Abstract
The synthesis of DNA, RNA, and de novo proteins is fundamental for early development of the seedling after germination, but such processes release pyrophosphate (PPi) as a byproduct of ATP hydrolysis. The over-accumulation of the inhibitory metabolite PPi in the cytosol hinders these biosynthetic reactions. All living organisms possess ubiquitous enzymes collectively called inorganic pyrophosphatases (PPases), which catalyze the hydrolysis of PPi into two orthophosphate (Pi) molecules. Defects in PPase activity cause severe developmental defects and/or growth arrest in several organisms. In higher plants, a proton-translocating vacuolar PPase (H+PPase) uses the energy of PPi hydrolysis to acidify the vacuole. However, the biological implications of PPi hydrolysis are vague due to the widespread belief that the major role of H+PPase in plants is vacuolar acidification. We have shown that the Arabidopsis fugu5 mutant phenotype, caused by a defect in H+PPase activity, is rescued by complementation with the yeast cytosolic PPase IPP1. In addition, our analyses have revealed that increased cytosolic PPi levels impair postgerminative development in fugu5 by inhibiting gluconeogenesis. This led us to the conclusion that the role of H+PPase as a proton-pump is negligible. Here, we present further evidence of the growth-boosting effects of removing PPi in later stages of plant vegetative development, and briefly discuss the biological role of PPases and their potential applications in different disciplines and in various organisms.
Keywords: fugu5 mutant, compensation, gluconeogenesis, H+-pyrophosphatase, leaf development, oilseeds, sucrose
Biosyntheses of macromolecules in living cells are characteristically accompanied by liberation of pyrophosphate (PPi), a byproduct of ATP hydrolysis.1-3 PPi is formed in a variety of biosynthetic reactions and is immediately hydrolyzed to orthophosphate by pyrophosphatase (PPase); thus, the cytosolic concentration of PPi is thought to be tightly regulated.4 Nevertheless, the physiological role of PPases remains unclear due to the drastic phenotypes that have been observed in the several PPase loss-of-function mutants of various organisms including Arabidopsis thaliana (Arabidopsis, hereafter).5-8
Role of H+-PPase in Plant Seedling Development
PPases fall into two major classes, soluble PPases and membrane-bound H+translocating PPases (H+PPase), and show no sequence similarity with each other.9 Our previous analysis of the Arabidopsis fugu5 mutant revealed that it is defective in the vacuolar type H+PPase AVP1.10 fugu5 has an altered morphology associated with decreased cell number and increased cell size,11 a phenotype that we have previously called “compensation,”12-15 which is restricted to the cotyledons and the first pair of rosette leaves.10 Interestingly, sucrose alone is sufficient to rescue both the visible and cellular fugu5 mutant phenotypes.10 Moreover, the fugu5 mutant does not show detectable PPi hydrolysis activity, and the vacuolar H+ATPase protein level and its activity do not change, indicating that fugu5 phenotypes are specifically caused by the loss of H+PPase activity. Biochemical analyses have also shown that the amount of sucrose decreases in germinating seedlings