Abstract
RACK1 is a scaffold protein with the ability to interact in a regulated manner with a diverse number of ligands from distinct signal-transduction pathways. This assessment allowed us to infer that it may be involved in different processes such as nodulation. In a recent study we showed by silencing, that PvRACK1 has a pivotal role in cell expansion and in symbiosome and bacteroid integrity during nodule development in Phaseolus vulgaris. On the other hand, we have also observed that its overexpression provokes a dramatic phenotype in: (a) seedlings that have been exposed to heat, in which systemic necrosis is induced; and (b) in Agrobacterium rhizogenes-transformed roots, where nodulation is strongly inhibited and nodules show early senescent symptoms. These findings indicate that PvRACK1 may be an integrator of diverse signal-transduction pathways in processes as varied as nodulation, cell expansion, heat stress responses, and systemic activation of necrosis.
Keywords: P. vulgaris, inhibition, necrosis, nodulation, over-expression, silencing, systemic
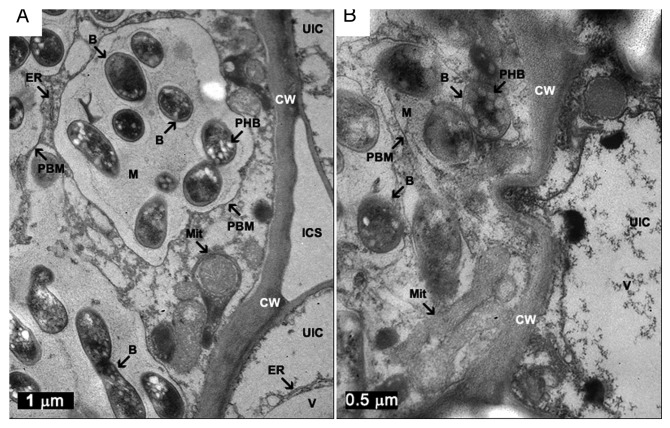
The first RACK1 (Receptor for Activated C Kinase) from plants was found in tobacco BY2 cells in 1993,1 and from then on, it has been found ubiquitously in eukaryotic cells. Furthermore, its role as a kinase receptor has been shown to be only the tip of the iceberg, and multiple ligands as well as its involvement in multiple pathways have also been documented.2,3 Its peculiar βpropeller structure allows its regulated interaction with multiple ligands in various plant signal-transduction pathways. This capacity makes RACK1 an interesting protein candidate to study the way in which diverse signal-transduction pathways are integrated in different living processes. Plant RACK1 homologs have been involved in hormone responses, development, and environmental stress;4,5 ROS production, and innate immunity in rice;6 and is a structural component of the ribosome 40S subunit in Arabidopsis.7 However, in spite of the copious information acquired from plant RACK1 in recent years, there are still many questions with regard to its function. Because of its versatility, it is evident that RACK1 could not play a single specific function, but rather, it probably plays roles at important assembly parts for common mechanisms from various processes. This is also supported by the fact that RACK1 has a ubiquitous expression,4,5 that must be finely controlled to maintain the plant homeostasis. There is evidence that gradually inhibiting the expression levels of Arabidopsis RACK1, several important cellular mechanisms were affected; in particular, those involved in hormonal responses, seed germination and seedling development and growth.4 In a recent publication, we analyzed the PvRACK1 expression during hormonal treatment of root development and during nodulation.8 In this work, and based on a previous characterization that indicated the presence of only one gene in P. vulgaris,9 we silenced the PvRACK1 expression by RNAi in hairy roots of P. vulgaris induced by A. rhizogenes with the purpose of learning about its possible involvement and function in this process. Our findings indicated that mRNA accumulation of PvRACK1 in roots was induced by auxins, abscisic acid (ABA), cytokinin, and gibberellic acid (GA); and during nodulation it was highly accumulated at 12–15 d post-inoculation (Table 1). Furthermore, silenced transgenic roots formed 70–90% less nodules and silenced nodules were smaller because infected and non-infected cells did not expand. Moreover, the non-infected cells and symbiosomes showed significant defects in membrane structure (Fig. 1). The nodulation inhibition could arise due to an effect of less sensitivity to positive regulators of nodulation such as GA and BR (Brassinosteroids) when PvRACK1 is silenced;10 while it can be more sensitive to ABA, which is a negative regulator of nodulation and, at the same time, is negatively regulated by RACK1A and RACK1C in Arabidopsis.11 In the case of the silenced nodules, the small size provoked by the small cells within them, could be explained by a role of PvRACK1 in cell expansion. This is supported by the fact that PvRACK1 is expressed two-fold in 12–15 d post-inoculation nodules, which correspond to the cell expansion stage. These data suggest that PvRACK1 expression is controlled by hormones, in order for cell expansion and nodule development to occur appropriately.
