Abstract
The Arabidopsis EXORDIUM-LIKE1 (EXL1) gene (At1g35140) is required for adaptation to carbon (C)- and energy-limiting growth conditions. An exl1 loss of function mutant showed diminished biomass production in a low total irradiance growth regime, impaired survival during extended night, and impaired survival of anoxia stress. We show here additional expression data and discuss the putative roles of EXL1. We hypothesize that EXL1 suppresses brassinosteroid-dependent growth and controls C allocation in the cell. In-depth expression analysis of homologous genes suggests that the EXL2 (At5g64260) and EXL4 (At5g09440) genes play similar roles.
Keywords: anoxia, brassinosteroids, carbon availability, EXL1, EXL2, EXL4, growth, starvation
C-starvation and reduced energy supply is a serious problem for plants under various environmental conditions. Plant cells respond rapidly to the diminished energy status. Changes in sugar and nucleotide levels may deliver early endogenous signals1-3 that trigger inhibition of C utilization and anabolic pathways,4 induction of catabolic pathways that mobilize reserve compounds,5,6 and ultimately autophagic processes which degrade organelles in lytic vacuoles.7
Plants are able to avoid C deficiency under fluctuating environmental conditions because growth and development are synchronized with the supply and utilization of C.4,8 The balanced control of C assimilation, C storage, and C utilization is mandatory for plant growth during the diurnal cycle and for survival under adverse environmental conditions.9 In addition to a reduction in photosynthesis, energy deprivation can be a consequence of a reduction in respiration. For example, waterlogging and submergence reduce diffusion of gases and can critically reduce the oxygen level in plant cells.10
Identification of Genes Involved in the Adaptation to Low C and Energy Conditions
Different strategies were used to identify genes and regulatory pathways that allow growth and survival under low energy availability. Responses to C starvation conditions were intensely analyzed in artificial (but well-defined) experimental conditions such as extended night, low total irradiance (i.e., short light period and/or low light), low CO2 supply, and privation of exogenous sugars in synthetic medium. These studies revealed massive changes in gene expression patterns, metabolic pathways, and enzyme activities in response to C starvation or C supply.3 The rapid response to C depletion includes the inhibition of growth. Sustained periods of reduced C availability result in a higher rate of starch synthesis during the day and optimized use of available C. Thus, an ‘acute response’ may allow dealing with conditions of sudden C starvation, and an ‘acclimatory response’ may tune the balance between supply and demand to avoid C starvation and cessation of growth.9
The above-mentioned responses to the C status critically depend on information on the levels of cellular metabolites such as sugars and amino acids.11 Yeast has proven useful as a model for reverse genetics approaches in plants, and glucose sensors such as the hexokinases and central regulatory factors such as the SNF1-related protein kinases play major roles in both yeast and plants.2 Mutant screens identified numerous further loci that modify sugar responses. The level of T6P (trehalose 6-phosphate) reflects the tissue sucrose status and possibly links metabolism and control of development.12
Plant tissues with high metabolic activity or restrictions in oxygen supply can become hypoxic even in well-oxygenated surroundings (21% v/v oxygen).13,14 Under these conditions, plants have to decrease their oxygen consumption to avoid internal anoxia. The inhibition of respiration is rapid and occurs above the Km(oxygen) of cytochrome oxidase. Oxygen-sensing systems presumably control the rapid response.14 The decrease in the internal oxygen concentration is associated with a decrease in the ATP/ADP ratio. Low ATP production induces energy-conserving metabolic pathways. Under anoxic conditions when cytochrome oxidase activity becomes oxygen limited, glycolysis is stimulated and fermentation begins. This results in the accumulation of ethanol and organic acids such as lactate and an increase in the NADH/NAD+ ratio.14
Flooding is an environmental stress that is associated with a reduction of cellular oxygen content. Fundamental work in rice and other flood-tolerant species suggests that different types of flooding (e.g., shallow and prolonged or deep and ephemeral floods) require different types of adaptations [e.g., ethylene-driven shoot elongation and other changes (the ‘low-oxygen escape syndrom’) or maximised conservation of carbohydrates and energy (the ‘quiescence strategy’)].10 Rosette plants such as Arabidopsis cannot use the escape strategy, and can only survive flooding by using the quiescence strategy. Interestingly, Arabidopsis accessions differ significantly in submergence tolerance.15
C starvation also could result from low water availability, because CO2 supply is limited, and drought and salt stress trigger the production of sugars, compatible solutes, and protective proteins.16,17 However, experimental evidence in Arabidopsis suggests that adjustments to mild drought stress costs only a small fraction of the assimilated C. Since growth is reduced more than photosynthesis, drought may lead to a positive C balance.8,18 Thus, analysis of drought responses in Arabidopsis under mild stress conditions may barely result in the identification of factors involved in the C starvation response.
