Abstract
Berry formation is the process of ovary conversion into a functional fruit, and is characterized by abrupt changes in the content of several phytohormones, associated with pollination and fertilization. Much effort has been made in order to improve our understanding of berry development, particularly from veraison to post-harvest time. However, the period of berry formation has been poorly investigated, despite its importance. Phytohormones are involved in the control of fruit formation; hence it is important to understand the regulation of their content at this stage. Grapevine is an excellent fleshy-fruit plant model since its fruits have particularities that differentiate them from those of commonly studied organisms. For instance, berries are prepared to cope with stress by producing several antioxidants and they are non-climacteric fruits. Also its genome is fully sequenced, which allows to identify genes involved in developmental processes. In grapevine, no link has been established between pollination and phytohormone biosynthesis, until recently. Here we highlight relevant findings regarding pollination effect on gene expression related to phytohormone biosynthesis, and present results showing how quickly this effect is achieved.
Keywords: Vitis vinifera, VvGA20ox, VvNCED1, anthesis, berry formation, fruit set, grapevine, parthenocarpy, phytohormones, pollination
Grapevine, Vitis vinifera L., is a woody perennial plant with a reproductive cycle that extends for two consecutive growing seasons,1 and leads to the formation of fleshy fruits, known as berries. Coombe refers to fruits as an envelope that surrounds seeds.2 Likewise, we will refer here to berry as the fleshy portion without seeds, also known as pericarp, which is composed by three tissue layers: the exocarp or skin, the mesocarp, known as pulp, and the endocarp, the seed-surrounding tissue.3 Berry development is separated, to its growth pattern, in an initial active growth period (Phase I), a slow growth period (Phase II) and a second active growth period (Phase III), according to Coombe and Hale.4 Most studies have focused on processes occurring during Phase II and III, and berry formation has received little attention despite its importance.
Berry Formation: The Missing Piece of the Puzzle
Berry formation, the process of flower’s ovary conversion into a functional fruit, depends mainly on cell division starting at anthesis—the moment at which the pistil is receptive to pollen—and cell elongation. Initial berry growth is due exclusively to cell division occurring from anthesis to 5 d after anthesis (DAA) approximately.5 From now onwards cell enlargement and, to a lesser degree, cell division increase berry size and are responsible for the so called “fruit set” occurring at 7–15 DAA, which is defined as “the changeover from the static condition of the flower’s ovary to the rapidly growing condition of the young fruit” according to Coombe.6 Early events, occurring few days after anthesis, are decisive for berry formation. For instance, in the fleshless berry (flb) grapevine mutant, cell proliferation is affected as early as 3 DAA, which results in an extremely thin pericarp.7
Berry formation depends on two initial stimuli: pollination and fertilization, the latter occurring at 2–3 DAA.8 Even though these stimuli normally trigger fruit set, unpollinated pistils may develop into seedless berries by the application of phytohormones, such as auxin an gibberellins,9 which is known as artificial parthenocarpy.10 This suggests that early events crucial for berry formation, such as pollination and fertilization, are mediated by phytohormones.
Phytohormones Control Grapevine Berry Formation
Coombe identified for the first time auxin- and gibberellin-like compounds with growth promoter activity during the initial period of grapevine berry development.11 Further studies have shown that auxin is present at high levels mainly at anthesis and fruit set, while gibberellin content increases within the cell enlargement period.12-16 On the other hand, there is some evidence showing that abscisic acid (ABA) and cytokinins are high at anthesis and fruit set and decline thereafter.13,16
In grapevine, evidence suggests that phytohormones could be a substitute for pollination and fertilization, regarding their effect on berry set and berry size. For instance, unpollinated grape bunches treated with auxin or gibberellin at anthesis, develop parthenocarpically.9 With respect to gibberellin, Lavee suggested that this phytohormone compensates for the lack of seeds.17 In agreement with this, the size of seedless berries can be increased by applying gibberellin mainly at the cell enlargement period.18 Cytokinins produce a similar effect when they are applied at fruit set.19 On the other hand, application of auxin at flowering increases berry set20 and seedless cultivars expressing an ovule-specific auxin-synthesizing transgene possess higher levels of auxin at fruit set in correlation with an increased number of berries per bunch.21 This evidence suggests that there is a connection between early events of berry formation and phytohormones, as it has been shown in other organisms.22-26 This connection could be the regulation of gene expression related to phytohormone biosynthesis.
