number of somatic embryos per mm of root (mean ± SEM) was scored after lugol staining for the embryogenic hybrid (A) or for the recalcitrant C. intybus cv Pe´vèle (B) after 12 d (white bars) or 20 d (gray bars) of culture. The media were conditioned by culturing roots of the hybrid for 0, 2 and 4 d in standard conditions. Scoring of embryos was done on two independent experiments each involving ten root explants.
The effect of CM on the embryogenic hybrid led to greatly increase the ISE and the D4-CM already led to double the number of somatic embryos produced. In the same way, the D4-CM promoted the ISE of recalcitrant cv Pe´vèle root explants. Together our results show that condition media contain SE-promoting molecules that likely accumulate progressively during induction of SE.
Involvement of AGPs in SE
HPLC analyses of soluble AGPs profiles
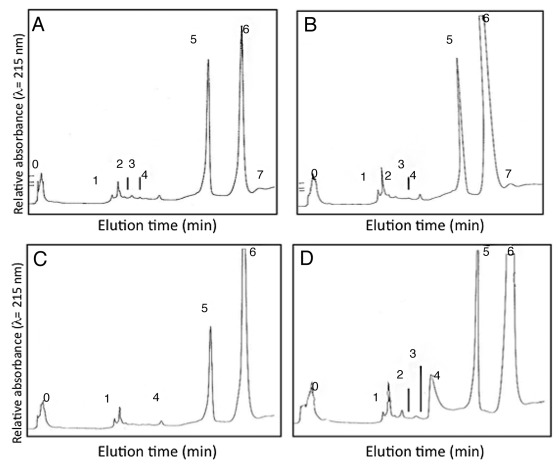
Earlier works in different SE models have suggested that soluble ArabinoGalactan Proteins (AGPs) might be responsible for the SE-promoting activity.27,28 To identify a soluble AGP fraction responsible for promoting SE in our system we analyzed 100 μg of the soluble AGPs of leaf and root explants of both the embryogenic hybrid and the recalcitrant cv Pe´vèle by liquid chromatography.
Besides three minor peaks (2–3 and 7) that are absent in cv Pe´vèle, the AGP profiles obtained for roots of the hybrid (Fig. 4A) and cv Pe´vèle (Fig. 4C) did not exhibit major differences. Similarly, besides the peak 4 which was slightly greater in cv Pe´vèle, there were also no critical differences between the AGP profiles obtained for leaf explants of the two genotypes (Fig. 4B and D). Thus, despite a difference in the quantity of AGPs released in the medium, the same AGP fractions were detected in the same explants of the two chicories.
Figure 4. HPLC profiles of extracellular AGPs isolated after 12 d of culture of roots (A) and leaf (B) of the embryogenic hybrid, or of root (C) and leaf (D) of the recalcitrant cv Pe´vèle. Eight fractions (T0, T1, T2, T3, T4, T5, T6, T7) were collected from 0 to 45 min of elution.
Quantitation of AGPs released in the culture medium
We have previously shown that AGPs are released in the culture medium of embryogenic and non-embryogenic chicories but no major difference in the soluble AGP profiles was detected.33,38 We have also shown earlier that complexation of soluble AGPs with β-D Glc Yariv reagent inhibits mitosis while cell reactivation still occurs.33,38 To test whether the difference in the ISE of the embryogenic hybrid and of the cv Pe´vèle was correlated with differences in the quantity of released AGPs, we determined the mean quantity of soluble AGPs released in the culture media for ten independent experiments. The culture media of the recalcitrant cv Pe´vèle accumulated 6–7 fold less soluble AGPs (1.77 ± 0.83 μg mL−1 for root explants and 0.80 ± 0.05 μg mL−1 for leaf explants) than the embryogenic hybrid (10.69 ± 0.85 μg mL−1 for root explants and 5.70 ± 0.05 μg mL−1 for leaf explants). These quantitations of soluble AGPs released in the medium correlate with the different degrees of ISE of the two genotypes and suggest that the quantity of AGPs released in the media are, at least in part, responsible for the development of SE .
Exogenous addition of amino sugars and L-Proline
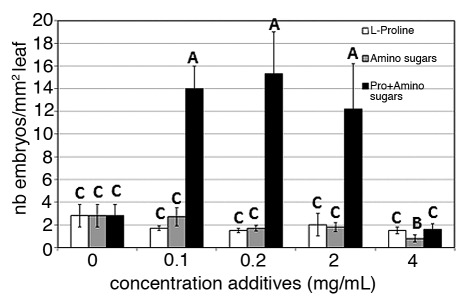
To provide additional evidences that the quantity of AGPs released in the media are responsible for increased ISE, we supplemented the culture media with AGP precursors: a mix of different sugars and L-Proline (Pro).
Compared with the control, the addition of amino sugars or of Pro alone did not lead to increase the ISE of the embryogenic hybrid (Fig. 5). As we anticipated however, the addition of both amino sugars and Pro led to increase the ISE by 4–5 fold depending on the concentrations used. Thus, these results clearly show that addition of both AGP precursors to the culture media increase the ISE of the embryogenic hybrid. These results also suggest that a supposed direct increase in the quantity of AGPs produced by addition of its precursors increases the ISE.
Figure 5. Effects of L-Proline (white bar), of amino sugars (gray bar), or of a combination of L-Proline and amino sugars (black bar) concentrations on the incidence of SE of our embryogenic chicory hybrid. The number of somatic embryos per mm2 of leaf was scored after 12 d of culture. Results of Student-Neuman Keuls range test are shown where means followed by different letters are significantly different at the 5% probability level.