of fugu5, whereas PPi accumulates.10 Together, these findings suggest that the elevated level of PPi (~2.5fold increase) inhibits gluconeogenesis (~50% decrease in sucrose levels) in the fugu5 mutant. Finally, we have demonstrated that fugu5 mutant phenotypes are rescued by yeast cytosolic PPase IPP1 expression, which actively hydrolyzes cytosolic PPi but has no effect on vacuolar acidification. Based on these findings, we concluded that the major role of Arabidopsis vacuolar H+PPase during early seedling development is the removal of the inhibitory metabolite PPi rather than vacuole acidification.10
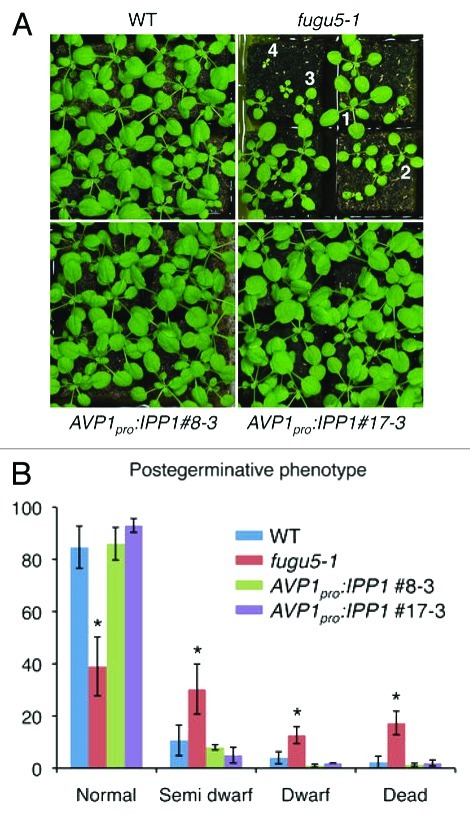
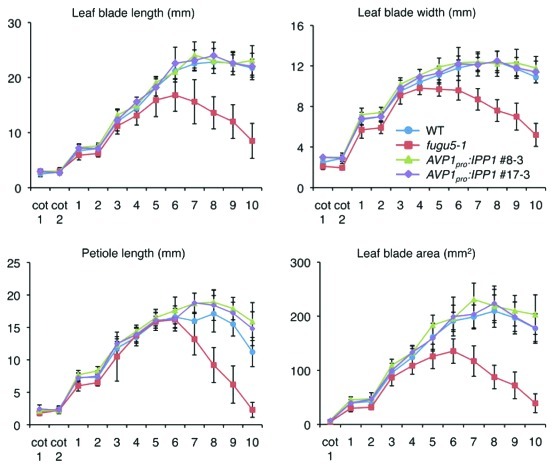
The postgerminative growth phenotype was determined to further assess the effect of the IPP1 gene on plant growth in later stages of vegetative development (Fig. 1). fugu5 plants exhibited delayed growth, whereas this phenotype was totally recovered by introducing the AVP1Pro:IPP1 transgene (Fig. 1).10 We measured leaf blade length, width, area, and petiole length in the first 10 rosette leaves (Fig. 2). Interestingly, our results revealed that the growth of transgenic plants totally recovered to wild-type levels (Fig. 2). These results suggest that fugu5 mutant defects during the early stages of postgerminative development drastically hinder overall plant growth.
Figure 1. Heterologous IPP1 expression rescues fugu5 gross phenotypes. (A) Gross morphology of the wild type, fugu5–1, and two representative lines of AVP1Pro:IPP1 transgenic plants, AVP1pro:IPP1 #8–3 and AVP1pro:IPP1 #17–3, 28 d after sowing (DAS). Plants numbered 1–4 represent those used to determine postgerminative phenotypes. (1) Normal; (2) Semi dwarf; (3) Dwarf; (4) Dead. Normal, plants with the highest growth; semi-dwarf, plant size reduced by ~50% compared with normal plants; dwarf, plant size reduced by ~75% compared with normal plants. (B) Distribution of postgerminative phenotype determined at 21 DAS. Results are means ± SDs (n = 6). Asterisk, p < 0.01 (two-tailed Student ttest).
Figure 2. Heterologous IPP1 gene expression restores the growth delay at later stages of vegetative development. Wildtype, fugu5–1, AVP1pro:IPP1 #8–3, and AVP1pro:IPP1 #17–3 were grown on rockwool, and the cotyledons and the first 10 rosette leaves were collected 31 d after sowing (DAS) to determine leaf blade length, width, area, and petiole length. The results are means ± SDs (n = 10). Note that growth of the fugu5–1 was severely retarded.
In a previous report, the loss-of-function mutant avp1–1 has been reported to show severe phenotypes that could be interpreted as seedling lethal.8 The reported phenotypes were not observed in our fugu5 mutant alleles.10 Two plausible explanations of such striking differences are as follows: First, the TDNA insertion line avp1–1 may have more than one TDNA insertion site. Second, a large genomic region at the vicinity of AVP1 gene was lost and/or rearranged during the TDNA insertion process. As a straightforward way to address this issue, the above possibilities should be carefully checked using the avp1–1 allele. Alternatively, whether AVP1 gene can rescue the avp1–1 mutant phenotypes or not, is fundamentally important to clarify this ambiguous situation.
