Abstract
Aberrant microtubule organization has been recently recorded in dividing root cells of fra2 and lue1 p60-katanin Arabidopsis thaliana mutants. Here, we report similar defects in the bot1 and ktn1-2 mutants of the same plant, proposing that they constitute a consistent phenotype of p60-katanin mutants. In addition, we show that the Targeting Protein for Xklp2 (TPX2) protein co-localizes with microtubules on the surface of prophase nuclei of the mutants, probably participating in multipolar spindle assembly. As microtubule organization defects are not observed in metaphase/anaphase spindles and initiating phragmoplasts, we also discuss the putative association of the observed aberrations with the nuclear envelope and we emphasize on the mechanism of bipolar metaphase spindle organization in the mutants. It seems that chromosome-mediated spindle assembly, probably minimally dependent on microtubule severing by p60-katanin, dominates after nuclear envelope breakdown, restoring bipolarity.
Keywords: Arabidopsis thaliana, cell division, katanin, microtubules, TPX2
Microtubule Organization Defects in Dividing Root Cells of p60-Katanin Mutants
Microtubule severing by p60-katanin has been proven crucial for cortical microtubule organization and cell morphogenesis.1,2 We have shown recently that it is also essential for the organization of microtubules in dividing root cells.3 The following defects were recorded in dividing root cells of fra24,5 and lue16 p60-katanin Arabidopsis thaliana mutants: (1) Early preprophase bands consisted of poorly aligned microtubules, while few mature preprophase bands were asymmetrically organized. In addition, preprophase band microtubules persisted during prometaphase. (2) The prophase spindles were multipolar. (3) The microtubules of expanding phragmoplasts were long, bended and attached to the daughter nuclei, exhibiting a particular “double-arrow” configuration. The frequently observed oblique cell divisions in the mutant roots were mainly attributed to the multipolarity of prophase spindles.3
Microtubule organization defects, similar to those of fra2 and lue1 dividing root cells, were also recorded in dividing root cells of two more p60-katanin mutants, bot17 and ktn1–28 (Fig. 1). It can therefore be assumed that they are universal among p60-katanin mutants of Arabidopsis thaliana. Studies on the dgl19 mutant of Oryza sativa might further establish these observations as a consistent phenotype among katanin mutants of diverse plant species.
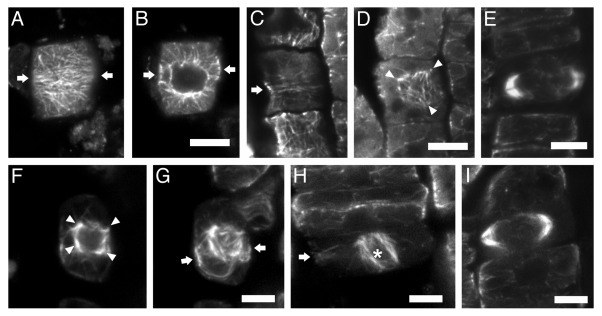
Figure 1. CLSM sections after α-tubulin immunostaining, as described in reference 3, of bot1 (A–E) and ktn1–2 (F–I) roots. The seeds of bot1 were purchased from NASC, while those of ktn1-2 mutant were offered by Dr. Masayoshi Nakamura and Prof. Takashi Hashimoto (Graduate School of Biological Sciences; Nara Institute of Science and Technology; Japan). (A, B) Early preprophase cell, at cortical (A) and intranuclear (B) section. The arrows point to the division plane. The preprophase band consists of poorly aligned microtubules (A), while the nucleus is already surrounded by numerous microtubules (B). (C and D) Late preprophase/prophase cell CLSM sections, through the cortical cytoplasm (C) and at the surface of the nucleus (D). The preprophase band is loose (C, arrow), while perinuclear microtubules exhibit various orientations (D), converging to multiple foci (arrowheads in D). (E) “Double-arrow” shaped expanding phragmoplast in cytokinetic bot1 cell. (F and G) Late preprophase/prophase cell at intranuclear CLSM section (F) and maximum projection of 8 successive sections (G). The arrows in (G) point to the division plane. The perinuclear microtubules exhibit various orientations, converging to multiple foci (arrowheads in F). (H) Prometaphase cell (the asterisk indicates the spindle) with preprophase band remnants at the cell cortex (arrow). (I) “Double-arrow” shaped expanding phragmoplast in cytokinetic ktn1–2 cell. Scale bars: 5 μm.