Discussion
The different physiological assays described here show that the ISE of the embryogenic chicory ‘474’ can be promoted and that of usually recalcitrant commercial chicories can be unraveled. The term “recalcitrant” was recently proposed to define “a plant species or a developmental phase of a plant [in which] tissue-culture procedures fail to result in SE or organogenesis.”30 The identification of unconventional SE induction methods is hypothesized to be able to overcome such recalcitrance in commercial chicories of the endivia and intybus species. The results presented here suggest that SE-promoting molecules are released in the culture medium and that these molecules, supposedly soluble AGPs, can be collected to promote SE of primary chicory explants.
Pretreatments of donor plants
Plasmolysis is known to induce mechanical abiotic stresses. We have shown here that immersion of chicory leaves in a 330 mM glycerol solution lead to enhance the ISE. As it was demonstrated in the SE models Citrus and chicory, glycerol acts both as an osmotic agent and as a carbon source.6,31,32 Addition of other osmotica, such as mannitol or sucrose, was also shown to promote SE (Colocasia esculenta, Cucumis melo, Cucumis sativus, Eleutherococcus sentocosus, Pinus taeda, Saccharum officinarum, or androgenesis (wheat).22,33-38 The high osmolarity in the culture medium can also be a consequence of hydrolysis of the carbon source that might lead to enhance glucose and fructose levels, for example; what should be also considered during the course of SE induction.31,39
ABA is a plant growth regulator often associated with totipotency and its endogenous concentration should be low to promote SE in Daucus carota, Medicago or Nicotiana14,40-42 while it should be high to promote SE in other models (Cucumis melo, Daucus carota, Pennistum purpureum).34,43,44 Exogenous addition of ABA led to improve the ISE in monocots (Cocos nucifera, Cynodon hybrid Musa sp.)9,11,13 and in dicots (Daucus carota, Hevea).8,10,12 Our experiment in chicory established that 50 μM ABA promoted the ISE whereas 100 μM had a slightly negative effect. Thus, our results are in agreement with results already described in the literature.
The addition of spermine strongly promoted SE in chicories, especially when it was used in combination with 330 mM glycerol and 50 μM ABA. During SE in Cichorium hybrid, an increase of free PA content, especially put, was reported by Couillerot et al.4 Moreover, totipotency was strongly reduced when put and related PAs synthesis was inhibited by addition of α-difluoromethylarginine (DFMA), a specific and irreversible inhibitor of the PA biosynthesis. On the other hand, the exogenous addition of put to DFMA treated cultures restored the spd and spm contents and therefore totipotency.5 A correlation was suggested between the ISE of the Cichorium embryogenic hybrid and the PA content as it was later established in C. intybus cv Lucknow pluripotency (i.e. organogenesis pattern).45 During SE induction, totipotent cells reached a fully reactivated status and were prepared to re-entry the cell cycle.1,46 A re-initiation of cell division in sugar beet cell suspension was obtained by adding PA and it was suggested that PA controlled gene expression, especially cell division.47
Recent works have shown that the competence for totipotency of our chicory embryogenic interspecific hybrid was inherited from the C. intybus parent.47 Our results indicate that none of the C. endivia tested exhibited totipotency whatever the pretreatments applied. In contrast, some cultivars from C. intybus developed a low number of SE. In consequence, overcoming recalcitrance was not completely possible in our standard SE protocol for all chicories. Other conditions have already been successfully identified for the chicory embryogenic hybrid.1 For conifers, Bonga et al.30 have suggested possible causes of recalcitrance. Among them, an effect of the cytoplasm in maintaining the nucleus of somatic cells in a state non-reprogrammed for SE, inhibition by cell-to-cell contact, or the non-activation of embryogenic genetic mechanism are attractive possibilities to be investigated. Moreover, the presence of molecular determinants could also be linked with totipotency competence that could be revealed in some models. For example, AGPs could also play a critical role in hybrid fir (presence of specific Gal4-AGP epitopes) and banana (MAC204-AGP epitopes) SE.48,49
Effect of co-culture
Organ-dependent molecules released in the medium promoted preferentially SE in the same organs of the embryogenic hybrid and the recalcitrant cv Pe´vèle. Despite the putative accumulation of inhibitory factors in CM, co-cultures were successful in the recalcitrant cv Pe´vèle (Fig. 2). When root explants of cv Pe´vèle were cultivated in 4D-CM obtained from embryogenic hybrid-root led to unravel their totipotency (Fig. 3).
High cell density could inhibit SE as it was reported in carrot probably due to nutrient consumption.50,51 Standard conditions for chicory SE induction consist to use 20 mL medium for nearly 6 cm2 leaves or for one complete root system.31,52 In our co-culture experiments, the volume available for each chicory was lower. As a consequence, the nutrients available for each chicory might become a limiting factor. However, Umehara et al.53 have established that the inhibitory effect of CM was due to the accumulation of inhibitory factors. This inhibition, characterized by the suppression of rapid cell division, is reversible and does not affect the embryogenic potential.54 Nevertheless, Osuga et al.55 were able to improve the ISE at a high cell density when the medium was partially renewed, and in our SE standard conditions, explants were transferred onto a new medium after 4D of culture.31
Co-culture is sometimes performed to overcome a deficiency of embryo development and could show a positive effect when it is a nurse culture.56 The authors developed a method where isolated wheat zygotes were co-cultured with nurse-microspores. This method allowed them to develop and produce fertile plants. Co-culture was also achieved by Meijer et al.57 in a heterologous system similar to the one we use for chicory. The authors co-cultured Arabidopsis cell suspension in which somatic embryos were arrested at the globular stage, and carrot embryogenic cultures. Unexpected inhibition of carrot SE was noticed by the release of the Arabidopsis soluble auxin 2,4-D. In the chicory co-culture system, a slight inhibition of SE was also observed in the embryogenic hybrid but it could not be related to such a 2,4-D. As for CM, unknown inhibitory factors could be released and accumulated in the medium, as the culture was processing.