Table 1. PvRACK1 mRNA accumulation.
| Treatment | Days post-treatment/inoculation (dpt/dpi) | Fold induction |
|---|---|---|
| Auxins (25 mM) |
1 dpt |
x14 |
| ABA (10 mM) |
1 dpt |
x10 |
| Cytokinin (25 mM) |
1 dpt |
x5 |
| Gibberellin (10 mM) |
1 dpt |
x13 |
| R. tropici inoculation | 12–15 dpi | x2 |
The table shows the times-fold induction of the PvRACK1 mRNA accumulation. Three-day old roots were treated with hormones or inoculated with Rizobium tropici, and mRNA accumulation was determined by q-PCR in roots and nodules.
Figure 1. Ultrastructural features of control and PvRACK1-knockdown Phaseolus vulgaris nodules by electron microscopy. (A) 26 dpi control nodule. (B) 26 dpi PvRACK1-knockdown nodule. B, bacteroids; PBM, peribacteroid membrane; M, matrix; V, vacuole; CW, cell wall; UIC, uninfected cell; ICS, intracellular space; Mit, mitochondria; PHB, polyhydroxybutyrate granule; and ER, endoplasmic reticulum. The lysis of bacteroid membranes and disorganized symbiosome are evident in the infected cell of the knockdown nodule (B).
A more recent study on overexpression of PvRACK1 showed that such overexpression caused severe damage to seedlings inoculated with A. rhizogenes carrying the PvRACK1 overexpression construct (Islas-Flores et al. in preparation). Transformed seedlings showed systemic necrosis at 4–5 d after inoculation when exposed to heat, and no callus was formed at the inoculation zone. Furthermore, the seedlings never progressed to form transgenic roots (Islas-Flores et al. in preparation). This indicates that the excessive PvRACK1 expression has a dramatic effect over the control of the plant response to heat stress. On the other hand, when the transformation was performed under greenhouse conditions at 26–28°C, there was a progression toward the development of hairy roots, but nodulation was decreased (80% less nodules than in the control). Overexpressing nodules showed a severely affected morphology compared with controls (Islas-Flores et al. in preparation). This dramatic phenotype indicated that overexpression of PvRACK1 affects multiple biological functions, from developmental processes to stress responses.
From all the reported evidence, the relationship between hormonal responses and PvRACK1 expression appears to be the key to understanding the acute loss of control of an, albeit moderate, heat stress response in common bean. As discussed above, ABA induces PvRACK1 expression. In addition, ABA is negatively regulated by RACK1A, B and C in Arabidopsis, and it modulates stress responses.11 Thus, a cross-regulation between ABA and RACK1 may exist in order to keep hormonal and RACK1modulated interactions in planta. In addition to RACK1 involvement in stress, OsRACK1 in rice is induced by biotic stress, and its overexpression promotes the production of ROS.6 In rice, OsRACK1 forms an immune complex with Rac1, NADPH oxidase Rboh, RAR1 (Required for Mla12 Resistance), and SGT1 (Suppressor of the G2 allele of skp1); and activates the NADPH oxidase Rboh to produce ROS; thus, overexpression of OsRACK1 enhances ROS production.6 In Arabidopsis, RbohD mediates a rapid systemic signal triggered by wounding, heat, cold, high-intensity light, and salinity stresses. As expected, the signal propagation was accompanied by the concomitant accumulation of ROS.12 Therefore, it is possible that a PvRACK1 excess could cause an enhanced ROS production, and this is maximized by the heat stress which, in turn, could activate a rapid systemic response that results in the systemic necrosis. From all this, we hypothesize that the overexpression of PvRACK1 may mediate the stress response through ABA and ROS, in which excessive ROS causes an acute oxidative stress which results in severely damaged tissues, provoking a generalized apoptosis in the whole plant.
