Abstract
Plant viral symptoms are rarely explained by direct molecular interaction between a viral protein and a host factor, but rather understood as a consequence of arms race between host RNA silencing and viral silencing suppressors. However, we have recently demonstrated that the 2b protein (2b) of Cucumber mosaic virus (CMV) HL strain could bind to Arabidopsis catalase that is important in scavenging cellular hydrogen peroxide, leading to the induction of distinct necrosis on Arabidopsis. Because we previously used virulent strains of subgroup I CMV in the study, we here further analyzed mild strains of subgroup II CMV, which share 70 to 80% sequence homology with subgroup I, to understand whether the necrosis induction is a general phenomenon to compromise host defense system mediated by catalase in the pathosystem of any CMV strains and Arabidopsis. Based on the results, we concluded that 2bs of subgroup II could also bind to catalase, resulting in decrease in catalase activity and weak necrosis on Arabidopsis. Because the 2b-catalase interaction did not prevent CMVs from spreading, it may eventually operate in favor of CMV.
Keywords: Cucumber mosaic virus, 2b, Arabidopsis, catalase, necrosis
The molecular mechanism for viral pathogenicity can be explained mainly by host RNA silencing (RS) and viral RS suppressors (RSSs). The major viral symptom is mosaics of light and dark green areas on the infected leaves. In the dark green areas, cells are resistant to subsequent viral infection due to host RS. Viral systemic symptoms are produced by a balance between RS and suppression of RS by viral RSSs. It is likely that the perturbation of the miRNA pathway, which controls plant differentiation, causes some symptom expression. Differences in symptoms between viral strains are attributed to differences in the interaction between viral RSSs and the miRNA pathway.1,2
On the other hand, there are rarely some cases where a viral protein interferes directly with the intrinsic function of a specific host protein, leading to some symptom production. In one such example, Zhu et al. (2005)3 previously demonstrated that the P2 protein of Rice dwarf virus (RDV) directly interacted with rice ent-kaurene oxidases, which play a role in the biosynthesis of gibberellin, and that this interaction led to a decrease in the hormones and stunting of the infected rice. Recently we also provided evidence that the 2b protein of CMV can bind to Catalase3 (CAT3) that is important in scavenging cellular hydrogen peroxide, resulting in the induction of a specific necrosis in the leaves of Arabidopsis.4,5 2b is known to be a RSS1,6,7 and also involved in viral movement and symptom induction (reviewed in ref. 8).
CMV contains three genomic RNAs (RNAs 1 to 3) and produces two subgenomic RNAs, RNA 4 for coat protein and RNA 4A for 2b.8 Based on the sequence homology, CMVs are classified into three subgroups, IA, IB and II.9 Subgroup I CMVs are found to be more virulent than subgroup II strains, and the 2b gene is the major determinant of viral virulence.10 The 2b protein of a subgroup I strain tends to show stronger RSS activity than that of a subgroup II strain.11 We recently showed that 2b of a lily strain of CMV (CMV-HL), which belongs to subgroup I, could induce distinct necrotic spots on infected Arabidopsis leaves, and that 2bs of other subgroup I strains such as CMV-Y could also induce similar spots but they were not as distinct as those induced by 2b of CMV-HL (HL2b).12 This raises the question of whether milder CMV strains of subgroup II can still induce necrosis on Arabidopsis, and thus whether any CMVs can, in general, induce necrosis on Arabidopsis. Here we investigated the ability of two 2b proteins (Q2b and M2b) belonging to subgroup II CMVs to cause necrosis on Arabidopsis leaves and compared them with HL2b.