The above-mentioned experimental conditions vary substantially. In a direct comparison of the genomic responses to submergence and an unanticipated shift to darkness, the shift to darkness altered the global expression patterns much more than the submergence, although the submergence treatment also was performed in darkness.19 The submergence-responsive mRNAs only partially overlapped with the dark-regulated transcripts.
EXL1, EXL2, and EXL4 Expression Depends on the C and Energy Status
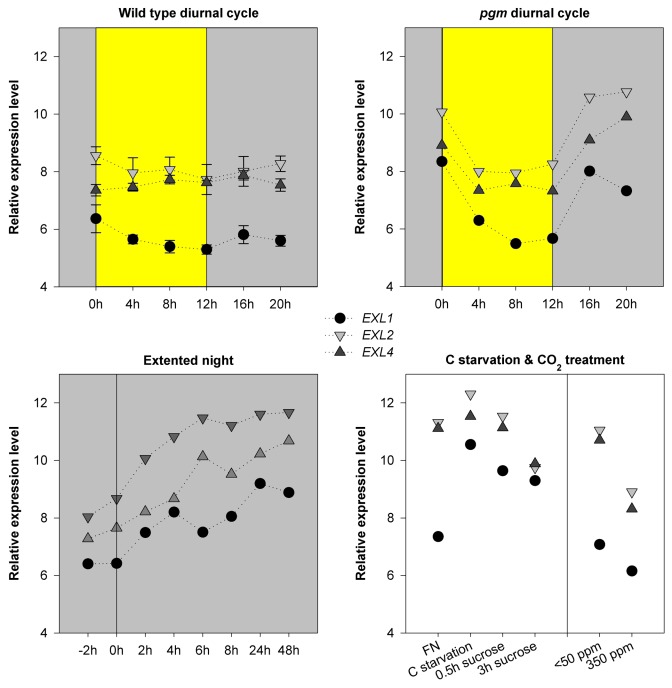
Publicly available gene expression profiles allowed the analysis of EXL1 expression in various situations. EXL1 transcript levels decreased slightly during the light period and increased during the night. This diurnal cycling was intensified in the starch-deficient pgm mutant. EXL1 expression was induced under several conditions that were characterized by low C and energy availability: extended night, low supply of sucrose or glucose to synthetic medium, and low CO2 supply.20-23 Overexpression of SnRK1.1/KIN10 resulted in elevated EXL1 transcript levels.24 EXL1 protein levels were analyzed in parallel to EXL1 transcript levels. An increase in the EXL1 mRNA level was associated with an increase in the protein level under several conditions. Thus, transcriptional regulation of EXL1 confers an adjustment of EXL1 protein levels to the C status.25
EXL1 expression is also induced by hypoxia. Mustroph et al.26 identified ribosome-associated mRNAs by immunoprecipitation of a FLAG-epitope tagged ribosomal protein (FLAG-RPL18). EXL1 expression was hypoxia-induced in all cell types in the shoot, root, and root tip (Fig. 1). Furthermore, Lee et al.19 identified a core of 34 highly induced anaerobic response genes in several hypoxia and submergence samples. This cluster of genes included EXL1 [also termed HYPOXIA-RESPONSIVE UNKNOWN PROTEIN 46 (HUP46)].
Figure 1.EXL1, EXL2, and EXL4 expression in response to hypoxia. Ribosome-associated mRNAs were immunopurified from specific cell populations in the shoot, whole root, or root tip from 7-d-old Arabidopsis seedlings expressing a FLAG-epitope tagged ribosomal protein.26 Seedlings were cultured in the presence (control) or absence of air (2 h hypoxia). Affymetrix ATH1 CEL files (accession number GSE14578) were normalized and log2 transformed using RMA-Express.27 (A) Signal log ratios of relative transcript levels in different root tip cell types (hypoxia vs. control). (B) Signal log ratios of relative transcript levels in different root cell types (hypoxia vs. control). (C) Signal log ratios of relative transcript levels in different shoot cell types (hypoxia vs. control).