Exogenous addition of amino sugars and L-Proline
AGPs, which belong to the Hydroxyproline Rich GlycoProtein family58 can exhibit glucosamine and N-acetylglucosamine residues that makes them sensitive to EP3 endochitinase.59 For these reasons, some molecules (amino sugars, included glucosamine and N-acetylglucosamine, and l-proline) present in the AGPs core and glycosylated fractions, were added in the culture medium of the embryogenic hybrid. The addition of a combination of amino sugars and Pro led to greatly enhance the ISE of our chicory while amino sugars or Pro alone had no effect. Beside the putative direct effect on increasing AGPs synthesis, Pro accumulation might also act as a stress molecule during abiotic stress.60,61 In our culture conditions, no glycerol was added as osmoticum to promote SE and the mix of amino sugars cannot induce osmotic stress. The positive interaction between Pro and the mix of amino sugars remains unclear but efficient. The accumulation of Pro in stressed plants reported by others might indicate an adaptation to osmotic stresses.62,63
Conditioned medium and AGP involvement in totipotency acquisition
As we also report in our system here, promotive-mitogenic factors are released in conditioned medium.64 For example, phytosulfokine α,21,22 cell wall remodeling enzymes,65-68 and parietal fragments such as AGPs,31-37,69 or oligogalacturonide pectic fragments have been reported.68
The use of CM obtained from wild type carrot cell suspension favored embryo development in the thermosensitive mutant ts11 that normally produced somatic embryo arrested at the globular stage.71 The plating efficiency of protoplasts is significantly increased onto an agarose obtained with CM-dilution (Lolium, Chrysanthemum).64,72 Our results established a promoting effect of 4D-CM obtained from culture of root explants of the embryogenic hybrid. This promoting effect of was clearly observed on root explants from both chicories (Fig. 3).
Inhibitory fractions of AGPs could be probably present in older CM as shown by Toonen et al.73 We noticed a slight decrease of the embryogenic potential in the hybrid with the use of 4D-medium or older conditioned medium (data not shown), but 4D-CM significantly improved SE in cv Pe´vèle. Beside released AGPs, other inhibitory factors such as 4-hydroxybenzyl,74-76 vanillyl benzyl ether75 might also accumulate in the medium at later time point. Promoting phytosulfokin α might compete with such inhibitory factors.76 The determination of the optimal age of CM will therefore a pre-requisite since older CM might have stronger inhibitory effects.
The embryogenic hybrid released more AGPs in the medium than the recalcitrant chicory C. intybus cv Pe´vèle. The relatively high amount of AGPs observed in root culture medium might be due to the disorganization of root tissue or to the absence of cuticle. Although our data indicate a role of the quantity of soluble AGP released, comparisons of several embryogenic and non-embryogenic genotypes taken from non-commercial population of C. intybus have indicated that slight or even no significant differences were found in the quantity of AGPs released in the medium (AS Blervacq, I Habarugira and M Demilly, unpublished results).77 Thus, the capacity to release AGPs in the medium alone might not be the sole criteria determining the SE capacity of a given genotype.
It is assumed that the sets of AGPs were organ-dependent (see review).58 First experiments were therefore performed on extracellular AGPs pattern recovered in the culture medium of both embryogenic hybrid and recalcitrant cv Pe´vèle. Similar chromatographic profiles were obtained whatever the organs cultured and the chicories considered. As a consequence, no significant peak could be related to the competence for totipotency. Numerous columns and solvent conditions were applied to improve separation of eight fractions; each one contained at least 2 to 4 subfractions (D. Windels, unpublished results). This could be explained by AGPs differing in their protein core and/or in their degree of glycosylation (maturation) or of their degradation. Moreover, stressed environment could modify AGPs gene-expression.29,78,79
Finally, the set of released AGP could also reflect the regulation of gene expression that resulted from different stresses due to in vitro culture (collecting, fragments, liquid culture, orbital shaking…), but also resulted from the competence of each genotype to commit into SE. Legrand et al. established that one AGP gene was upregulated in the high embryogenic chicory (C. intybus, Kospool Hungarian cultivar) compared with the low embryogenic one.77 In these genotypes, Lucau-Danila et al. also showed that at least eight genes, including the previously identified AGP gene (DT212818), were differentially expressed in both genotypes.29 They were probably involved in cell fate determination in chicory (i.e. acquisition of totipotency).
Conclusions
Our results established that pretreatments of tissues before culture with combinations of polyamines and ABA, as well as the use of conditioned medium could reveal or release SE recalcitrance in some Cichorium varieties. Soluble SE-promoting factors, comprising soluble AGPs and their putative precursors, also stimulated SE in both recalcitrant and embryogenic chicories.
Material and Methods
Growth of plant material
Plantlets of the embryogenic hybrid (Cichorium endivia L., var latifolia x Cichorium intybus L., var sativum) clone ‘474’ were grown in vitro as previously described in Bellettre et al.52 Seeds of Cichorium intybus L., var sativum (cultivars Cassel, Orchies and Pe´vèle) and of Cichorium intybus L., var Witloof (cultivar Flash) were respectively obtained from Florimond Desprez (Cappelle en Pe´vèle, France) and from the Institut National de la Recherche Agronomique (INRA, France), surface-sterilized in 0.1% HgCl2 for 10 min, washed several times in water and germinated on solid Heller mineral medium.80
Standard culture conditions for induction of SE
Leaves of 2-mo old plantlets were cultured in 20 mL SE medium supplemented with 330 mM glycerol, which acts as osmoticum and carbon source, for 4 d.31 Explants were then prepared and cultured into 20 mL SE medium for 7–8 d.52,81 Explants were cultured in the dark at 35°C under orbital rotation (160 rpm). For each plantlet, 6 leaf fragments (representing 6 cm2 each) or × cm long fragments of the entire root system were used. The number of somatic embryos per cm2 of leaves and per cm of roots was scored after 8 and 12 d, respectively, for the hybrid and after 20 d for commercial chicories.