2b of subgroup II CMVs can also induce necrosis in Arabidopsis
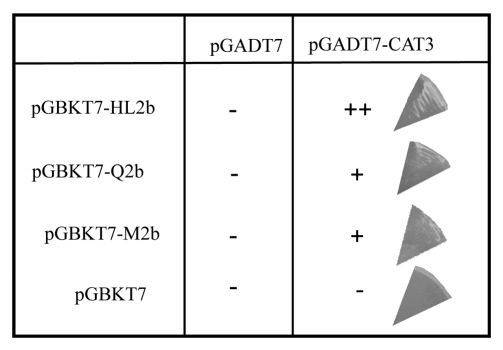
To answer the above questions, we first conducted inoculation experiments. Because the 2b protein has been found to be the determinant for the necrosis on Arabidopsis,12 we cloned two 2b genes of CMV-Q13 and CMV-m2,14 which belong to subgroup II and then inserted the clones into the CMV RNA2-based vector H1 that lacks the entire 2b gene15 to create H1-Q2 and H1-M2, respectively. We can thus avoid the possible involvement of other viral genes in the necrosis and compare the necrosis-inducing ability of 2b in the same viral backbone. To produce infectious CMV RNAs, we used in vitro transcribed viral RNAs 1 to 3 from the plasmids containing the viral full-length cDNAs16; the transcript from H1-Q2 (or H1-M2) was synthesized, and mixed with the transcripts of CMV-Y RNAs 1 (Y1) and 3 (Y3) for inoculation. The vector H1 itself did not induce necrosis on Arabidopsis (Fig. 1A). When Arabidopsis (Col-0) plants were inoculated with Y1H1-Q2Y3 or Y1H1-M2bY3, as shown in Figure 1B, the infected plants actually developed weak necrosis on the upper leaves, which was as distinct as those induced by CMV-Y (subgroup I). This observation suggests that 2bs of even subgroup II CMVs, which normally cause much milder symptoms on their hosts, have the potential to induce necrosis in Arabidopsis. We next tested the ability of Q-2b and M2–2b to interact with CAT3 in yeast two-hybrid assay as demonstrated for subgroup I CMV strains by Inaba et al. (2011).12 Figure 2 showed that both Q2b and M2b could indeed bind to CAT3 although the binding was weaker than that between CAT3 and HL2b (subgroup I) in the assay. To analyze the effect of 2b’s binding to CAT3 on catalase activity, we then measured catalase activity in virus-infected leaves. As expected, catalase activity was decreased at 13 dpi in the plants infected both with Y1H1-Q2bY3 and with Y1H1-M2bY3 (Fig. 3). When compared with the mock- or H1-infected control plants,12 decrease levels of catalase activity were similar to that in CMV-Y-infected plants but not comparable with CMV-HL because in CMV-HL-infected plants, the catalase level was decreased down to less than 50% of the control. These results suggest that 2bs of subgroup II CMVs can decrease host catalase activities at least as efficiently as 2b of CMV-Y (Y2b, subgroup I).
Figure 1.
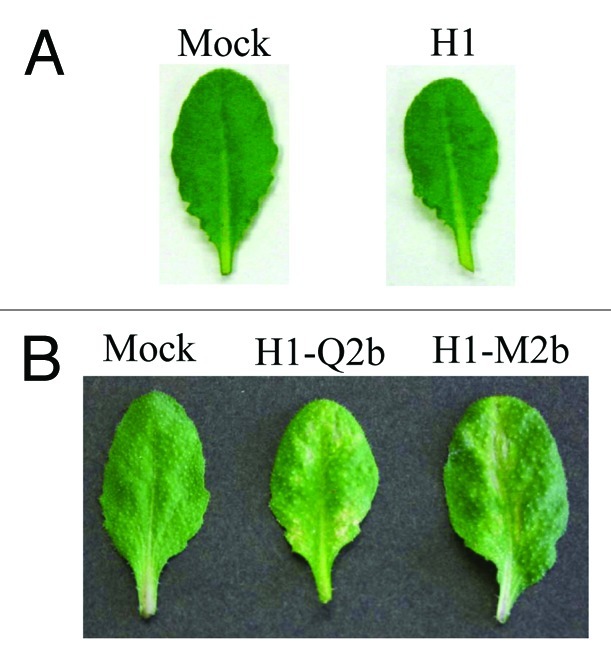
Necrotic symptoms on Arabidopsis Col-0 plants infected with mock, H1, H1-Q2b and H1-M2b at 13 d post-inoculation (dpi). (A) The CMV vector H1 did not induce necrosis at 13 dpi. (B) H1-Q2 and H1-M2b are H1 containing CMV-Q 2b and CMV-M 2b, respectively.