Similar to EXL1, the EXL2 and EXL4 transcript levels decreased during the light period, increased during the night and in extended night, and increased upon C starvation (Fig. 2 and Fig. 3). Furthermore, EXL2 transcript was enriched in the ribosome-associated mRNA fraction during hypoxia (Fig. 1). However, tissue and cell type specific expression of EXL2 and EXL4 differs from the EXL1 expression pattern in the absence of stress. For example, EXL2 and EXL4 were strongly expressed in flower organs, and could play a prominent role in reproductive tissues (Fig. 4).
Figure 2.EXL1, EX2 and EXL4 expression in response to C availability. Publicly available expression profiles were normalized and log2 transformed using RMA-Express.27 The means of two or three replicates are shown. The standard error (SE) is shown if three replicates were available. Expression of EXL1 is shown for comparison. FN, full nutrition; C-starv, C starvation.
Figure 3. Quantitative RT-PCR analysis of EXL2 and EXL4 transcript levels in wild-type (Col-0) shoots. The mean of the CT (cycle threshold) values of three reference genes (eIF1α, PDF1, and LRS1) was subtracted from the respective CT value of the gene of interest. Subsequently, differences were subtracted from an arbitrary value (i.e., 40). Higher numbers indicate higher transcript levels. A difference of one unit indicates a fold change of approximately two. Error: SE of gene of interest in three technical replicates. eN: end of night; eD: end of day. (A) Plants were grown in soil under long-day conditions (16 h light/8 h night) and subjected to extended night. RNA was extracted from 28-d-old plants. (B) Plants were grown in soil under a low total irradiance regime [4 h light (60 µmol m−2 s−1)/20 h night]. RNA was extracted from shoots of 28-d-old plants.
Figure 4.EXL1, EXL2 and EXL4 expression in different organs and developmental stages. Wild-type expression profiles of the development series were downloaded from AtGenExpress and normalized and log2 transformed using RMA-Express.27,28 The mean and SD of three replicates are shown. Expression of EXL1 is shown for comparison.
The EXL2 and EXL4 proteins are structurally similar to EXL1. The PHI1 conserved region (PFAM entry PF04674) comprises almost the complete primary structure. It may be composed of several distinct motifs (supplemental material). The molecular function of the region and its putative motifs is unknown. The proteins form a putative N-terminal signal peptide and were identified as extracellular proteins.29,30
EXL1 is Essential for Growth and Survival under Anoxia and C Starvation
Plant growth depends on numerous signaling pathways, intact metabolic pathways, proper allocation and flux of C, nitrogen, and other nutrients, and countless additional processes that ultimately control cell division and cell expansion. The available data do not allow determining the precise mode of EXL1 action. However, the conditional exl1 phenotype, the extracellular localization of the EXL1 protein, and specific EXL1 expression pattern may allow prioritization of hypotheses.
EXL1 promotes growth under conditions that are characterized by low C and energy availability. For example, the exl1 loss of function mutant produced significantly less biomass under a low total irradiance growth regime (4 h light/60 µmol m−2 s−1, 20 h dark) and in synthetic medium supplemented with low sugar concentrations such as 0.2% w/v sucrose.25
The rapid accumulation of EXL1 transcript under starvation conditions corresponds to the essential role of EXL1 under these conditions. This is best exemplified by anoxia experiments. Oxygen-deprivation rapidly induces EXL1 expression in all analyzed organs and cell types (Fig. 1). Survival of the exl1 mutant under oxygen deficiency is significantly impaired. In contrast, survival of the exl1-D knock-on mutant is significantly improved. The exl1 mutant also is impaired in the long-term adaptation to C deprivation. Survival rates under extended night were significantly reduced in comparison to the wild type.25 Thus, EXL1 is involved both in a primary response to energy deprivation and in the long-term adaptation to C starvation.
Putative Function of EXL1
In the context of a sudden C starvation, an ‘acute response’ may serve to rapidly shut down growth and anabolism.9 EXL1 may suppress biosynthetic pathways and the flux of C intermediates into resource-consuming processes. Thus, EXL1 could control C allocation in the cell. Such an ‘acute response’ may be similarly relevant during oxygen deprivation, although the primary requirement during hypoxia is the reduction of oxygen consumption rather than the reduction of C consumption.