Pollination Alters Gene Expression Related to Phytohormone Biosynthesis During Berry Formation
Until the work of Dauelsberg and coworkers, the effect of pollination on gene expression had not been investigated in grapevine.27 In this study, the effect of pollination on the expression of VvGA20ox, VvGA2ox-like, VvASB1-like and VvIPT1, which are involved in gibberellin, auxin and cytokinin biosynthesis, respectively, was evaluated during berry formation. In pollinated pistils, fruit set was achieved at 21 d after emasculation (DAE; corresponding to 15 DAA), since the major increase in berry size was recorded here. Prior to fruit set, the expression of VvASB1-like, VvGA20ox and VvIPT1 was induced in these pistils with respect to those unpollinated. In the latter, fruit set was achieved later, at 24 DAE (18 DAA), and VvASB1-like and VvIPT1 upregulation with respect to pollinated pistils was registered prior to fruit set, at 15 DAA. Interestingly, an upregulation of VvGA2ox-like, a gene coding for a putative gibberellin deactivating enzyme, was registered only in unpollinated pistils at 15 DAA.
Pollination Effect on Gene Expression—How Rapid is it?
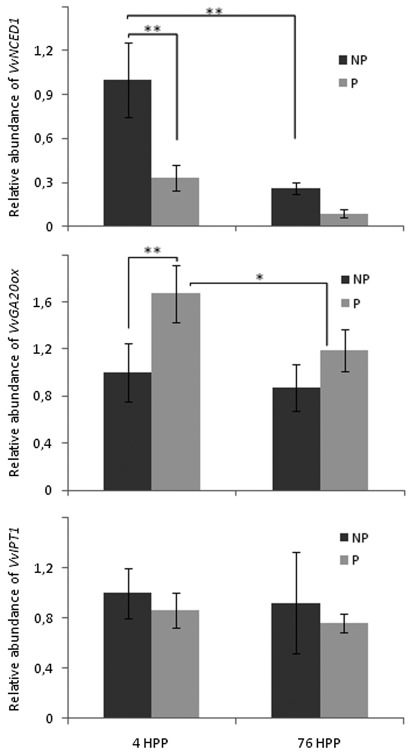
Based on the above results of Dauelsberg's research performed in our laboratory, we asked how quickly pollination exerts its effect on gene expression. Therefore, we compared the expression of VvNCED1, VvGA20ox and VvIPT1 in pollinated and unpollinated pistils 4 h post-pollination (HPP) and 76 HPP (Fig. 1). VvNCED1 is a gene coding a key enzyme of ABA biosynthesis28 and possibly involved in the inhibition of fruit formation.26 At 4 HPP, VvNCED1 was upregulated while VvGA20ox was downregulated in unpollinated pistils with respect to pollinated ones. At 76 HPP, VvNCED1 expression was low in both treatments. VvIPT1 expression was not affected by pollination at any time (Fig. 1).
Figure 1. Rapid effect of pollination on gene expression related to phytohormone biosynthesis during berry formation. Transcript abundance of VvNCED1, VvGA20ox and VvIPT1 in pollinated (P) and unpollinated (NP) pistils, measured at 4 h post-pollination (4 HPP) and 76 HPP, relative to VvUBI1 gene expression. Values are the mean of 3 biological and three technical replicates, and are relative to the emasculated not pollinated 4 HPP sample, which was set to 1.0 arbitrarily. Bars represent means ± STERR (n = 3). Asterisks indicate significant differences (*p ≤ 0.05; **p ≤ 0.01).
