Abstract
Salicylic acid is well known phytohormone, emerging recently as a new paradigm of an array of manifestations of growth regulators. The area unleashed yet encompassed the applied agriculture sector to find the roles to strengthen the crops against plethora of abiotic and biotic stresses. The skipped part of integrated picture, however, was the evolutionary insight of salicylic acid to either allow or discard the microbial invasion depending upon various internal factors of two interactants under the prevailing external conditions. The metabolic status that allows the host invasion either as pathogenesis or symbiosis with possible intermediary stages in close systems has been tried to underpin here.
Keywords: abiotic-stress, microbes, pathogenesis, salicylic acid, symbiosis, systemic acquired resistance
Introduction
Hormones are the biochemical language of living systems. These in either of the plant or animal system perform fundamentally similar function; the integration of system at basal economy level. Classically, plant hormones have been defined as low molecular weight organic compounds governing pleotropic physiological responses within plants, but distant from the sites of their synthesis. Their extreme low (mM to µM) concentrations are sufficient to elicit multifaceted responses in different tissues types that shares expression of specific receptors within cytosol and/or on membranes. Where on one hand their binding to receptors elicits amplified downstream signal cascades, the population of receptors expressed inter-/intracellularly play crucial role to sense, thereby, subsequently determine the extent/speed of response in tissue types.
Passing decades coarsely witnessed three types of hormonal systems embodied by animals viz. endocrinal, pericrinal and autocrinal based on the distance they travel to site of action. In plants with the introduction of new class of regulators inconsistencies emerged regarding the assignment of their places in the category of plant hormones. The blurred boundaries of these new-comers asked reconsideration of previous definition of phytohormones. However, plants studies recently leaped to introduce new regulators which not only act at short distance, their µM concentrations are sufficient to elicit marked physiological and molecular alterations. Interestingly, some other of them shown to travel in air par-systematically mimicking as volatile ‘plant-pheromones’ (regulating defense e.g., methyl salicylate, jasmonates, strigolactones) or as gaseous molecules (ethylene, NO) switching other essential developmental traits within a part, complete plant or even whole population in-chorus to regulate defense.1-4 Plants being sessile have to withstand a plethora of biotic invasions coupled with abiotic stressors. The synergy of later with either of plant or microbes disables the interaction of one with another, or may sometimes the equilibrium of trio persist as a long-duration-compromise. Inconsistencies arise with the elucidation of role and mechanism of action of certain short distance regulators viz. steroids; nitric acid and ROS; and intersystemic regulators as salicylic acid, jasmonic acid and strigolactones etc.
These glycosides of salicylic alcohol were first isolated by Johann Buchner in 1828 and called as Salicine has traveled long way since their discovery to establish themselves as distinct class of plant hormone,5 the salicylic acid. The ubiquitous distribution both in monocots and dicots reflects their importance among plant kingdom.6 Salicylic acids are peculiar to their type govern plant growth and development under natural and abiotically stressed environmental regimes. These phenolics seems to be transported from the site of application5 in their transportable form and enhance plant growth and yield, message inter-/intra-cellularly, systemically, and even par systemically under biotic stress when challenged with invading microbes to prevent the loss of host population. These reportedly interact with different classical and newly recognized plant hormones to regulate diverse metabolic and physiological function7 in metabolically active tissues or those which are under stress to maintain cellular redox homeostasis from oxidative stress. The elucidation of role of salicylic acid is a challenge in understanding their natural selection and evolution in establishment of two opposite phenomenon, the symbiosis (-a positive interaction) and pathogenesis (-the negative interaction) through all their intermediates (-incompatible interactions, secondary host, attenuation, facultatism etc.). The knowledge how they regulate interplant signaling in different pathogenic and symbiotic relationships is scarce and still elusive.