On the other hand, the AVP1 overexpression has been found to boost plant growth,8 and to confer tolerance against salt and/or drought stress in several plant species including Arabidposis.16-18 Still, it is difficult to discriminate between the contribution of vacuolar acidification and PPi hydrolysis and the observed phenotypes, provided that both activities should be upregulated in the AVP1OX lines. Interestingly, transgenic lines expressing the IPP1 gene alone under the control of AVP1 promoter in fugu5 showed a slightly improved growth that sometimes exceeded that of the wild type.10 Taken together, a direct comparative analysis of the effects of IPP1 and AVP1 overexpression on plant growth and/or stress tolerance should represent a powerful system to properly address the points above.
H+-PPase as a Master Regulator of Cytosolic PPi Homeostasis
The Arabidopsis genome contains three genes that encode two types of H+PPase enzymes, a single gene for the type I enzyme AtVHP1;1/AVP1,8,10 which is exclusively localized on the tonoplast, and two genes for the type II enzyme, AtVHP2;1 and AtVHP2;2, which are exclusively localized on the Golgi apparatus with an extremely low expression level compared with that of AtVHP1;1.19 The physiological contribution of the type II H+PPases in vacuolar acidification and cytosolic PPi hydrolysis is fairly insignificant. This explains the absence of detectable PPi hydrolysis activity in the total membrane fraction of fugu5.10 Therefore, it is evident that AVP1/FUGU5 is a master PPase regulator of cytosolic PPi levels, which is an important result for future studies related to PPi homeostasis in plants.
Molecular cloning and characterization of H+PPase has been also performed in several species of monocots such as barley (Hordeum vulgare) and rice (Oryza sativa).20,21 For example, in the rice genome there are at least 6 genes encoding the type I H+PPase (OsVHP1;1 to OsVHP1;6); with OsVHP1;3 being markedly enhanced in deepwater rice by submersion.21 In the starchy grains of cereals, for example, carbon is stored in the endosperm mainly in the form of starch, but the embryo, scutellum (single large cotyledon) and aleurone layer tissues that constitute a very small part of the total seed weight are enriched in triacylglycerol (TAG).22,23 Therefore, it will be fundamentally important to determine whether H+PPase(s) plays a similarly critical role during the mobilization of starch and/or oil stores in nonoilseed plants.
Implication of H+-PPase in Postgerminative Oilseed Metabolism
Oilseed plants, including Arabidopsis and several food crop plants, such as Helianthus annuus (sunflower), Brassica napus (oilseed rape), Arachis hypogaea (peanut) and Glycine max (soybean) produce seeds that contain major oil reserves in the form of TAG.24 After germination, these lipid stores are broken down providing carbon source for sucrose de novo synthesis in the cytosol (gluconeogenesis), and also intermediate metabolites used for energy production in the mitochondria (respiration).
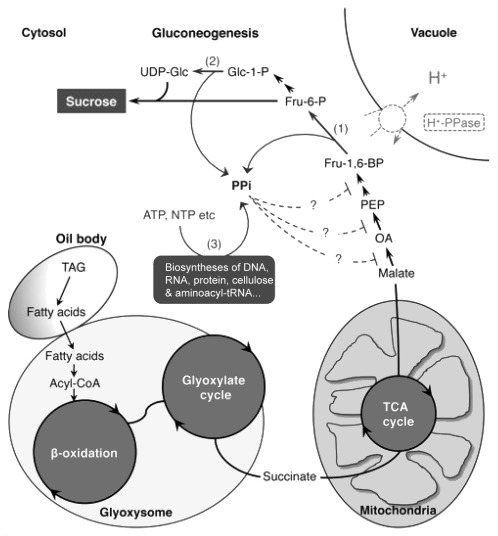
Specialized metabolic pathways involving the βoxidation, the glyoxylate cycle and gluconeogenesis play central role to ensure the efficient and rapid use of seed oil reserves to sustain seedling growth during its transition from heterotrophic to photoautotrophic growth. The enzymes involved in the above pathways have been extensively studied and elucidated. In spite of that, very recently, analyses of the fugu5 mutants have provided the opportunity to discover the metabolic and physiological contribution of the H+PPase during germination and postgerminative growth. Indeed, the removal of cytosolic PPi by the H+PPase has a major contribution for sustaining gluconeogenesis.10 The inhibitory effect of cytosolic PPi accumulation on several biosynthetic reactions has been generally recognized (Fig. 3). For the particular case of gluconeogenesis, the limiting step for sucrose synthesis under high PPi levels has to be determined.