The control of C- and energy-consuming biosynthetic pathways could also allow optimal growth under low light or low C supply. Limited supply of light and C requires more efficient use of the available resources and preferential allocation of C into essential processes. Wild-type levels of hexoses, sucrose, and starch were detected in exl1 under low light conditions.25 The exl1 mutant presumably does not suffer from reduced carbohydrate production or from improper diurnal control of C metabolism. However, it cannot be excluded that metabolic flux rates are altered. The reduced growth of the exl1 mutant under low C supply could be a consequence of suboptimal C allocation.
Growth is controlled by phytohormones such as auxin, brassinoteroids (BR), cytokinins, and gibberellins. EXL1 presumably does not have a broad impact on phytohormone levels, because expression levels of phytohormone-responsive genes were not significantly altered in the mutant. However, EXL1 modifies the response to exogenous BR. BR-induced growth was analyzed in synthetic medium supplemented with low or medium sugar levels (i.e., 0.2% or 1% sucrose). The growth response was significantly stronger in the exl1 mutant in comparison to the wild type in the presence of 0.2% sucrose. In contrast, growth was identical to the wild type in the presence of 1% sucrose.25 Thus, EXL1 reduced BR-dependent growth under low C availability.
BR action appears to be intimately associated with C metabolism and C allocation. BR action requires protein synthesis and stimulates cell expansion. BR increases sink strength and stimulates photosynthesis.31-34 Protein biosynthesis and provision of cell wall components require extensive supply of energy and biosynthetic precursors. Increasing photosynthetic capacity is unprofitable under low light or in darkness, and increasing sink strength may be inappropriate under conditions such as anoxia and extended night. Thus, the limitation of BR-dependent processes presumably results in an altered C allocation, reduced C and energy consumption, and may ultimately cause improved survival of anoxia and extended night.
In this way, EXL1 could not only shut down C and energy consumption in frame of an ‘acute’ response, but also tune the balance between supply and demand to optimize the capacity for sustained growth in frame of an ‘acclimatory response’.9 The induction of EXL2 expression by C starvation and hypoxia suggests a similar function of the EXL2 protein. EXL4 expression was strongly induced by C starvation and extended night, but hypoxia stress barely resulted in altered EXL4 mRNA levels. EXL4 function may therefore be less relevant under oxygen deficiency.
Future Perspectives
The available data suggest a role of EXL1 in the control of growth and C allocation. Future time series experiments will analyze the metabolism and BR-dependent growth of exl1 plants under low C and energy availability. The identification of interacting proteins may help to reveal the molecular mode of action of the EXL1 protein.
Materials and Methods
Gene expression analysis
Sequences of primers used for RT-PCR analysis were as follows: EXL2-fw 5′ GCC GTT TCA CCA GCC GAT TTA C 3′, EXL2-rev 5′ TTC CGT CAA CTC CAA CGT CAC C 3′, EXL4-fw 5′ ACG AGT GGA GCG AGT TAC AAC G 3′, and EXL4-rev 5′ AAG TCT GAG GAT CCC ACA ACG C 3′. The eIF1α, PDF1, and LRS1 genes were used to normalize the expression levels. Sequences of primers were as follows: eIF1α-fw 5′ TTG ACA GGC GTT CTG GTA AGG, 3′eIF1α-rev 5′ CAG CGT CAC CAT TCT TCA AAA A 3′, PDF1-fw 5′ ACG TCG CTA AAG TAC TTC AAT CCC 3′, PDF1-rev 5′ CGA ATC GTC TTC TCC ACA ACC G 3′, LRS1-fw 5′ ATG GGC ATT TGA CGA GGA TGC G3′, and LRS1-rev 5′ CGT CGT TCA CCC AGT CAA CAT GAG 3′.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (MU 1738/4–3). We thank Martin Steup for critical reading of the manuscript.