Evolutionary Importance of Appearance of Salicylic Acid and Their Analogs
Plant physiology encompasses the functional domain of metabolic manifestation of biochemical processes within the plant which in turn get regulated at molecular level. Despite being visibly demarked as compartmentalized cells, tissue types and different systems etc., a remarkable coherence is obvious within such subsystems. The appearance of integrative key molecules initially served here the crucial part of “systemic integration” that backed the complex life to supersede their evolutionary ancestors in the race of competitive selection of survival in aquatic ecosystems and on land later on. Nature, though in part, kept on imparting the selective pressure over generations to sieve the best fit in the long run of evolutionary race, the secondary metabolites, hence, come forth to reserve their place and evolve the secondary metabolic pathways with their active defense molecules.8 Their expression, in most cases, albeit remain under regulation of inducible promoter. The boundaries of this integrated system to establish the plant to its functional niche does not culminated here, it ensured its survival extending its signaling to message inter-systemically now studied under inter-plant, plant-microbe and plant-predator relationships. Jasmonates and salicylates are special in their role as they not only regulate the plant physiological functions and systemic immunity, these also prime defense signals9 even in the population of their ecological community. These signals evidenced to talk with insects and pests in biochemical language counteracting in rhizosphere or phylloplane in complex co-evolved fashion. Nevertheless, the time of bargain under unfavorable environment could compromise the cost of invasion; altering the intensity of attack (-virulence) and resistance level to woven the dual life (pathosystem, parasitism, helotism, commensalism, exo-/endosymbiosis etc.).
Plant, Invader and Abiotic Stressors (Duration/Intensity): A Synergy of Trio Determines the Nature of Relationship
Several examples in nature represent different stages of interaction of organism of two drastically different taxa, from kingdom to species. It appears positive interaction evolved as adjustment descended from early negative interaction diversified polyphylatically. At first site certain examples appears to be at the stage of establishing positive interactions in favor of host/plant, for instance; lichens, mycorrhizae, corallaoid roots of Cycas, Nostoc colonies in Anthoceros thallus, Rhizobium-legume nodules, Agrobacterium tumifaeciens mediated tumors, endosymbiont Chlorella in ciliates, endosymbiotic bacteria (which later on accommodated themselves as chloroplast and mitochondria) during evolution of eukaryotic cell, viral genome integration in bacterial and other eukaryotes (-transposons sequences) etc. However, negative interactions of plant with microbes are also very common reducing plant growth, or even survival. A condition unfavorable to either of the interacting organisms renders the two to adjust for the time being till the favorable time returns. Although, single/simple-celled microbes seems quickly adjust these altered conditions as compared with integrated cell systems (here plant system). However, these hostile conditions may persist for long time to imprint the memory in the genome of either of the interactants.
The population of plant systems when shows healthy state it represents their favorable growth conditions. On contrary disease is facilitated by the unfavorable conditions of either of the hostile environment, nutritional scarcity alone or along with beneficiary pathogenic encounter to be termed appropriately as pathogenesis. The synergy of two (abiotic and biotic stressors) deteriorates plant physiology more than either of stress. The favorable conditions of invasion viz. nutritional scarcity, temperature, moisture/humidity, inoculum density and virulence of invader with reference to age/physiological condition and susceptibility of host plant appears to be the critical factors responsible to prime the successful ingression and microbial (/pathogen = disease) establishment, often resulting in mal-physiology and reduced yield/growth of plant. In the pyramid of disease progression suggests that ‘time’ factor imparts critical role in ascertaining the fate of relation between invaders with its host. For instance; (a) relatively quick response of host often leads to restricted damage of host tissue rendering the pathogen incompatible for further growth and establishment (-total resistance), (b) a delayed response may favor invader to grow progressively within the host tissue pushing defense responses and rendering it semi-compatible (c). However, a lack of time-tuned appropriate response and signal components (as in case of sensitive host variety) provided aggressive invasion (virulent strain) may completely indispose plant to total failure against invasion (as evident in disease progression) aiding to establishment of pathosystem (pathogenesis).
Precise synchrony of phytohormonal network may leads to total discard of invader at site with minimal tissue/cell loss reassuring its nutritional supply after a due phase of lagged growth (case a). However, same could not be the case with compatible system (case c) where a remarkable diversion of nutritional supply hijacked through molded gradient of phytohormonal gradient facilitate invaders’ growth. The hijackers could express extended phenotype at genomic (integration of DNA segment facilitated by protein regulators, siRNA mediated gene silencing) or proteomic (intruder’s effector molecules alter the metabolic pathways favoring its establishment) level.10 The nonmetabolizable microbial produce (-pathotoxins) may lead to severe setback of plant growth progressive tissue death. Between these two extremes a semi-compatible system (b) appears to exist to be considered as quasi/semipathosystem. Several secondary hosts could be considered under this category lacking the essential components to support the growth of invader negatively. Plants appear to signal their stressed state inviting these intruders under nutritional or abiotically stressed regimes. Krouk and coworkers11 opinioned nitrogen signals crucially play with the hormonal feedback during nitrogen acquisition and assimilation and also in root development.