Exogenous supply of amino sugars and l-proline
Leaf fragments were cultured in SE culture medium deprived of glycerol for x days. L-Proline (Pro) (Sigma), a mixture of amino sugars (galactosamine, glucosamine, N-acetyl galactosamine, N-acetyl glucosamine provided by Sigma), and a combination of both in equal concentrations (ranging from 0.01 to 4 µg mL−1) were added to the SE medium. The number of somatic embryos per cm of roots was scored after 12 d, Statistical significance was assayed using a Student-Neuman Keuls test. Means followed but different letters are significantly different at the 5% probability level.
Pretreatments of plantlets before induction
During pretreatments, 4 leaves-old plantlets were submerged in aseptic solutions containing either (a) water only, (b) 330 mM glycerol (Prolabo), (c) 0.5 mM polyamine (putrescine, spermidine or spermine purchased from Sigma), (d) 10, 50 or 100 µM (± ) cis, trans abscisic acid (ABA) (Sigma). Leaves were collected after 2 d for induction of SE.
Conditioned medium
Conditioned media (CM), consisting of filtered, glycerol-free SE culture media in which roots of the embryogenic “474” hybrid had been cultured for 0, 2, or 4 d, was used as induction medium. The number of somatic embryos per cm of roots was scored after 12 and 20 d.
Co-culture conditions
To detect the effect of one genotype on the ISE of another genotype, co-cultures were performed in GA-7 polycarbonate Magenta boxes (Sigma). Root or leaf explants were separated with a 25 µm polypropylene membrane (Sigma). To avoid mechanical stresses, 100 mL of medium was used. First test was done with the embryogenic hybrid as a reference. In vitro raised plantlets of the hybrid or of the commercial genotypes were used. In these cases, three complete root systems or ten leaf fragments were collected. Co-cultures were done either (1) only leaves, (2) only with roots, or, (3) with both types of explants. For the hybrid genotype, the ISE was estimated after 8 d of co-culture in the case of leaves and after 12 d in the case of roots. For the Pe´vèle cultivar, the ISE was estimated after 20 d in the case of both types of explants.
Estimation of somatic embryogenesis levels
Cultured explants were fixed in a FAE solution (formaldehyde/acetic acid/ethanol, 3.6/6.5/90, v/v/v) and stained with Lugol (2% I2, 6% KI, w/v). The number of typical brown-colored embryos larger than 100 µm was recorded under light microscope as described in Bellettre et al.31 The mean number of SE was calculated from the count of nine repetitions (10 microscope fields per fragments). Data were analyzed according to Student-Fischer law.
Quantification of AGPs secreted in the culture medium
Extracellular AGPs released after 12 d of culture for roots, and 8 d of culture for leaves were quantified according to Kreuger and Van Holst.82 In brief, the culture medium was lyophilized and the residues were dissolved in minimal sterile water volumes. AGPs were then precipitated with β 1,3-D Glc Yariv reagent which we prepared according to the protocol of Yariv et al.83 After centrifugation, AGPs were released from the complex with Na2SO4 and desalted with PD10 columns (Amersham). AGPs were stored in water at –20°C.
The concentrations of total AGP fractions were determined on (1%, w/v; 0.15M NaCl) agarose gel by comparing the signals obtained to a standard curve (0, 0.05, 0.1, 0.15 and 0.20 mg mL−1) of Arabic gum.84
HPLC analyses
One hundred micrograms of AGPs per sample were separated onto a monoQ H5 / 5 anion exchange column (Pharmacia Biotech), eluted with a discontinuous gradient (0 to 100% solvent B: 1M NaCl, 20 mM TRIS-HCl; solvent A: water, 20 mM TRIS-HCl; flow rate = 0.7 mL.min−1) and collected by fraction from the column (from 0–45 min). The chromatography was monitored by absorption at 215 nm.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest to disclose.
Acknowledgments
This work was supported by a “Contrat Plan Etat-Re´gion” to the UMR Lille1 INRA 1281, and a doctoral fellowship from the “Ministère de la Recherche et de l’Enseignement Supe´rieur” to D. Windels. We thank the M.Sc. students L. Batel and C. Masquelier for technical help with in vitro culture.
Glossary
Abbreviations:
- ABA
abscisic acid
- AGP
arabino galactan proteins
- CM
conditioned medium
- cv.