Figure 2.
Interaction between CMV 2b and CAT3 in the yeast two-hybrid assay. Each plasmid (pGBKT7-HL2b, pGADT7-Q2b, and pGADT7-M2b) was co-transferred with pGADT7 (control) or pGADT7-CAT3 into yeast strain AH109. The transformed AH109 cells were plated on the growth medium without leucine, tryptophan, histidine and adenine (-LWHA, right panel). + and – indicate that yeast growth was positive and negative, respectively. For the 2b’s interactions with pGADT7-CAT3, yeast colonies on each medium were indicated at right.
Figure 3.
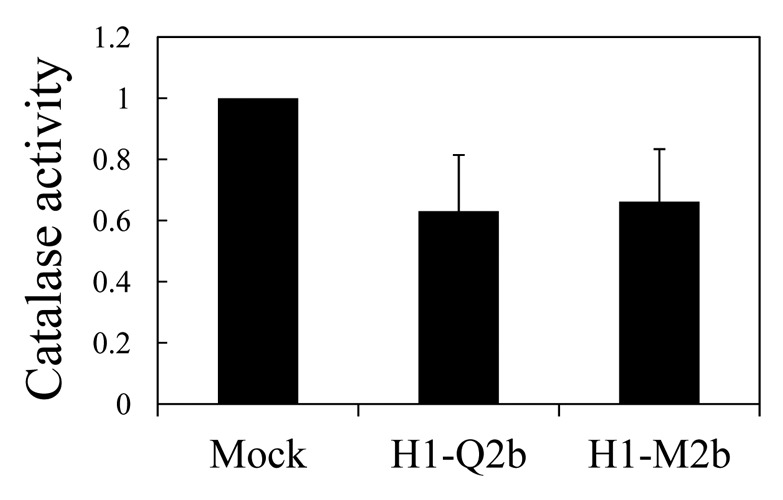
Catalase activity in the Col-0 plants infected with the recombinant CMVs that produce the 2b proteins of subgroup II CMVs. Catalase activity was determined in mock-, H1-M2b-, and H1-Q2b-inocualted plants by measuring the decrease in absorbance at 240 nm 13 dpi and expressed as mmol⋅−1min⋅−1mg protein. Three replicates were done. Error bars represent standard errors of the means. H1-Q2b and H1-M2b were inoculated together with Y1 and Y3. The catalase activities in mock-, H1- and H1-HL2b-inoculated plants have been previously compared (Fig. 4A in ref. 12). There was little difference in catalase activity between mock- and H1-inoculated plants, but the level of catalase activity was almost half in H1-HL2b-inoculated plants compared with the control.
Is such necrosis as a consequence of the CAT3–2b interaction in favor of virus or host?
We started this study when we observed very distinct necrotic spots on Col-0 plants infected with CMV-HL.12 In our previous inoculation experiments, all three Arabidopsis ecotypes (Col-0, C24 and Ler) used in the study were found to develop such necrosis; in Ler, the necrosis was most severe. Although we found that in addition to HL2b, Y2b could also induce necrosis on Col-0 but to a lesser extent, there was still the possibility that the necrosis was rather a specific reaction only to some particular CMV strains. Here we demonstrated that such necrosis was also induced by subgroup II CMVs, and thus this phenomenon was rather general in the pathosystem of CMV and Arabidopsis. We believe that this phenomenon is a consequence after a long arms race between CMV and Arabidopsis in their evolutionary histories.
