Abstract
Boron (B) is essential for plants, but is toxic in excess. Plants have to strictly regulate the uptake and translocation of B. In Arabidopsis thaliana root cells, a boric acid channel, NIP5;1, and a boric acid/borate exporter, BOR1, localize to the outer (facing soil) and inner plasma membrane domains, respectively, under B limitation. The opposite polar localizations of the importer and exporter would enable plant roots to transport B efficiently toward the xylem. In addition, accumulation of the B transporters is controlled by B conditions. When plants are shifted from low to high B conditions, NIP5;1 transcript accumulation is downregulated through mRNA degradation. The BOR1 protein is transported to the transGolgi network/early endosome and multivesicular body and finally degraded in the vacuole. We have recently shown that both the polar localization and the endocytic degradation of BOR1 are controlled by at least two tyrosine residues in a large loop located in the cytosol. We also showed that ubiquitination is required for the endocytic degradation of BOR1. Here, we analyzed possible involvement of an additional tyrosine residue (Y414) in the loop region and discuss the pathway of the BOR1 trafficking for polar localization and endocytic degradation of BOR1.
Keywords: endocytosis, membrane trafficking, mineral transporter, polar localization, tyrosine-based signal, Ubiquitination
Polar Localization of Boron Transporters
Transporters are required for uptake and translocation of essential minerals in plants. For the directional transport of minerals across root cells, polar localization of transporters in the plasma membrane (PM) appears to be important. For example, a potato phosphate transporter, PT2, and a maize ferricphytosiderophore transporter, YS1, localize in the outer PM domain (facing soil) of root epidermal cells.1,2 This outer polar localization would be beneficial for uptake of minerals from the soil solution. Furthermore, there are examples of opposite polar localization of an importer and an exporter in root cells. A silicic acid channel, Lsi1 (OsNIP2;1), and a silicon exporter, Lsi2, localize to the outer and inner PM domains, respectively, in both the exodermis and endodermis of rice roots.3 A boric acid channel NIP5;1 and a borate exporter BOR1 also localize to outer and inner PM domains, respectively, in various types of root cell in Arabidopsis thaliana.4 The polar localization of importers and exporters indicates the radial transport route of nutrients from soil to xylem, and possibly represents a common system for other mineral nutrients.
How is outer/inner polarity maintained in the PM of plant cells? One possibility is a separation of outer and inner PM domains accompanying the polar trafficking of the transporters. In differentiated endodermal cells in roots, the Casparian strip functions as an apoplastic diffusion barrier and also as a diffusion barrier for the PM.5,6 The Casparian strip clearly separates outer and inner PM domains when visualized with fluorescent-protein tagged NIP5;1 and BOR1, respectively. Importantly, transporters including NIP5;1 and BOR1 show outer/inner polarity also in cells without an apparent diffusion barrier,4,7,8 suggesting that additional mechanisms are present for retention or recycling. These mechanisms are largely unknown but may be similar to those for apical/basal polarity of auxin transporters.9
Boron-Dependent Degradation of Boron Transporters
Another interesting feature for localization of boron (B) transporters is B-dependent degradation of BOR1. When plants were transferred from low B conditions to high B conditions, BOR1GFP was transported from the PM to the multivesicular body (MVB)/late endosome (LE), and then to the vacuole for degradation.4,10 Immunogold electron microscopy detected BOR1GFP in the transGolgi network (TGN)/early endosome (EE) and intralumenal vesicles of the MVB/LE after high B supply.11 These data suggest high B-induced trafficking of BOR1 from the PM to TGN/EE, then to intralumenal vesicles of the MVB/LE, and finally to the vacuolar lumen for degradation. In contrast, accumulation of NIP5;1 is regulated mainly through B-dependent degradation of NIP5;1 mRNA.12 Consistently, disappearance of GFPNIP5;1 after high B supply was slower than that of BOR1GFP.4 The downregulation of B transporters is apparently important for preventing accumulation of B to toxic levels.
Specific Tyrosine Residues in BOR1 Play Important Roles in BOR1 Trafficking
In our previous report, we demonstrated that the polar localization of BOR1 requires tyrosine residues in a large loop region that is most probably located in the cytosol.4 Interestingly, the same residues are also required for the B-dependent degradation of BOR1. These tyrosine residues (Y373, Y398, and Y414) of BOR1 probably function as tyrosine-based signals. The tyrosine-based signal YxxΦ, where Y is tyrosine, x is any amino acid, and Φ is any bulky hydrophobic residue, is well established as a membrane protein signal for endocytosis, sorting to lysosomes, and also polar PM sorting in mammals.13,14 The tyrosine-based signals are recognized by a medium (µ) subunit of adaptor protein (AP) complexes for sorting into coated vesicles and different postGolgi transport pathways.