TPX2 and Multipolar Prophase Spindle Organization
In wild-type prophase Arabidopsis thaliana cells, perinuclear microtubules exhibit uniform “meridian-like” orientation, perpendicular to the division plane, while in the p60-katanin mutants, microtubules with various orientations persist around the prophase nucleus. We have suggested that in the wild type, any microtubules that do not follow the “meridian” arrangement are removed and/or reoriented by severing, resulting in bipolar prophase spindles. As unaligned microtubules are not severed in the mutants, they converge in multiple poles at any side of the prophase nucleus.3 TPX2, a central regulator of spindle assembly in vertebrate cells, has been shown to participate in spindle organization also in plants, exported from the nucleus during prophase, interacting with Aurora kinases and probably initiating microtubules.10 Importantly, TPX2 was immunolocalized in the multipolar prophase spindles of p60-katanin mutants (Fig. 2). It seems, therefore, that TPX2 cannot per se achieve prophase spindle bipolarity. TPX2 may rather participate in the convergence of aligned perinuclear microtubules of wild-type cells or unaligned perinuclear microtubules of mutant cells to bipolar or multipolar spindles, respectively.
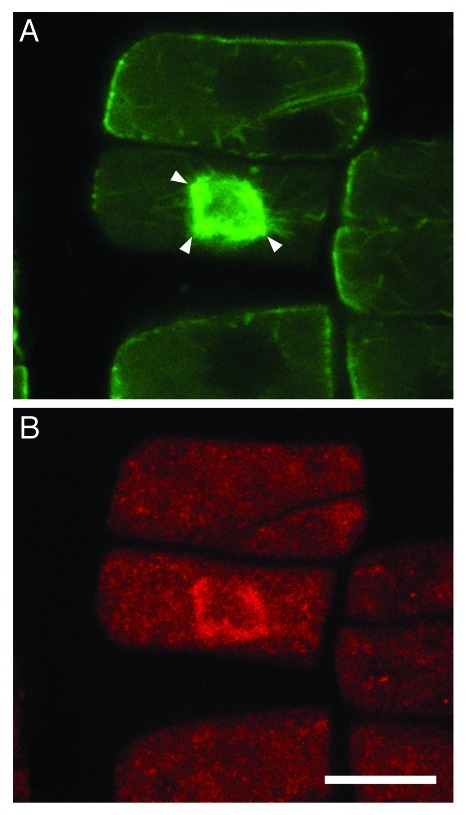
Figure 2. Co-localization of microtubules (A) and TPX2 (B) at the prophase spindle of a fra2 root cell. The anti-TPX2 antibody was a generous gift of Prof. Anne-Catherine Schmit (Institut de Biologie Mole´culaire des Plantes, CNRS, Strasbourg, France). Double immunostaining was performed as described in references 3 and 18 (with the addition of a 30 min and a successive overnight step of incubation with 0.5% NaBH4 before enzyme treatment, and a 20 min step in cold methanol just afterwards) with monoclonal anti-α-tubulin and polyclonal anti-AtTPX2.10 The prophase spindle, at intranuclear CLSM section, appears to consist of microtubules converging to more than two poles (arrowheads in A). TPX2 signal (B) is coincident with the prophase spindle microtubules (compare with A). Scale bar: 10 μm.
Metaphase Spindle Assembly Seems to be Independent from p60-Katanin Function
As reported by GFP-katanin localization,3 p60-katanin is associated with every cell cycle-specific array. Interestingly, the microtubule arrays that assemble after nuclear envelope breakdown, at late prophase, until its reassembly at telophase, i.e., metaphase/anaphase spindle and early phragmoplast, are typical in organization in the p60-katanin mutants. On the contrary, nuclear envelope-associated microtubules, i.e., unaligned perinuclear microtubules at prophase and nuclear envelope-connected minus ends of phragmoplast microtubules at late telophase, are not severed, resulting in the observed aberrations. It seems thus that severing by p60-katanin is necessary on and/or around the nuclear envelope for normal microtubule organization. Accordingly, although microtubule-dependent microtubule nucleation by γ-tubulin complexes may occur almost anywhere in the plant cell8,11,12 it seems that the nuclear envelope may possess some special centrosomal properties (see also ref. 13). In this context, its engagement with severing by p60-katanin seems to represent an analog to the centrosome-katanin duet of animal cells. Experiments interfering with nuclear envelope assembly/disassembly are required to further elucidate this point.