These results are remarkable since they demonstrate that within few hours pollination alters gene expression, which has not been reported in fleshy fruits to date. VvNCED1 downregulation at 4 HPP (0 DAA) in pollinated pistils is in agreement with findings reported in tomato, showing downregulation of LeNCED1 expression 1 d after pollination.29 On the other hand, at 76 HPP (3 DAA) differences in VvGA20ox expression between pollinated and unpollinated pistils are less marked than at 4 HPP. This suggests that pollination has an immediate effect on gene expression, which is reduced at 3 DAA. Finally, the independence of VvIPT1 expression on pollination concurs with the results of Dauelsberg et al.27
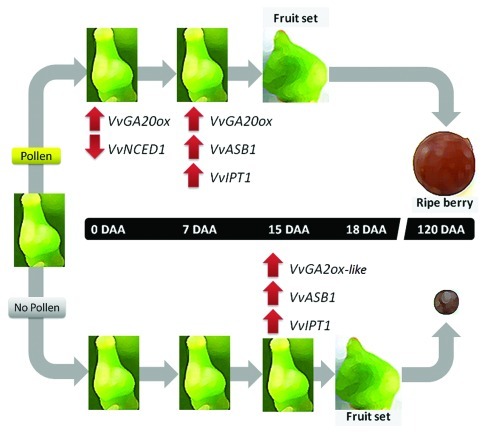
In sum, few hours after pollination differences in the expression of genes related to ABA and gibberellin biosynthesis between pollinated and unpollinated pistils are observed (Fig. 2). Thereafter, prior to fruit set, low levels of VvGA20ox transcript and induction of VvGA2ox-like expression, a gene coding for a putative gibberellin deactivating enzyme, are observed in unpollinated pistils prior to fruit set, in correlation with their inability to form berries (Fig. 2). These results support the crucial role of pollination in the control of gene expression related to phytohormone biosynthesis, which occurs as early as 4 h after pollination. It would be interesting to analyze this effect from a more global perspective and at earlier time points.
Figure 2. Schematic diagram showing relevant findings regarding pollination effect on transcript accumulation of genes related to phytohormone biosynthesis during the initial period of berry formation. Only the time points that present significant differences are shown. Differences in transcript accumulation at each time point between pollinated and unpollinated pistils are indicated with arrows. A downward-pointing arrow indicates downregulation and upwards-pointing arrows indicate upregulation of the corresponding genes. At 0 DAA, the effect of pollen 4 h post-pollination (4 HPP) on transcript accumulation is shown.
Materials and Methods
Plant material and treatments
To evaluate the effect of pollination on pistil gene expression, 20 pre-capfall (fused petal) inflorescences from two grapevine plants (Vitis vinifera L. cv Moscatel Rosada) were selected from an experimental field located in the Curacavi´ Valley, Chile, during the 2010/2011 growing season. Single flowers from each inflorescence were chosen from the cluster third fraction closest to the rachis. The inflorescences were manually decapped and emasculated. After emasculation, 10 inflorescences were manually pollinated (P) at anthesis, with pollen collected from the same cultivar, and 10 inflorescences were kept unpollinated (NP). P and NP inflorescences were covered with paper bags. P and NP inflorescences were collected 4 and 76 h post-pollination (4 HPP and 76 HPP, respectively), at the same time of day. Pools were formed from five inflorescences taken from both plants (two or three from each one). Three pools were formed for each treatment and date, corresponding to three biological samples, which were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
RNA extraction and cDNA synthesis
Total RNA was extracted from 0.5 g of floral samples using a CTAB-Spermidine extraction buffer, as described by Poupin et al.32 For cDNA synthesis, total RNA (1.5 µg) was reverse transcribed with random hexamer primers using SuperScript™ II reverse transcriptase (Invitrogen), according to the manufacturer’s instructions.
Quantitative analysis of gene expression
Real time RT-qPCR was performed as described by Dauelsberg et al.27 A fragment of the VvUBIQUITIN1 gene was utilized as a housekeeping gene as described by Downey et al.30 Primers chosen for VvUBIQUTIN1 (VvUBI1), VvGA20ox and VvIPT1 amplification were those described by Dauelsberg et al.27 Primers selected for VvNCED1 amplification were those described by Hayes et al.31 Real-Time RT-qPCR was performed on a MX3000P qPCR Machine (Stratagene), using the SensiMixPlus SYBR commercial kit (Quantace). Relative gene expression calculations were conducted as described in the software manufacturer’s instructions. An accurate ratio between the expression of the gene of interest (GOI) and the housekeeping gene (VvUBI1) was generated according to 2−(ΔCt GOI-VvUBI1) equation. Ct values for VvUBI1 varied no more than 1.4 units between all samples analyzed for each experiment. Gene expression levels were normalized to the expression of the emasculated not pollinated 4 HPP sample, which was set to 1.0, arbitrarily. All experiments were performed with three biological replicates and three technical replicates. Statistical analysis was made using ONE-WAY ANOVA test and media was compared by tukey's test using a p < 0.01.