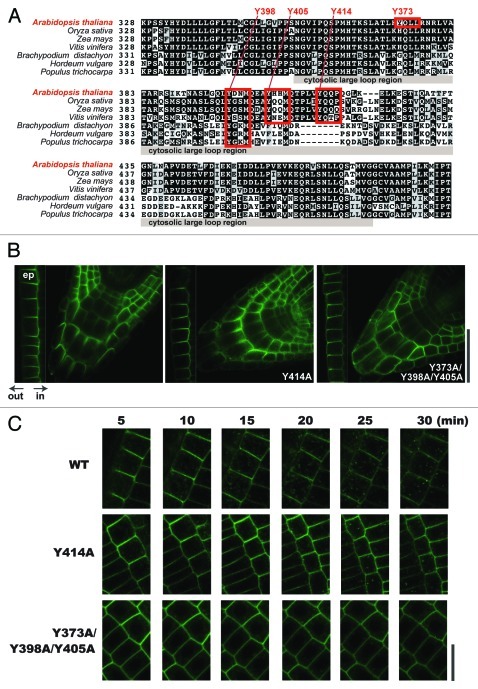
To further investigate the regulatory mechanisms of BOR1 trafficking, we compared amino acid sequences of the cytosolic large loop region of BOR1 to those of BOR1 homologs in various plant species (Fig. 1A). Consistent with the results that Y398 and Y405 are especially important for BOR1 trafficking,4 Y398 and Y405, but not Y373, were highly conserved among the BOR1 homologs. We also noticed that a fourth tyrosine residue, Y414, in the large loop was highly conserved. Y414QQP may function as a tyrosine-based signal or a tyrosine phosphorylation site. In mammal and yeast systems, Src kinase recognizes YxxP motifs and tyrosine phosphorylation is involved in endocytosis of membrane proteins.15,16 Although there is no full-length homolog of Src kinase in the Arabidopsis genome, there are genes that encode src homology3 (SH3) -containing proteins.17
Figure 1. Effect of Y414A substitution on the polar localization and endocytic degradation of BOR1. (A) Amino acid sequences of the cytosolic large loop region of BOR1 and its homologs. The sequences were aligned using the Clustal W program (clustalw.ddbj.nig.ac.jp/). Putative tyrosine-based signals are highlighted. (B) Involvement of tyrosine-based signals in polar localization of BOR1. BOR1GFP with tyrosine–to–alanine substitution in elongating epidermal cells (ep; left end of each panel) and root tips under low B conditions. Transgenic lines expressing BOR1GFP(WT), BOR1(Y414A)GFP, and BOR1(Y373A/Y398A/Y405A)GFP under control of the BOR1 promoter are shown. Plants were grown on vertically placed solid medium containing 0.3 µM boric acid, 1% sucrose, and 1.5% Gellan gum (Wako Pure Chemicals) for 5 d. Other growth conditions were the same as in the previous work.4 (C) Time-course analysis of the subcellular localization of BOR1GFP(WT), BOR1(Y414A)GFP, and BOR1(Y373A/Y398A/Y405A)GFP after high B supply. Plants were grown on solid medium containing 0.3 µM boric acid for 5 d. The plants were then transferred to solid medium containing 100 µM boric acid and 0.5% Gellan gum. Time after shifting to high B medium is indicated. Laser scanning confocal microscopy was performed using a Zeiss LSM510 META. Excitation and detection wavelengths for GFP were 488 nm and 505–530 nm (band path), respectively. Scale bars indicate 50 µm (B) and 20 µm (C), respectively.
To investigate the possible importance of the Y414 residue, we generated transgenic plants expressing BOR1(Y414A)GFP under control of the BOR1 promoter. Under low B conditions, the BOR1(Y414A)GFP showed polar localization indistinguishable from wild-type BOR1GFP in root cap cells and elongating endodermal cells, while the BOR1(Y373A/Y398A/Y405A)GFP showed apolar localization, as previously reported (Fig. 1B). Then we developed a method for time-course analysis of degradation after shifting to high B medium in epidermal cells. Plants grown on solid media containing 0.3 µM B were transferred to glass-bottom dishes, and then the roots were covered with media solidified with 0.5% Gellan gum containing 100 µM B and observed with an inverted microscope. Under this condition, the speckled endosomes stained with wild-type BOR1GFP apparently increased 15 min after the high B supply, illustrating the rapid response to B (Fig. 1C). BOR1(Y414A)GFP also showed rapid internalization, while BOR1(Y373A/Y398A/Y405A)GFP was not internalized. Therefore, Y414 does not seem to be involved in polar localization or vacuolar trafficking.