Figure 3. Schematic model of the impact of H+PPase loss-of-function on postgerminative metabolism in oilseeds. The metabolic pathways shown here were taken and modified from Ferjani et al. (2011). TAGs from oil bodies are broken down into fatty acids by the action of lipases, then transported into the glyoxysomes where they are converted to acetyl-CoA by βoxidation and then to succinate by the glyoxylate cycle. Succinate passes from the glyoxysome to the mitochondria and enters the tricarboxylic acid (TCA) cycle, where it is converted to malate. Malate exported to the cytosol is oxidized to oxaloacetate (OA) and then sequentially converted to phosphoenolpyruvate (PEP), fructose 1,6bisphosphate (Fru-1,6-BP); fructose 6phosphate (Fru-6-P); Glucose 1phosphate (Glc-1-P); and finally to UDP-glucose (UDP-Glc). During gluconeogenesis, PPi is generated by the reactions of PPidependent phosphofructokinase (1) and by the reaction of UDPGlc pyrophosphorylase (2). Syntheses of macromolecules, such as cellulose and proteins also generate PPi as a by-product (3). PPi in the cytosol is usually consumed by the H+-PPase. H+-PPase loss-of-function causes an overaccumulation of PPi in the cytosol.10 PPi inhibits cellular metabolism such as reactions (1) and (2) resulting in partial arrest of gluconeogenesis.10 Reaction (3) is also susceptible to PPi inhibiton leading to either arrest or retardation of syntheses of macromolecules. Other metabolic reactions immediately upstream (dotted lines) or downstream of reaction (1) might be a potential target of PPi inhibition.
Focus on Metabolic Significance of PPase Enzymes Toward Unraveling Their Role in Animal Systems
Our findings on plant PPase also provide clues for understanding the roles of animal PPases. In plants, H+PPase activity is generally high in young tissues such as leaf primordia, which are characterized by high proliferative activity.25 It is interesting to note that increased PPase expression in humans is associated with hyperthyroidism26 and many types of cancer.27-29 For example, cytosolic PPase is expressed 7.6fold higher, and three isoforms of triosephosphate isomerase (a key component of the glycolytic pathway) are upregulated 2.1- to 2.7fold higher, in human lung tumors than in normal lung tissues.29 This suggests that the human cytosolic PPase may help clear toxic byproducts resulting from elevated metabolism in these tumors, such as PPi, to sustain cell cycling and other cellular activities. In contrast, the null mutant Caenorhabditis elegans pyp-1 shows developmental arrest at the L2 larval stage and exhibits gross defects in intestinal morphology and function.7 This could explain the PYP1::GFP expression pattern in the intestines and nervous system, as the intestines are highly metabolic organs involved not merely in digestion but also in storage and macromolecule synthesis.7 Therefore, it appears that active synthesis of macromolecules, as a key process, may represent a common target of PPi inhibition leading to the observed phenotypes, given its central role in cellular and organismal growth. By focusing on the metabolic significance of PPases, which we believe are rather well conserved among all living things, we may gain valuable insights into PPase function in different organisms including vertebrates. Alternatively, PPases may represent a novel potential target of many cancers, which would allow the development of new promising specific inhibitors or drug-delivery systems to cure this lethal disease.