cultivar
- D
day
- ISE
incidence on somatic embryogenesis
- PA
polyamine
- put
putrescine
- Pro
L-proline
- SE
somatic embryogenesis
- spd
spermidine
- spm
spermine
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18637
References
- 1.Blervacq AS, Lucau-Danila A, Couillerot JP, Morcillo F, Aberlenc-Bertossi F. Hawkins et al. Stem cell-like cells and plant regeneration. In: Generation and characterization of stem cells. In: LV Bernhardt ed. Advances in Medicine and Biology, vol 15, New York, Nova Science Publishers, 2011:1-60. [Google Scholar]
- 2.Vasseur J, Dubois J, Hilbert JL, Couillerot JP. Somatic embryogenesis in chicory (Cichorium species). In: Bajaj YS ed. Biotechnology in agriculture and forestry, Berlin, Springer Verlag, 1995:125-137. [Google Scholar]
- 3.Alcázar R, Marco F, Cuevas JC, Patron M, Fernando A, Carrasco P, et al. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–76. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 4.Couillerot JP, Decout E, Warnot F, Dubois J, Vasseur J. Evolution des polyamines libres en relation avec la source carbone´e et l’embryogenèse somatique chez un Cichorium hybride. C R Acad Sci Paris. 1993;316:299–305. [Google Scholar]
- 5.Helleboid S, Couillerot JP, Hilbert JL, Vasseur J. Inhibition of direct somatic embryogenesis by ´-difluoromethylarginine in a Cichorium hybrid: effects on polyamine content and protein patterns. Planta. 1995;196:571–6. doi: 10.1007/BF00203658. [DOI] [Google Scholar]
- 6.Wu XB, Wang J, Liu JH, Deng XX. Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J Plant Physiol. 2009;166:52–62. doi: 10.1016/j.jplph.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Habibi N, Suthar RK, Purohit SD. Role of PGrs and inhibitors in induction and control of somatic embryogenesis in Themeda quadrivalvis. Indian J Exp Biol. 2009;47:198–203. [PubMed] [Google Scholar]
- 8.Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y. Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta. 2000;211:756–9. doi: 10.1007/s004250000387. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Qu R. In vitro somatic embryogenesis in turf-type bermudagrass: roles of abscisic acid and gibberellic acid, and occurrence of secondary somatic embryogenesis. Plant Breed. 2002;121:155–8. doi: 10.1046/j.1439-0523.2002.00684.x. [DOI] [Google Scholar]
- 10.Ogata Y, Iizuka M, Nakayama D, Ikeda M, Kamada H, Koshiba T. Possible involvement of abscisic acid in the induction of secondary somatic embryogenesis on seed-coat-derived carrot somatic embryos. Planta. 2005;221:417–23. doi: 10.1007/s00425-004-1449-5. [DOI] [PubMed] [Google Scholar]
- 11.Sholi NJY, Chaurasia A, Agraval A, Sarin NB. ABA enhances plant regeneration of somatic embryos derived from cell suspension cultures of plantain cv. spambia (Musa sp.) Plant Cell Tissue Organ Cult. 2009;99:133–40. doi: 10.1007/s11240-009-9585-z. [DOI] [Google Scholar]
- 12.Rai MK, Shekhawat NS, Gupta HAK, Phulwaria M, Ram K, Jaiswal U. The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tissue Organ Cult. 2011;106:179–90. doi: 10.1007/s11240-011-9923-9. [DOI] [Google Scholar]
- 13.Samaj J, Baluska F, Bobak M, Volkmann D. Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monclonal antibody JIM4. Plant Cell Rep. 1999;18:369–74. doi: 10.1007/s002990050588. [DOI] [Google Scholar]
- 14.Chapman A, Helleboid S, Blervacq AS, Vasseur J, Hilbert JL. Removal of the fibrillar network surrounding Cichorium somatic embryos using cytoskeleton inhibitors: analysis of proteic components. Plant Sci. 2000;150:103–14. doi: 10.1016/S0168-9452(99)00185-5. [DOI] [Google Scholar]
- 15.Abedin M, King N. Diverse evolutionary paths to cell Adhesion. Trends Cell Biol. 2010;20:734–42. doi: 10.1016/j.tcb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifert GJ, Blaukopf C. Irritable Walls: The Plant Extracellular Matrix and Signaling. Plant Physiol. 2010;153:467–78. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attree SM, Moore D, Sawhney VR, Fowke LC. Enhanced maturation and desiccation tolerance of white spruce (Picea glauca [Moench.] Voss) somatic embryos: effects of non-plasmolyzing water stress and abscisic acid. Ann Bot (Lond) 1991;68:519–25. [Google Scholar]
- 18.Ling You X, Seon Y, Choi EY. Cellular change and callose accumulation in zygotic embryos of Eleutherococcus senticosus caused by plasmolysisng pretreatment result in high frequency of single-cell-derived somatic embryos. Protoplasma. 2006;227:105–12. doi: 10.1007/s00709-006-0149-3. [DOI] [PubMed] [Google Scholar]
- 19.Matsubayashi Y, Sakagami Y. Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cell of Asparagus officinalis L. Proc Natl Acad Sci USA. 1996;93:7623–7. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Eun CH, Hanai H, Matsubayashi Y, Sakagami Y, Kamada H. Phytosulfokin-α, a peptidyl plant growth factor, stimulates somatic embryogenesis in carrot. J Exp Bot. 1999;50:1123–8. [Google Scholar]