Thus, in the P. vulgaris plant, where it is highly likely that only one RACK1 gene exists in its genome, silencing of PvRACK1 expression affects directly the normal cellular expansion processes that lead to the development of a normal nodule. On the other hand, overexpression of the same transcript leads to an increased susceptibility to heat stress, and also negatively influences normal nodule development. The altered level of expression, either up- or downregulated, would have the common denominator of an altered hormonal response which leads to defects in normal plant and nodule growth and development. This is a situation difficult to contend with, by a plant that does not have additional RACK1 genes to complement its normal function. Finally, an additional player would be the increased ROS production leading to activation of a rapid systemic response resulting in systemic necrosis and cell death.
Acknowledgments
We thank G. Zavala from the Electron Microscopy facility of the Institute of Biotechnology-UNAM for her technical help. This work was supported by grant 83324 from CONACyT and grant IN-208407 from PAPIIT-UNAM. T. Islas-Flores was supported by a Ph.D. scholarship (176434) from CONACyT.
Glossary
Abbreviations:
- RACK1
receptor for activated C kinase
- PKC
protein kinase C
- ROS
reactive oxygen species
- BY2
bright yellow-2
- RAR1
required for Mla12 resistance
- SGT1
suppressor of the G2 allele of skp1
- ABA
abscisic acid
- GA
gibberellic acid
- BR
brassitonsteroids
- Rboh
respiratory burst oxidase homologs
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18485
References
- 1.Ishida S, Takahashi Y, Nagata T. Isolation of cDNA of an auxin-regulated gene encoding a G protein β subunit-like protein from tobacco BY-2 cells. Proc Natl Acad Sci USA. 1993;90:11152–6. doi: 10.1073/pnas.90.23.11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–73. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta J, Nilsson J, Gursky R, Spahn CM, Nissen P, Frank J. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol. 2004;11:957–62. doi: 10.1038/nsmb822. [DOI] [PubMed] [Google Scholar]
- 4.Chen JG, Ullah H, Temple B, Liang J, Guo J, Alonso J, et al. RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J Exp Bot. 2006;57:2697–708. doi: 10.1093/jxb/erl035. [DOI] [PubMed] [Google Scholar]
- 5.Ullah H, Scappini EL, Moon AF, Williams LV, Armstrong DL, Pedersen LC. Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein Sci. 2008;17:1771–80. doi: 10.1110/ps.035121.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima A, Chen L, Thao NP, Fujiwara M, Wong HL, Kuwano M, et al. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell. 2008;20:2265–79. doi: 10.1105/tpc.107.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang I-F, Szick-Miranda K, Pan S, Bailey-Serres J. Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol. 2005;137:848–62. doi: 10.1104/pp.104.053637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islas-Flores T, Guille´n G, Alvarado-Affantranger X, Lara-Flores M, Sánchez F, Villanueva MA. PvRACK1 loss-of-function impairs cell expansion and morphogenesis in Phaseolus vulgaris L. root nodules. Mol Plant Microbe Interact. 2011;24:819–26. doi: 10.1094/MPMI-11-10-0261. [DOI] [PubMed] [Google Scholar]
- 9.Islas-Flores T, Guille´n G, Islas-Flores I, San Rom´n-Roque C, Sánchez F, Loza-Tavera H, et al. Germination behavior, biochemical features and sequence analysis of the RACK1/arcA homolog from Phaseolus vulgaris. Physiol Plant. 2009;137:264–80. doi: 10.1111/j.1399-3054.2009.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Y, Oldroyd GE. Positioning the nodule, the hormone dictum. Plant Signal Behav. 2009;4:89–93. doi: 10.4161/psb.4.2.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Wang J, Xi L, Huang WD, Liang J, Chen JG. RACK1 is a negative regulator of ABA responses in Arabidopsis. J Exp Bot. 2009;60:3819–33. doi: 10.1093/jxb/erp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Sign. 2009;2:ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]