Final Remarks
We recently showed that removing PPi is essential to sustain cell proliferation during Arabidopsis germination, a stage in which vacuolar acidification seems to have no contribution.10 However, the remainder of the compensation mechanism (i.e., excessive cell enlargement) remains unclear and will be addressed in future research. In our analyses, plants were always grown under standard culture conditions. Therefore, it would be of great interest to determine whether H+PPase plays a role as a proton pump under particular growth conditions, such as drought or other kinds of abiotic stress. These results might deepen our understanding of the evolutionary significance of having a vacuolar membrane-bound H+PPase rather than a cytosolic PPase in plants.
Acknowledgments
This work was supported by Grant-in-Aid for Young Scientists (B) (to A.F.), Creative Scientific Research (to H.T.), Scientific Research (A, to H.T. and G.H.; B, to M.M.), Scientific Research on Priority Areas (to H.T.), Young Scientists (B) and Exploratory Research (to G.H.), and the Ministry of Education, Culture, Sports, Science, and Technology of Japan, as well as grants from the Bio-Design Program of the Ministry of Agriculture, Forestry, and Fisheries of Japan (to H.T.) and the Toray Science Foundation (to H.T.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18573
References
- 1.Maeshima M. Vacuolar H+-pyrophosphatase. Biochim Biophys Acta. 2000;1465:37–51. doi: 10.1016/S0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 2.Heinonen JK. Biological Role of Inorganic Pyrophosphate. Boston/Dordrecht/London: Kluwer Academic Publishers 2001. [Google Scholar]
- 3.Kornberg A. On the metabolic significance of phosphorolytic and pyrophosphorolytic reactions. In: Kasha H, Pullman B (eds) Horizons in biochemistry. Academic Press, New York, 1962; pp 251-264. [Google Scholar]
- 4.Josse J, Wong SCK. Inorganic pyrophosphatase of Escherichia coli In: Boyer PD (ed) The enzymes (3rd ed), Academic Press, New York, 1971; pp 499-527. [Google Scholar]
- 5.Chen J, Brevet A, Fromant M, Le´vêque F, Schmitter JM, Blanquet S, et al. Pyrophosphatase is essential for growth of Escherichia coli. J Bacteriol. 1990;172:5686–9. doi: 10.1128/jb.172.10.5686-5689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundin M, Baltscheffsky H, Ronne H. Yeast PPA2 gene encodes a mitochondrial inorganic pyrophosphatase that is essential for mitochondrial function. J Biol Chem. 1991;266:12168–72. [PubMed] [Google Scholar]
- 7.Ko KM, Lee W, Yu JR, Ahnn J. PYP-1, inorganic pyrophosphatase, is required for larval development and intestinal function in C. elegans. FEBS Lett. 2007;581:5445–53. doi: 10.1016/j.febslet.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, et al. Arabidopsis H+-PPase AVP1 regulates auxin mediated organ development. Science. 2005;310:121–5. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 9.Islam MK, Miyoshi T, Kasuga-Aoki H, Isobe T, Arakawa T, Matsumoto Y, et al. Inorganic pyrophosphatase in the roundworm Ascaris and its role in the development and molting process of the larval stage parasites. Eur J Biochem. 2003;270:2814–26. doi: 10.1046/j.1432-1033.2003.03658.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H. Keep an eye on PPi: The vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell. 2011;23:2895–908. doi: 10.1105/tpc.111.085415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferjani A, Horiguchi G, Yano S, Tsukaya H. Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol. 2007;144:988–99. doi: 10.1104/pp.107.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukaya H. Relationship between shape of cells and shape of the organ. In Phytohormones and Cell Shape. H. Imazeki and H. Shibaoka, eds (Tokyo: Gakkai-shuppann Center), 1998; pp. 177-184 [In Japanese]. [Google Scholar]