- 21.Hanai H, Matsuno T, Yamamoto M, Matsubayashi Y, Kobayashi T, Kamada H, et al. A secreted peptide growth factor, phytosulfokine, acting as a stimulatory factor of carrot somatic embryogenesis formation. Plant Cell Physiol. 2000;41:27–32. doi: 10.1093/pcp/41.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Eun CH, Ko SM, Matsubayashi Y, Sakagami Y, Kamada H. Phytosulfokine-alpha requires auxine to stimulate carrot non-embryogenic cell proliferation. Plant Physiol Biochem. 2003;41:447–52. doi: 10.1016/S0981-9428(03)00052-4. [DOI] [Google Scholar]
- 23.Tsuwamoto R, Fukuoka H, Takahata Y. Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta. 2007;225:641–652. doi: 10.1007/s00425-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 24.De Vries SC, Booij H, Meyerink P, Huisman G, Wilde HD, Thomas TL, et al. Acquisition of embryogenic potential in carrot-cell suspension. Planta. 1988;176:196–204. doi: 10.1007/BF00392445. [DOI] [PubMed] [Google Scholar]
- 25.Komamine A, Matsumoto M, Tsukahara M, Fujiwara A, Kawahara R, Ito M, et al. Mechanism of somatic embryogenesis in cell-cultures physiology, biochemistry and molecular biology. In: Nijkamp HJJ, Van der Plas LHW, Van Aartrijk J eds. Progress in plant cellular and molecular biology. Current Plant Science and Biotechnology in Agriculture, vol. 9, Dordrecht, Kluwer, 1990:307-317. [Google Scholar]
- 26.Egertsdotter U, von Arnold S. Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies) Physiol Plant. 1995;93:334–45. doi: 10.1111/j.1399-3054.1995.tb02237.x. [DOI] [Google Scholar]
- 27.Chapman A, Blervacq AS, Vasseur J, Hilbert JL. Arabinogalactan-proteins in Cichorium somatic embryogenesis: effect of beta-glucosyl Yariv reagent and epitope localisation during embryo development. Planta. 2000;211:305–14. doi: 10.1007/s004250000299. [DOI] [PubMed] [Google Scholar]
- 28.Tang XC, He YQ, Wang Y, Sun MX. The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L. cv. Topas. J Exp Bot. 2006;57:2639–50. doi: 10.1093/jxb/erl027. [DOI] [PubMed] [Google Scholar]
- 29.Lucau-Danila A, Laborde L, Legrand S, Huot L, Hot D, Lemoine Y, et al. Identification of novel gene potenially involved in somatic embryogenesis in chicory (Cichorium intybus L.) BMC Plant Biol. 2010;10:122. doi: 10.1186/1471-2229-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonga JM, Klimaszewska KK, von Aderkas P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. 2010;100:241–54. doi: 10.1007/s11240-009-9647-2. [DOI] [Google Scholar]
- 31.Bellettre A, Couillerot JP, Vasseur J. Effect of glycerol on somatic embryogenesis in Cichorium leaves. Plant Cell Rep. 1999;19:26–31. doi: 10.1007/s002990050705. [DOI] [PubMed] [Google Scholar]
- 32.Deng Z, Zhang W, Wan S. High frequency of somatic embryogenesis and plant regeneration from cellular calli and protoplasts in Citrus. Acta Biol Exp Sin. 1990;23:143–5. [Google Scholar]
- 33.Deo PC, Taylor M, Harding RM, Tyagi AP, Becker DK. Initiation of embryogenic cell suspensions of taro (Colocasia esculenta var. esculenta) and plant regeneration. Plant Cell Tissue Organ Cult. 2010;100:283–9. doi: 10.1007/s11240-009-9648-1. [DOI] [Google Scholar]
- 34.Nakagawa H, Saijyo T, Yamauchi N, Shigyo M, Kako S, Ito A. Effects of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L.) expanded cotyledon. Sci Hortic (Amsterdam) 2001;90:85–92. doi: 10.1016/S0304-4238(00)00259-4. [DOI] [Google Scholar]
- 35.Lou H, Kako S. Role of high sugar concentrations in inducing somatic embryogenesis from cucumber cotyledons. Sci Hortic (Amsterdam) 1995;64:11–20. doi: 10.1016/0304-4238(95)00833-8. [DOI] [Google Scholar]
- 36.Pullman GS, Chase KM, Skyabina A, Bucalo K. Conifer embryogenesis tissue initiation: improvements by supplementation of medium with D-xylose and D-chiro-inositol. Tree Physiol. 2009;29:147–56. doi: 10.1093/treephys/tpn013. [DOI] [PubMed] [Google Scholar]
- 37.Gandonou C, Errabii T, Abrini J, Idaomar M, Chibi F, Senhaji NS. Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane (Saccharum spp.) Afr J Biotechnol. 2005;4:1250–5. [Google Scholar]
- 38.Maraschin SF, de Priester W, Spaink HP, Wand M. Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot. 2005;56:1711–26. doi: 10.1093/jxb/eri190. [DOI] [PubMed] [Google Scholar]
- 39.Dussert S, Verdeil JL, Rival A, Noirot M, Buffard-Morel J. Nutrient uptake and growth of in vitro coconut (Cocos nucifera L.) calluses. Plant Sci. 1995;106:185–93. doi: 10.1016/0168-9452(95)04079-A. [DOI] [Google Scholar]
- 40.Kikuchi A, Sanuki N, Higashi K, Koshiba T, Kamada H. Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta. 2006;223:637–45. doi: 10.1007/s00425-005-0114-y. [DOI] [PubMed] [Google Scholar]
- 41.Ivanova A, Velcheva M, Denchev P, Atanassov A, Van Onckelen HA. Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant. 1994;92:85–9. doi: 10.1111/j.1399-3054.1994.tb06658.x. [DOI] [Google Scholar]
- 42.Senger S, Mock HP, Conrad U, Manteuffel R. Immunomodulation of ABA function affects early events in somatic embryo development. Plant Cell Rep. 2001;20:112–20. doi: 10.1007/s002990000290. [DOI] [PubMed] [Google Scholar]