Acknowledgments
This work was supported by the Grapevine Breeding Project of the Technological Fruit Consortium, FONDECYT 1100709 and the Millennium Nucleus for Plant Functional Genomics (P06–009-F). N. Kühn is supported by a PhD fellowship from Conicyt. We thank Michael Handford (University of Chile) for critical reading of the manuscript.
Glossary
Abbreviations:
- ABA
abscisic acid
- DAA
days after anthesis
- DAE
days after emasculation
- HPP
hours post-pollination
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18353
References
- 1.Carmona MJ, Chaib J, Marti´nez-Zapater JM, Thomas MR. A molecular genetic perspective of reproductive development in grapevine. J Exp Bot. 2008;59:2579–96. doi: 10.1093/jxb/ern160. [DOI] [PubMed] [Google Scholar]
- 2.Coombe BG. The development of fleshy fruits. Annu Rev Plant Physiol. 1976;27:507–28. doi: 10.1146/annurev.pp.27.060176.001231. [DOI] [Google Scholar]
- 3.Hardie WJ, O’Brien TP, Jaudzems VG. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust J Grape Wine Res. 1996;2:97–142. doi: 10.1111/j.1755-0238.1996.tb00101.x. [DOI] [Google Scholar]
- 4.Coombe BG, Hale CR. Hormone content of ripening grape berries and effects of growth substance treatments. Plant Physiol. 1973;51:629–34. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojeda H, Deloire A, Carbonneau A, Ageorges A, Romieu C. Berry development of grapevines: relations between the growth of berries and their DNA content indicate cell multiplication and enlargement. Vitis. 1999;38:145–50. [Google Scholar]
- 6.Coombe BG. The effect of removing leaves, flowers and shoot tips on fruit-set in Vitis vinifera L. J Hortic Sci. 1962;37:1–15. [Google Scholar]
- 7.Ferńndez L, Romieu C, Moing A, Bouquet A, Maucourt M, Thomas MR, et al. The grapevine fleshless berry mutation. A unique genotype to investigate differences between fleshy and nonfleshy fruit. Plant Physiol. 2006;140:537–47. doi: 10.1104/pp.105.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt C. Reproductive anatomy in cultivated grapes: A review. Am J Enol Vitic. 1971;22:93–109. [Google Scholar]
- 9.Weaver RJ, McCune SB. Furthur studies with gibberellin in Vitis vinifera grapes. Bot Gaz. 1960;121:155–62. doi: 10.1086/336060. [DOI] [Google Scholar]
- 10.Gustafson FG. Parthenocarpy: natural and artificial. Bot Rev. 1942;8:599–654. doi: 10.1007/BF02881046. [DOI] [Google Scholar]
- 11.Coombe BG. Relationship of growth and development to changes in sugars, auxins and gibberellins in fruit of seeded and seedless varieties of Vitis vinifera L. Plant Physiol. 1960;35:241–50. doi: 10.1104/pp.35.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Böttcher C, Keyzers RA, Boss P, Davies C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot. 2010;61:3615–25. doi: 10.1093/jxb/erq174. [DOI] [PubMed] [Google Scholar]
- 13.Inaba A, Ishida M, Sobajima Y. Changes in endogenous hormone concentrations during berry development in relation to the ripening of Delaware grapes. J Japan Soc Hort Sci. 1976;45:245–52. doi: 10.2503/jjshs.45.245. [DOI] [Google Scholar]
- 14.Iwahori S, Weaver RJ, Pool RM. Gibberellin-like activity of berries and seedless ‘Tokay’ grapes. Plant Physiol. 1968;43:333–7. doi: 10.1104/pp.43.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pe´rez FJ, Viani C, Retamales J. Bioactive gibberellins in seeded and seedless grapes: Identification and changes in content during berry development. Am J Enol Vitic. 2000;51:315–8. [Google Scholar]