BOR1 Trafficking Pathway
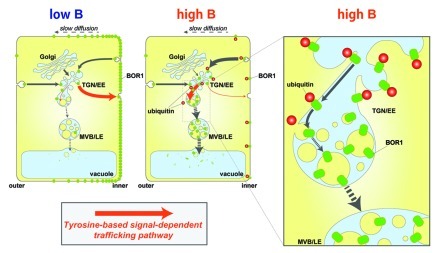
We have shown that at least two well-conserved tyrosine residues of BOR1 are required for both polar localization and B-dependent degradation of BOR1.4 Furthermore, we recently demonstrated the requirement for ubiquitination at K590 in a C-terminal region for B-dependent degradation but not for polar localization.18 Ubiquitination of membrane proteins functions as a signal for endocytosis (internalization from PM) and MVB sorting (entry into the intraluminal vesicles of an MVB) in animal and yeast cells.19,20 We reported that inhibition of the recycling pathway by brefeldin A visualized accumulation of both BOR1(Y373A/Y398A/Y405A)GFP and BOR1(K590A)GFP in endosomal aggregation similar to the case for wild-type BOR1GFP,4,18 suggesting that the tyrosine-based signals and ubiquitination are not required for endocytosis from the PM. Although there are various possibilities, we propose a model of BOR1 trafficking considering a recent important finding that the MVB/LE matures from TGN/EE21 (Fig. 2). Under low B conditions, the tyrosine-based signals bind with an AP in the TGN/EE, and BOR1 is directed to the polar recycling pathway. Under high B conditions, the same tyrosine-based signals bind with another AP, and BOR1 is recruited toward a TGN/EE sub-compartment, which becomes the MVB/LE. Moreover, the ubiquitination of BOR1 at K590 accelerates MVB sorting. The MVB fuses with the vacuole21 and the intraluminal vesicles containing BOR1 are released into the vacuolar lumen.
Figure 2. A hypothetical model of the BOR1 trafficking pathway. Under low B conditions, the polar localization of BOR1 to the inner PM domain is maintained by polar recycling from the transGolgi network/early endosome (TGN/EE) and the pathway is dependent on the tyrosine-based signals. Relatively slow diffusion in the PM4 also seems to be important for the polar localization. Under high B conditions, BOR1 is transported to a sub-compartment of the TGN/EE, which becomes a multivesicular body/late endosome (MVB/LE). This pathway also requires the tyrosine-based signals. BOR1 is ubiquitinated under high B conditions and the ubiquitination accelerates BOR1 sorting into the intraluminal vesicles of an MVB. The MVB fuses with vacuoles and the intraluminal vesicles containing BOR1 are released into the vacuolar lumen.
Clearly, identification of regulators for the polar trafficking of BOR1 and the components of Bsensing machinery is required to further elucidate BOR1 trafficking mechanisms. The study of B transporters will help to reveal common mechanisms of transporter trafficking and endocytic pathways in plant cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge excellent technical assistance by Kayo Konishi and Yuko Kawara, and critical reading by Shinji Wakuta. This work is supported in part by the NEXT program form the Japanese Society for the Promotion of Science (JSPS, to J.T.) and a Grant-in-Aid for Scientific Research S from the Ministry of Education, Culture, Sports, Science and Technology (to T.F.).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18527
References
- 1.Ueno D, Yamaji N, Ma JF. Further characterization of ferric-phytosiderophore transporters Zm YS1 and HvYS1 in maize and barley. J Exp Bot. 2009;60:3513–20. doi: 10.1093/jxb/erp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon-Weeks R, Tong YP, Davies TGE, Leggewie G. Restricted spatial expression of a high-affinity phosphate transporter in potato roots. J Cell Sci. 2003;116:3135–44. doi: 10.1242/jcs.00615. [DOI] [PubMed] [Google Scholar]
- 3.Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, et al. An efflux transporter of silicon in rice. Nature. 2007;448:209–12. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 4.Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, et al. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA. 2010;107:5220–5. doi: 10.1073/pnas.0910744107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alassimone J, Naseer S, Geldner N. A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci USA. 2010;107:5214–9. doi: 10.1073/pnas.0910772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roppolo D, De Rybel B, Tendon VD, Pfister A, Alassimone J, Vermeer JE, et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature. 2011;473:380–3. doi: 10.1038/nature10070. [DOI] [PubMed] [Google Scholar]
- 7.Łangowski L, Růzicka K, Naramoto S, Kleine-Vehn J, Friml J. Trafficking to the outer polar domain defines the root-soil interface. Curr Biol. 2010;20:904–8. doi: 10.1016/j.cub.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 8.Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. Plants tolerant of high boron levels. Science. 2007;318:1417. doi: 10.1126/science.1146634. [DOI] [PubMed] [Google Scholar]
- 9.Grunewald W, Friml J. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29:2700–14. doi: 10.1038/emboj.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takano J, Miwa K, Yuan L, von Wire´n N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA. 2005;102:12276–81. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–57. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Takano J, Chiba Y, Lombardo F, Ogasawara Y, Onouchi H, et al. Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. Plant Cell. 2011;23:3547–59. doi: 10.1105/tpc.111.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–45. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 15.Simpson F, Hussain NK, Quaimann B, Kelly PB, Kay BK, McPherson PS, et al. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol. 1999;1:119–24. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz D, Gygi SP. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol. 2005;23:1391–8. doi: 10.1038/nbt1146. [DOI] [PubMed] [Google Scholar]
- 17.Lam BC, Sage TL, Bianchi F, Blumwald E. Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell. 2001;13:2499–512. doi: 10.1105/tpc.010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasai K, Takano J, Miwa K, Toyada A, Fujiwara T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem. 2011;286:6175–83. doi: 10.1074/jbc.M110.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, et al. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–60. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 20.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 21.Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hilmer S, Frigerio L, Robbinson DG, Pimpl P, Schumacher K. Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell. 2011;23:3463–81. doi: 10.1105/tpc.111.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]