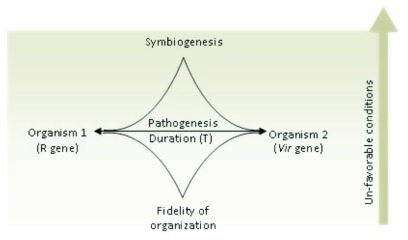
However, with the due passage of time host may acclimate to withstand (facultative) invasion to sustain consecutive relationship (obligatory). The ‘microbial produce’ may be metabolized by host to support its vital functions over generations to develop symbiotic relationship in future (Fig. 1).
Figure 1. Multiple factors determine the fate of relationship between two interactants (macrobiont and microbiont) i.e., environmental conditions, genotype of two and the time duration.
Functional Validation of Salicylic Acid as a Plant Defense Hormone
Studies revealed the major role of salicylic acid in plants counteracting the biotic stress responses. White12 first evidenced the involvement of salicylic acid in plant defense. Internal increase in salicylic acid levels during pathogenic plant-microbe interaction often facilitates the building up of the resistance and systemic resistance.13 Salicylic acid induced resistance against many necrotic or systemic viral, bacterial and fungal pathogens in a variety of plants.14 Durrant and Dong9 clearly reviewed the demonstration of salicylic acid accumulation and subsequent role in signaling using mutant and transgenic plants. Bacterial salicylic acid degrading enzyme salicylate dehydroxylase (NahG) when overexpressed in transgenic Arabidopsis and tobacco plants developed inefficient defense response and showed more susceptibility to pathogen infection damage.15
The role of salicylic acid in plants was noticed in the induction of SAR against pathogens.16 Although, their analogs have also been proved outstanding, defending plant against abiotic stresses17 to secure their development18 under suboptimal conditions. Huang et al.19 analyzed quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter species ADP1. The naturally occurring salicylic acid responsive saloperon in the strain was constructed with ADPWH_lux which induced bioluminescence sensing salicylic acid even in concentration as low as 5 nM. The spatial and temporal pattern of increased free salicylic acid and MeSA accumulation in leaves was reported before and after the appearance of the HR elicited by TMV in tobacco. Salicylic acid accumulation was also detected during compatible and incompatible interactions between tobacco and Pseudomonas syringeae. The increased accumulation of salicylic acid in pre-necrotic tissue corresponds to preHR physiological effects such as local increases in temperature, transpiration rate and alterations in chlorophyll fluorescence.20,21
Salicylic Acid Regulates the Fundamental Processes of Plant, the Photosynthesis and Energy Economy
It is recognized that salicylic acid potentially generates a wide array of metabolic responses in plants and also affects photosynthetic parameters and plant water relations. These responses could be either pathogen induced3,4 mal-physiological responses; restricting plant growth and survival based upon pathogen type, could be root mal-nutritional responses11 or due to plant incompetency to catch up well compatible level of plant hormone network to push abiotic stress(es) as indicative with several studies showing favorable responses of stage, and dose specific responses of different plant hormones; or could be combinations of either of these cases.
A range of favorable physiological responses have been generated by external application to incompatible (sensitive) plants or while these were promoted in compatible (resistant) cultivars. These responses include shift in active biosynthesis of pigments and their subsequent accumulation,7,22-24 photosynthesis related parameters25-27 and the activity of associated enzymes22 and energy economy at the cost of ROS production (discussed later in detail).