- 13.Tsukaya H. Interpretation of mutants in leaf morphology: Genetic evidence for a compensatory system in leaf morphogenesis that provides a new link between cell and organismal theories. Int Rev Cytol. 2002;217:1–39. doi: 10.1016/S0074-7696(02)17011-2. [DOI] [PubMed] [Google Scholar]
- 14.Ferjani A, Yano S, Horiguchi G, Tsukaya H. Control of leaf morphogenesis by long- and short-distance signaling: Differentiation of leaves into sun or shade types and compensated cell enlargement. In Plant Cell Monographs: Plant Growth Signaling, L. Bögre and G.T.S. Beemster, eds (Berlin, Heidelberg, Germany: Springer Berlin Heidelberg), 2008; pp. 47-62. [Google Scholar]
- 15.Ferjani A, Horiguchi G, Tsukaya H. Organ size control in Arabidopsis: Insights from compensation studies. Plant Morphology. 2010;22:65–71. doi: 10.5685/plmorphol.22.65. [DOI] [Google Scholar]
- 16.Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, et al. Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol J. 2011;9:88–99. doi: 10.1111/j.1467-7652.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, et al. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA. 2005;102:18830–5. doi: 10.1073/pnas.0509512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, et al. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA. 2001;98:11444–9. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segami S, Nakanishi Y, Sato MH, Maeshima M. Quantification, organ-specific accumulation and intracellular localization of type II H+-pyrophosphatase in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:1350–60. doi: 10.1093/pcp/pcq096. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y, Chiba K, Maeda M, Maeshima M. Molecular cloning of cDNA for vacuolar membrane proton-translocating inorganic pyrophosphatase in Hordeum vulgare. Biochem Biophys Res Commun. 1993;190:1110–4. doi: 10.1006/bbrc.1993.1164. [DOI] [PubMed] [Google Scholar]
- 21.Muto Y, Segami S, Hayashi H, Sakurai J, Murai-Hatano M, Hattori Y, et al. Vacuolar proton pumps and aquaporins involved in rapid internode elongation of deepwater rice. Biosci Biotechnol Biochem. 2011;75:114–22. doi: 10.1271/bbb.100615. [DOI] [PubMed] [Google Scholar]
- 22.Cornah JE, Smith SM. Synthesis and function of glyoxylate cycled enzymes. In Plant Peroxisomes, A. Baker and I.A. Graham, eds (London: Kluwer Academic Publishers), 2002; pp. 57-101. [Google Scholar]
- 23.Ekman A, Hayden DM, Dehesh K, Bülow L, Stymne S. Carbon partitioning between oil and carbohydrates in developing oat (Avena sativa L.) seeds. J Exp Bot. 2008;59:4247–57. doi: 10.1093/jxb/ern266. [Erratum in: J Exp Bot 2009; 60: 350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastmond PJ, Graham IA. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001;6:72–8. doi: 10.1016/S1360-1385(00)01835-5. [DOI] [PubMed] [Google Scholar]
- 25.Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. J Exp Bot. 2007;58:83–102. doi: 10.1093/jxb/erl183. [DOI] [PubMed] [Google Scholar]
- 26.Koike E, Toda S, Yokoi F, Izuhara K, Koike N, Itoh K, et al. Expression of new human inorganic pyrophosphatase in thyroid diseases: its intimate association with hyperthyroidism. Biochem Biophys Res Commun. 2006;341:691–6. doi: 10.1016/j.bbrc.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Tomonaga T, Matsushita K, Yamaguchi S, Oh-Ishi M, Kodera Y, Maeda T, et al. Identification of altered protein expression and post-translational modifications in primary colorectal cancer by using agarose two-dimensional gel electrophoresis. Clin Cancer Res. 2004;10:2007–14. doi: 10.1158/1078-0432.CCR-03-0321. [DOI] [PubMed] [Google Scholar]
- 28.Lexander H, Palmberg C, Auer G, Hellstrom M, Franzen B, Jornvall H, et al. Proteomic analysis of protein expression in prostate cancer. Anal Quant Cytol Histol. 2005;27:263–72. [PubMed] [Google Scholar]
- 29.Chen G, Gharib TG, Huang CC, Thomas DG, Shedden KA, Taylor JM, et al. Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin Cancer Res. 2002;8:2298–305. [PubMed] [Google Scholar]