Although the necrosis seems to be associated with host defense response because mRNA levels of some pathogenesis-related (PR) proteins were elevated,12 the observed cell death is not effective against CMV at all, suggesting that 2b’s binding to catalase may be working mainly for sequestering catalase in cells in favor of viral infection. However, there is also the possibility that the induced resistance may function for other pathogens including bacteria and fungi; in this case, necrosis may operate in favor of host.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18526
References
- 1.Zhang X, Yuan Y-R, Pei Y, Lin S-S, Tuschl T, Patel DJ, et al. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006;20:3255–68. doi: 10.1101/gad.1495506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimura H, Pantaleo V. Viral induction and suppression of RNA silencing in plants. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagrm.2011.04.005. In press. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Gao F, Cao X, Chen M, Ye G, Wei C, et al. The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol. 2005;139:1935–45. doi: 10.1104/pp.105.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du YY, Wang PC, Chen J, Song CP. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol. 2008;50:1318–26. doi: 10.1111/j.1744-7909.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 5.Contento AL, Bassham DC. Increase in catalase-3 activity as a response to use of alternative catabolic substrates during sucrose starvation. Plant Physiol Biochem. 2010;48:232–8. doi: 10.1016/j.plaphy.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ji L-H, Ding S-W. The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant Microbe Interact. 2001;14:715–24. doi: 10.1094/MPMI.2001.14.6.715. [DOI] [PubMed] [Google Scholar]
- 7.Goto K, Kobori T, Kosaka Y, Natsuaki T, Masuta C. Characterization of silencing suppressor 2b of Cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol. 2007;48:1050–60. doi: 10.1093/pcp/pcm074. [DOI] [PubMed] [Google Scholar]
- 8.Palukaitis P, García-Arenal F. Cucumoviruses. Adv Virus Res. 2003;62:241–323. doi: 10.1016/S0065-3527(03)62005-1. [DOI] [PubMed] [Google Scholar]
- 9.Roossinck MJ. Evolutionary history of Cucumber mosaic virus deduced by phylogenetic analyses. J Virol. 2002;76:3382–7. doi: 10.1128/JVI.76.7.3382-3387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi BJ, Palukaitis P, Symons RH. Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol Plant Microbe Interact. 2002;15:947–55. doi: 10.1094/MPMI.2002.15.9.947. [DOI] [PubMed] [Google Scholar]
- 11.Ye J, Qu J, Zhang JF, Geng YF, Fang RX. A critical domain of the Cucumber mosaic virus 2b protein for RNA silencing suppressor activity. FEBS Lett. 2009;583:101–6. doi: 10.1016/j.febslet.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Inaba J, Kim BM, Shimura H, Masuta C. Virus-Induced Necrosis is a consequence of direct protein-protein interaction between a viral RNA-silencing suppressor and a host catalase. Plant Physiol. 2011;156:2026–36. doi: 10.1104/pp.111.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding SW, Anderson BJ, Haase HR, Symons RH. New overlapping gene encoded by the cucumber mosaic virus genome. Virology. 1994;198:593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita M, Shigemune N, Kikuhara K, Furuya N, Takanami Y. Spatial analysis for exclusive interactions between subgroups I and II of Cucumber mosaic virus in cowpea. Virology. 2004;328:45–51. doi: 10.1016/j.virol.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Hong J-S, Tabayashi N, Ito A, Masuta C, Matsumura T. Development of Cucumber mosaic virus as a vector modifiable for different host species to produce therapeutic proteins. Planta. 2007;225:277–86. doi: 10.1007/s00425-006-0346-5. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Kuwata S, Kataoka J, Masuta C, Nitta N, Takanami Y. Functional analysis of deletion mutants of cucumber mosaic virus RNA 3 using an in vitro transcription system. Virology. 1991;183:106–13. doi: 10.1016/0042-6822(91)90123-S. [DOI] [PubMed] [Google Scholar]