Glossary
Abbreviations:
- BR
brassinosteroids
- C
carbon
- EXL
EXORDIUM-LIKE
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18369
References
- 1.Gout E, Bligny R, Douce R, Boisson A-M, Rivasseu C. Early response of plant cell to carbon deprivation: in vivo 31P-NMR spectroscopy shows a quasi-instantaneous disruption on cytosolic sugars, phosphorylated intermediates of energy metabolism, phosphate partitioning, and intracellular pHs. New Phytol. 2011;189:135–47. doi: 10.1111/j.1469-8137.2010.03449.x. [DOI] [PubMed] [Google Scholar]
- 2.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 3.Stitt M, Gibon Y, Lunn JE, Piques M. Multilevel genomics analysis of carbon signalling during low carbon availability: coordinating the supply and utilisation of carbon in a fluctuating environment. Funct Plant Biol. 2007;34:526–49. doi: 10.1071/FP06249. [DOI] [PubMed] [Google Scholar]
- 4.Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, et al. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–62. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 5.Baena-Gonz´lez E, Sheen J. Convergent energy and stress signalling. Trends Plant Sci. 2008;13:474–82. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halford NG, Hey SJ. Snf1-related protein kinases (SnRK1) act within an intricate network that links metabolic and stress signalling in plants. Biochem J. 2009;419:247–59. doi: 10.1042/BJ20082408. [DOI] [PubMed] [Google Scholar]
- 7.Izumi M, Wada S, Makino A, Ishida H. The autophagic degradation of chloroplasts via Rubisco-containing bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis. Plant Physiol. 2010;154:1196–209. doi: 10.1104/pp.110.158519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010;154:357–72. doi: 10.1104/pp.110.157008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–49. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 10.Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–39. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 11.Smeekens S, Ma J, Hanson J, Rolland F. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol. 2010;13:274–9. doi: 10.1016/j.pbi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Paul M. Trehalose 6-phosphate. Curr Opin Plant Biol. 2007;10:303–9. doi: 10.1016/j.pbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Bailey-Serres J, Voesenek LACJ. Life in the balance: a signaling network controlling survival of flooding. Curr Opin Plant Biol. 2010;13:489–94. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Geigenberger P. Response of plant metabolism to too little oxygen. Curr Opin Plant Biol. 2003;6:247–56. doi: 10.1016/S1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 15.Vashisht D, Hesselink A, Pierik R, Ammerlaan JMH, Bailey-Serres J, Visser EJW, et al. Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytol. 2011;190:299–310. doi: 10.1111/j.1469-8137.2010.03552.x. [DOI] [PubMed] [Google Scholar]
- 16.Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 17.Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Muller B, Pantin F, Ge´nard M, Turc O, Freixes S, Piques M, et al. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot. 2011;62:1715–29. doi: 10.1093/jxb/erq438. [DOI] [PubMed] [Google Scholar]
- 19.Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, et al. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 2011;190:457–71. doi: 10.1111/j.1469-8137.2010.03590.x. [DOI] [PubMed] [Google Scholar]
- 20.Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, et al. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell. 2005;17:3257–81. doi: 10.1105/tpc.105.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibon Y, Usadel B, Bläsing OE, Kamlage B, Höhne M, Trethewey R, et al. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 2006;7:R76. doi: 10.1186/gb-2006-7-8-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, et al. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J. 2007;49:463–91. doi: 10.1111/j.1365-313X.2006.02979.x. [DOI] [PubMed] [Google Scholar]
- 23.Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, et al. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146:1834–61. doi: 10.1104/pp.107.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baena-Gonz´lez E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–42. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 25.Schröder F, Lisso J, Müssig C. EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 2011;156:1620–30. doi: 10.1104/pp.111.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustroph A, Zanetti ME, Jang CJH, Holtan HE, Repetti PP, Galbraith DW, et al. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:18843–8. doi: 10.1073/pnas.0906131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, et al. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–6. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 29.Jamet E, Canut H, Boudart G, Pont-Lezica RF. Cell wall proteins: a new insight through proteomics. Trends Plant Sci. 2006;11:33–9. doi: 10.1016/j.tplants.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Schröder F, Lisso J, Lange P, Müssig C. The extracellular EXO protein mediates cell expansion in Arabidopsis leaves. BMC Plant Biol. 2009;9:20. doi: 10.1186/1471-2229-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz M, Godt DE, Roitsch T. Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J. 2000;22:515–22. doi: 10.1046/j.1365-313x.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 32.Lisso J, Altmann T, Müssig C. Metabolic changes in fruits of the tomato dx mutant. Phytochemistry. 2006;67:2232–8. doi: 10.1016/j.phytochem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Schlüter U, Köpke D, Altmann T, Müssig C. Analysis of carbohydrate metabolism of CPD-antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ. 2002;25:783–91. doi: 10.1046/j.1365-3040.2002.00860.x. [DOI] [Google Scholar]
- 34.Wu CY, Trieu A, Radhakrishnan P, Kwok SF, Harris S, Zhang K, et al. Brassinosteroids regulate grain filling in rice. Plant Cell. 2008;20:2130–45. doi: 10.1105/tpc.107.055087. [DOI] [PMC free article] [PubMed] [Google Scholar]