Spindle assembly in plants may be achieved by a combination of nuclear envelope- and chromosome-mediated pathways.14 Our observations support that, as prophase spindle multipolarity does not influence metaphase spindle bipolarity, chromosome-mediated spindle assembly, probably minimally dependent on microtubule severing by p60-katanin, may take control after nuclear envelope breakdown, as an alternative mechanism, to correct any previous spindle defect. This also resembles to the chromosome-mediated spindle assembly in acentrosomal animal cells,15 supporting that in angiosperms metaphase spindle may not originate exclusively by the prophase spindle.16,17
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18358
References
- 1.Burk DH, Zhong R, Ye ZH. The katanin microtubule severing protein in plants. J Integr Plant Biol. 2007;49:1174–82. doi: 10.1111/j.1672-9072.2007.00544.x. [DOI] [Google Scholar]
- 2.Hamada T. Microtubule-associated proteins in higher plants. J Plant Res. 2007;120:79–98. doi: 10.1007/s10265-006-0057-9. [DOI] [PubMed] [Google Scholar]
- 3.Panteris E, Adamakis I-DS, Voulgari G, Papadopoulou G. A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1 Arabidopsis thaliana mutants. Cytoskeleton. 2011;68:401–13. doi: 10.1002/cm.20522. [DOI] [PubMed] [Google Scholar]
- 4.Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Burk DH, Ye ZH. Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell. 2002;14:2145–60. doi: 10.1105/tpc.003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouquin T, Mattson O, Naested H, Foster R, Mundy J. The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J Cell Sci. 2003;116:791–801. doi: 10.1242/jcs.00274. [DOI] [PubMed] [Google Scholar]
- 7.Bichet A, Desnos T, Turner S, Grandjean O, Höfte H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001;25:137–48. doi: 10.1046/j.1365-313x.2001.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M, Ehrhardt DW, Hashimoto T. Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat Cell Biol. 2010;12:1064–70. doi: 10.1038/ncb2110. [DOI] [PubMed] [Google Scholar]
- 9.Komorisono M, Ueguchi-Tanaka M, Aichi I, Hasegawa Y, Ashikari M, Kitano H, et al. Analysis of the rice mutant dwarf and gladius leaf 1. Aberrant katanin-mediated microtubule organization causes up-regulation of gibberellin biosynthetic genes indepedently of gibberellin signaling. Plant Physiol. 2005;138:1982–93. doi: 10.1104/pp.105.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos JW, Pieuchot L, Evrard J-L, Janski N, Bergdoll M, de Ronde D, et al. The plant TPX2 protein regulates prospindl assembly before nuclear envelope breakdown. Plant Cell. 2008;20:1783–97. doi: 10.1105/tpc.107.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of γ -tubulin in higher plants. Nat Cell Biol. 2005;7:961–8. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 12.Chan J, Sambade A, Calder G, Lloyd CW. Arabidopsis microtubules are initiated along, as well as branching from, existing microtubules. Plant Cell. 2009;21:2298–306. doi: 10.1105/tpc.109.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erhardt M, Stoppin-Mellet V, Campagne S, Canaday J, Mutterer J, Fabian T, et al. The plant Spc98p homologue colocalizes with γ-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J Cell Sci. 2002;115:2423–31. doi: 10.1242/jcs.115.11.2423. [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth P, Lee W-L, Murata T, Baskin TI. Variations on theme: spindle assembly in diverse cells. Protoplasma. 2011;248:439–46. doi: 10.1007/s00709-010-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd C, Chan J. Not so divided: the common basis of plant and animal cell division. Nat Rev Mol Cell Biol. 2006;7:147–52. doi: 10.1038/nrm1831. [DOI] [PubMed] [Google Scholar]
- 16.Zachariadis M, Galatis B, Apostolakos P. Study of mitosis in root-tip cells of Triticum turgidum treated with the DNA-intercalating agent ethidium bromide. Protoplasma. 2000;211:151–64. doi: 10.1007/BF01304483. [DOI] [Google Scholar]
- 17.Zachariadis M, Quader H, Galatis B, Apostolakos P. An inhibitor of the ATP-dependent endoplasmic reticulum Ca2+-pump affects spindle organization in dividing cells of the angiosperm Triticum turgidum but not in species of gymnosperms and pteridophytes. J Biol Res. 2004;2:3–19. [Google Scholar]
- 18.Adamakis I-DS, Panteris E, Eleftheriou EP. Tungsten affects the cortical microtubules of Pisum sativum root cells: experiments on tungsten-molybdenum antagonism. Plant Biol. 2010;12:114–24. doi: 10.1111/j.1438-8677.2009.00197.x. [DOI] [PubMed] [Google Scholar]