Inter-Systemic Regulation is Attributed to Methyl Salicylates
The interaction of microbes with plants can induce both local and systemic alterations in the plant.28 The elevated transcripts level of enzymes of phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS) was clearly implicated specifically in the cells enwrapping arbuscules29 suggesting local activation of key enzymes of secondary metabolic pathway in vicinity of fungus thalli. However, effect of salicylic acid as SAR led to realization that perhaps it travels through vasculature.30 However, Vernooji and coworkers31 strongly favored that salicylic acid is not the signal to induce SAR, though it is require in signaling. Park and others32 later elegantly demonstrated MeSA as a crucial mobile signal in activation of SAR against TMV. The role of plausible proteins with esterase activity (SABP2) in distal manifestation of SAR and accumulation of MeSA (SAMT1) utilizing inhibitor and mutant study has also been elucidated.32,33 However, not all plants deploy this strategy of MeSA and JA mediated manifestation of SAR.34 Those who employ them as a mediator of SAR transport signals through both xylem and phloem.35 It was seen that in leaves of pathogen infested Arabidopsis most (97%) of the MeSA produced has been emitted from plant. However, volatilization of signals could be pathogen induced ploy to mild the SAR induced defense or it could be the plant signaling molded to induce inter-systemic regulation of defense priming SAR in en masse.34 Gutierrez-Luna et al.36 recently investigated that even PGPR emit volatile organics to modulate root architecture and plant growth. In addition Arabidopsis incorporates the JA signaling in response to avirulent pathogen through vasculature conduction.37 Either of host and microbe seems to overwhelm to counteract each other. The examples of pathogen mediated manipulation of plant defense responses have been suggested time to time.38
Inter-/Intra-Cellular Signaling: Reactive Oxygen Species and Salicylic Acids
During cellular metabolic processes, toxic level of reactive oxygen species (ROS) such as superoxide anion (O2-), hydroxyl ions (OH·), and hydrogen peroxides (H2O2) are generated in aerobic conditions and can cause damage to these cells, a phenomenon known as oxidative stress. When plants are exposed to various environmental stresses they produce range of ROS in large quantities sufficient to disrupt cellular and metabolic functions of the plant. To prevent oxidative injury by the toxic, reactive oxygen species, the activity of antioxidant enzymes such as SOD, APX, GR, POX, CAT and low molecular weight compounds like AsA and GSH is increased normally. For the survival of the plants appropriate functioning of the antioxidant system is important i.e., to maintain a balance between ROS and production and scavenging system.39 Stress tolerance is therefore related with improved antioxidant system in plants. Previous studies imprints salicylic acid enhances plant antioxidant protective system following logistic growth curve thereby inducing stress tolerance. However, several studies show that higher endogenous concentration of salicylic acid interplays with ROS pushing the cell toward death. Higher concentration of salicylic acid appears to deactivate the antioxidant activity in synergy with ROS. The analysis of peroxysomal antioxidant enzymes viz. SOD, CAT, GPX (Glutathione peroxidase) and AsAGSH cycle activities in tomato infected with B. cineria40 clearly indicated that initial infection induced increase in SOD, CAT and GPX indicating antioxidant defense activation. It was followed by a progressive inhibition concomitant with disease symptom development.
Accumulating evidence during the last few years raised the idea that salicylic acid interplays with ROS to signal genetic controlled defense reaction such as PCD and expression of stress defense genes. The idea of interplay between salicylic acid and ROS defending stress is now considered crucial for local and systemic defense response.9,41 Initial rise in endogenous level of salicylic acid supported the idea of upregulating (overexpression and activation) the antioxidative defense system to alter the activity of transcription factors and cellular signaling. However, the supra-optimal level is reported to harm the plant metabolism26 suggesting oxidation of thiol buffers and activity of low molecular weight transcription factors, thereby suppressing the activity of antioxidant system supporting oxidative burst of ROS. ROS and salicylic acid which accumulate at the high level during HR42 appears to aid signal amplification for cell death propagation41 (Fig. 4). Apoplastic ROS production43 in addition to mitochondrial production39 facilitates PCD. Besides, activating ROS production by several mechanisms44 salicylic acid also inhibits the activity of antioxidative enzymes such as APX and CAT leading to an over-accumulation of ROS.45 It has been shown that the accumulation of H2O2 is essential for the induction of plant disease resistance, but alone H2O2 could not be sufficient to induce program cell death. Exogenous application of salicylic acid causes an increase in H2O2 accumulation in plant tissues and their higher H2O2 concentration has been proposed to act as a signal inducing HR and SAR against pathogens.46
Burst of ROS: Local Defense and Systemic Acquired Resistance
As with the increase or continuous irritation of biotic/metal stress, accumulation of reactive oxygen and nitrogen species increases, the shift in cellular pH and redox state of cytoplasm toward more oxidizing and energetic state elicits change in phosphorylated pool of low molecular weight proteins. Change in oxidative state of cellular components are also facilitate by secondarily produced organic free radicals aids the altered property of membrane and further supporting the decline in pH. Recent technologies emerged to assess the phosphorylated state of the cell cytoplasm under altered conditions what is called as phospho-proteomics. A change in phosphorylated state and Cys residue disulfide bond39 (oxidation) alters the conformation, thereby, the activity of these low molecular weight proteins. Several of these molecules circumvent the low molecular weight thiol buffers constituting the antioxidant system and transcription factors. The local resistance is facilitated by the initial rise of H2O2, augment the defense gene expression.