- 43.Kiyosue T, Satoh S, Kamada H, Harada H. Puri□cation and immunohistochemical detection of an embryogenic cell protein in carrot. Plant Physiol. 1991;95:1077–83. doi: 10.1104/pp.95.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasekaran K, Hein MB, Vasil IK. Endogenous abscisic acid and indole-3-acetic acid and somatic embryogenesis in cultures leaf explants of Pennisetum purpureum Schum. Plant Physiol. 1987;84:47–51. doi: 10.1104/pp.84.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bais HP, Sudha GS, Ravishankar GA. Putrescine and silver nitrate influences shoot multiplication, in vitro flowering and endogenous titers of polyamines in Cichorium intybus L. cv. Lucknow Local. J Plant Growth Regul. 2000;19:238–48. doi: 10.1007/s003440000012. [DOI] [PubMed] [Google Scholar]
- 46.Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci. 2007;12:245–52. doi: 10.1016/j.tplants.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Fowler MR, Kirby MJ, Scott NW, Slatter A, Elliott MC. Polyamine metabolism and gene regulation during the transition of autonomous sugar beet cell in suspension culture from quiescence to division. Physiol Plant. 1996;98:439–46. doi: 10.1111/j.1399-3054.1996.tb05697.x. [DOI] [Google Scholar]
- 48.Samaj J, Salaj T, Matusova R, Salaj J, Takac T, Samajova O, et al. Arabinogalactan-protein epitope Gal4 is differentially regulated and localized in cell lines of hybrid fir (Abies alba x Abies cephalonica) with different embryogenic and regeneration potential. Plant Cell Rep. 2008;27:221–9. doi: 10.1007/s00299-007-0429-1. [DOI] [PubMed] [Google Scholar]
- 49.Pan X, Yang X, Lin G, Zou R, Chen H, Samaj J, et al. Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ‘Yueyoukang 1’) Physiol Plant. 2011;142:372–89. doi: 10.1111/j.1399-3054.2011.01478.x. [DOI] [PubMed] [Google Scholar]
- 50.Osuga K, Kamada H, Komamine A. Cell density is an important factor for synchronization of the late stage of somatic embryogenesis high frequency. Plant Tiss Cult Lett. 1993;10:180–3. doi: 10.5511/plantbiotechnology1984.10.180. [DOI] [Google Scholar]
- 51.Shigeta JI, Sato K, Mii M. Effects of initial cell density, pH and dissolved oxygen on bioreactor production of carrot somatic embryos. Plant Sci. 1996;115:109–14. doi: 10.1016/S0168-9452(96)04327-O. [DOI] [Google Scholar]
- 52.Belletre A, Couillerot JP, Blervacq AS, Aubert S, Gout E, Hilbert JL, et al. Glycerol affects both carbohydrate metabolism and cytoskeletal rearrangements during the induction of somatic embryogenesis in chicory leaf tissues. Plant Physiol Biochem. 2001;39:503–11. doi: 10.1016/S0981-9428(01)01263-3. [DOI] [Google Scholar]
- 53.Umehara M, Ogita S, Sasamoto H, Kamada H. Inhibitory factor(s) of somatic embryogenesis regulated suspensor differentiation in suspension culture of Japanese larch (Larix leptolepis Gordon) Plant Biotechnol. 2004;21:87–94. doi: 10.5511/plantbiotechnology.21.87. [DOI] [Google Scholar]
- 54.Kobayashi T, Higashi K, Saitou T, Kamada H. Physiological properties of inhibitory conditoning factor(s), inhibitory to somatic embryogenesis, in high-density cell cultures of carrot. Plant Sci. 1999;144:69–75. doi: 10.1016/S0168-9452(99)00062-X. [DOI] [Google Scholar]
- 55.Osuga K, Kamada H, Komamine A. Frequency improvement of somatic embryogenesis at high embryo density by partial replacement of medium in carrot suspension cultures. J Ferment Bioengineering 1997; 84:275-278; doi:10.1016/S0922-338X(97)82070-3.
- 56.Bakos F, Drako E, Ponya Z, Barnabas B. Regeneration of fertile wheat (Triticum aestivum) plants from isolated zygotes using wheat microspore cultures as nurse cells. Plant Cell Tissue Organ Cult. 2003;74:243–7. doi: 10.1023/A:1024071121083. [DOI] [Google Scholar]
- 57.Meijer EA, de Vries SC, Mordhorst AP. Co-culture with Daucus carota somatic embryos reveals high 2,4-D uptake and release rates of Arabidopsis thaliana cultured cells. Plant Cell Rep. 1999;18:656–63. doi: 10.1007/s002990050638. [DOI] [Google Scholar]
- 58.Schlutz C, Gilson P, Oxley D, Youl J, Bacic A. GPI-anchors on arabinogalactan-proteins: implications for signalling in plants. Trends Plant Sci. 1998;3:426–31. doi: 10.1016/S1360-1385(98)01328-4. [DOI] [Google Scholar]
- 59.van Hengel AJ, van Kammen A, de Vries SC. A relationship between seed development, Arabinogalactan-proteins (AGPs) and the AGP mediated promotion of somatic embryogenesis. Physiol Plant. 2002;114:637–44. doi: 10.1034/j.1399-3054.2002.1140418.x. [DOI] [PubMed] [Google Scholar]
- 60.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–9. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 61.Kong X, Sun L, Zhou Y, Zhang M, Liu Y, Pan J, et al. ZmMKK4 regulates osmotic stress through reactive oxygen species scavenging in transgenic tobacco. Plant Cell Rep. 2011;30:2097–104;. doi: 10.1007/s00299-011-1116-9. [DOI] [PubMed] [Google Scholar]
- 62.Verbruggen N, Villarroel R, Van Montagu M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol. 1993;103:771–81. doi: 10.1104/pp.103.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997;38:1095–102. doi: 10.1093/oxfordjournals.pcp.a029093. [DOI] [PubMed] [Google Scholar]