- 16.Zhang XR, Luo GG, Wang RH, Wang J, Himelrick DG. Growth and developmental responses of seeded and seedless grape berries to shoot girdling. J Am Soc Hortic Sci. 2003;128:316–23. [Google Scholar]
- 17.Lavee S. Effect of gibberellin on seeded grapes. Nature. 1960;185:395. doi: 10.1038/185395a0. [DOI] [Google Scholar]
- 18.Casanova L, Casanova R, Moret A, Agusti´ M. The application of gibberellic acid increases berry size of Emperatriz seedless grape. Span J Agric Res. 2009;7:919–27. [Google Scholar]
- 19.Peppi MC, Fidelibus MW. Effects of forchlorfenuron and abscisic acid on the quality of ‘Flame Seedless’ grapes. Hort Science. 2008;43:173–6. [Google Scholar]
- 20.Weaver RJ, McCune SB, Hale CR. Effect of plant regulators on set and berry development in certain seedless varieties of Vitis vinifera L. Vitis. 1962;3:84–96. [Google Scholar]
- 21.Costantini E, Landi L, Silvestroni O, Pandolfini T, Spena A, Mezzetti B. Auxin synthesis-encoding transgene enhances grape fecundity. Plant Physiol. 2007;143:1689–94. doi: 10.1104/pp.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellins metabolism in Arabidopsis. Plant J. 2009;58:318–32. doi: 10.1111/j.1365-313X.2008.03781.x. [DOI] [PubMed] [Google Scholar]
- 23.Mapelli S. Changes in cytokinins in the fruits of parthenocarpic and normal tomatoes. Plant Sci Lett. 1981;22:227–33. doi: 10.1016/0304-4211(81)90235-2. [DOI] [Google Scholar]
- 24.Ozga JA, Reinecke DM. Interaction of 4-chloroindole-3-acetic acid and gibberellins in early pea fruit development. Plant Growth Regul. 1999;27:33–8. doi: 10.1023/A:1006151401685. [DOI] [Google Scholar]
- 25.Serrani JC, Ruiz-Rivero O, Fos M, Garci´a-Marti´nez JL. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008;56:922–34. doi: 10.1111/j.1365-313X.2008.03654.x. [DOI] [PubMed] [Google Scholar]
- 26.Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 2008;177:60–76. doi: 10.1111/j.1469-8137.2007.02254.x. [DOI] [PubMed] [Google Scholar]
- 27.Dauelsberg P, Matus JT, Poupin MJ, Leiva-Ampuero A, Godoy F, Vega A, et al. Effect of pollination and fertilization on the expression of genes related to floral transition, hormone synthesis and berry development in grapevine. J Plant Physiol. 2011;168:1667–74. doi: 10.1016/j.jplph.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Soar CJ, Speirs J, Maffei SM, Loveys BR. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Funct Plant Biol. 2004;31:659–69. doi: 10.1071/FP03238. [DOI] [PubMed] [Google Scholar]
- 29.Nitsch LMC, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, et al. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1. Planta. 2009;229:1335–46. doi: 10.1007/s00425-009-0913-7. [DOI] [PubMed] [Google Scholar]
- 30.Downey MO, Harvey JS, Robinson SP. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.) Aust J Grape Wine Res. 2003;9:110–21. doi: 10.1111/j.1755-0238.2003.tb00261.x. [DOI] [Google Scholar]
- 31.Hayes MA, Feechan A, Dry IB. Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol. 2010;153:211–21. doi: 10.1104/pp.110.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poupin MJ, Frederici F, Medina C, Mattus JT, Timmermann T, Arce-Johnson P. Isolation of three grape sub-lineages of B-class MADS-box TM6, PISTILLATA and APETALA3 genes which are differentially expressed during flower and fruit development. Gene. 2007;404:10–24. doi: 10.1016/j.gene.2007.08.005. [DOI] [PubMed] [Google Scholar]