- 64.Zhou J, Wang B, Zhu L. Conditioned culture for protoplasts isolated from Chrysanthemum: an efficient approach. Colloids Surf B Biointerfaces. 2005;45:113–119. doi: 10.1016/j.colsurfb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Dyachok JV, Wiweger M, Kemme L, von Arnold S. Endogenous Nod-factor-like signal molecule promote early somatic embryo development in Norway spruce. Plant Physiol. 2002;128:523–33. doi: 10.1104/pp.010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tchorbadjieva M, Pantchev I, Harizanova N. Two-dimensional protein pattern analysis of extracellular proteins secreted by embryogenic and non-embryogenic suspension cultures of Dactylis glomerata. Biotechnol, Biotechnol Equip. 2004;18:20–7. [Google Scholar]
- 67.Wendt dos Santos AL, Steier N, Guerra MP, Zoglauer K, Moerschbacher BM. Somatic embryogenesis in Araucaria angustifolia. Biol Plant. 2008;52:195–9. doi: 10.1007/s10535-008-0044-1. [DOI] [Google Scholar]
- 68.Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Boutillier K, Bailleul F, Cle´ment C. Microspore embryogenesis in barley: anther pretreatment stimulates plant defence gene expression. Planta. 2009;229:393–402. doi: 10.1007/s00425-008-0838-6. [DOI] [PubMed] [Google Scholar]
- 69.Chapman A, Blervacq AS, Tissier JM, Delbreil B, Hendriks T, Vasseur J, et al. Cell wall differenciation during early somatic embryogenesis in plants. I. SEM and TEM study on somatic embryos originated from direct, indirect and adventitious pathway. Can J Bot. 2000;78:816–23. [Google Scholar]
- 70.Baldan B, Bertoldo A, Navazio L, Mariani P. Oligogalacturonide-induces changes in the developmental pattern of Daucus carota L. somatic embryos. Plant Sci. 2003;165:337–48. doi: 10.1016/S0168-9452(03)00193-6. [DOI] [Google Scholar]
- 71.Lo Schiavo F, Giuliano G, de Vries SC, Genga A, Bollini R, Pitto L, et al. A carrot cell variant temperature sensitive for somatic embryogenesis reveals a defect in the glycosylation of extracellular proteins. Mol Gen Genet. 1990;223:385–93. doi: 10.1007/BF00264444. [DOI] [PubMed] [Google Scholar]
- 72.Folling M, Madsen S, Olesen A. Effect of nurse culture and conditioned medium on colony formation and plant regeneration from Lolium perenne protoplasts. Plant Sci. 1995;108:229–39. doi: 10.1016/0168-9452(95)04146-L. [DOI] [Google Scholar]
- 73.Toonen MAJ, Schmidt EDL, van Kammen A, de Vries SC. Promotive and inhibitory effects of diverse arabinogalactan proteins in Daucus carota L. somatic embryogenesis. Planta. 1997;203:188–95. doi: 10.1007/s004250050181. [DOI] [Google Scholar]
- 74.Kobayashi T, Higashi K, Sasaki K, Asami T, Yoshida S, Kamada H. Purification from conditioned medium and chemical identification of a factor that inhibits somatic embryogenesis in carrot. Plant Cell Physiol. 2000;41:268–73. doi: 10.1093/pcp/41.3.268. [DOI] [PubMed] [Google Scholar]
- 75.Umehara M, Ogita S, Sasamoto H, Koshino H, Asami T, Fijioka S, et al. Identification of a novel factor, vanillyl benzyl ether, which inhibits somatic embryogenesis of japanese larch (Larix leptolepis Gordon) Plant Cell Physiol. 2005;46:445–53. doi: 10.1093/pcp/pci041. [DOI] [PubMed] [Google Scholar]
- 76.Umehara M, Ogita S, Sasamoto H, Eun CH, Matsubayashi Y, Sakagami Y, et al. Two stimulatory effects of the peptidyl growth factor phytosulfokine during somatic embryogenesis in japanese larch (Larix leptolepis Gordon) Plant Sci. 2005;109:901–7. doi: 10.1016/j.plantsci.2005.06.008. [DOI] [Google Scholar]
- 77.Legrand S, Hendriks T, Hilbert JL, Quillet MC. Characterization of expressed sequence tags obtained by SSH during somatic embryogenesis in Cichorium intybus L. BMC Plant Biol. 2007;7:27–39. doi: 10.1186/1471-2229-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I. CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiol. 2003;131:1450–9. doi: 10.1104/pp.015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun W, Kieliszewski MJ, Showalter AM. Overexpression of tomato LeAGP1- arabinogalactan protein promotes lateral branching and hampers reproductive development. Plant J. 2004;40:870–81. doi: 10.1111/j.1365-313X.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 80.Heller R. Recherches sur la nutrition mine´rale des tissues ve´ge´taux cultive´s in vitro. Ann Sci Nat Bot Biol Veg. 1953;14:1–223. [Google Scholar]
- 81.Dubois T, Dubois J, Guedira M, Vasseur J. Embryogenèse somatique directe sur les styles de Cichorium: effets de la tempe´rature et origine des embryoïdes. C R Acad Sci Paris. 1988;307:669–75. [Google Scholar]
- 82.Kreuger M, Van Holst GJ. Arabinogalactan-proteins epitopes in somatic embryogenesis in Daucus carota. Planta. 1995;197:135–41. doi: 10.1007/BF00239949. [DOI] [Google Scholar]
- 83.Yariv J, Rapport MM, Graf L. The interaction of glucosides and saccharides with antibody to the corresponding phenylglycosides. Biochem J. 1962;85:383–8. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Holst GJ, Clarke AE. Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal Biochem. 1985;148:446–50. doi: 10.1016/0003-2697(85)90251-9. [DOI] [PubMed] [Google Scholar]