The intensity of stress (oxidation) for a given duration of time on particular surface area is congregantly counteracted by the efficient management of available basal life sustaining component of cell (house-keeping metabolic processes plus counteracting buffer molecules/enzymes). The management is regulated by the active participation of released/translocated phyto-hormones. Once oxidative stress crosses the threshold (-a balance between oxidation and induced preventive reduction to prevent minimal required set up for basal metabolic processes), cell switches over toward PCD with simultaneous elicitation of defense mechanisms in neighborhood. Different combinations of hormones and signals (ROS, salicylic acid, JA, NO and H2O2) have been proposed to be involved in the initiation, propagation and containment phase of PCD or HR.41,47 Salicylic acids also mediate the lipid peroxidation, which play a key role in initiating defense response.48 ROS induced peroxidation of membrane lipids and proteins perturb the homeostasis and induce the decompartmentation of organelles. Induced ROS level not only have a toxic effect during cell death oxidizing cellular components but additionally constitute proximal (H2O2) and distal (methylated forms of salicylic acid and JA) signals to balance information between metabolism and environment.
Plant Interactions with Microbes
Within a community different populations belonging to different taxa interact. These interactions are defined as positive or negative interactions, obligate or facultative relations. The two interactants depending upon the size, mode of feeding and extent of damage reflect gradual trend of conversion of negative interaction to positive relationship through adjustment/attenuation with the passage of time. A highly coordinated molecular dialog between cells of plants and microbe determines the final outcome of the relationship, ranging from pathogenesis to symbiosis,49 for instance; competition, antagonism, necrotrophism, bionecrotrophism, biotrophism, parasitism, co-operation and endo- or exosymbiosis etc.
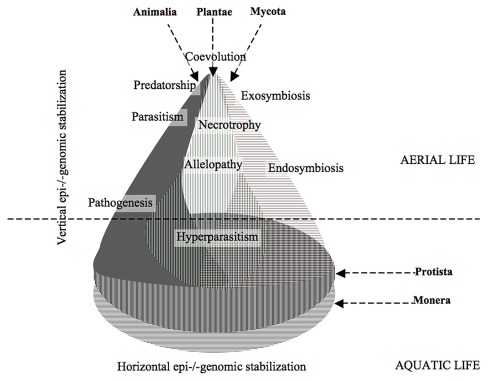
This adjustment involves enforced alteration of metabolism in either of candidate, discovering new signaling of adjustment to live together. The mild oxidative response, genomic and proteomic combat followed by comparative dominance of either of partner. The learning imprints the epigenome and/or genome for further chance interactions culminating into the permanent integration. Microbes are most abundant life forms, the simple structural base of all three major domains of multicellular life. In early intrusion host (-here plant) tries to ensure its organizational fidelity. The genomic, epigenomic or environmental bargaining results into different intermediate interactions to inculcating into more complex life (Fig. 2).
Figure 2. Major biological kingdoms interacts though simple organization/microbes (at base) more commonly with three multi-organized kingdoms to co-evolve chronologically diverse interactions in the nature. The horizontal genetic interchange is seems to be stabilized by the vertical gene transfer and epigenetic expressions under diverse environmental regimes on earth.
Symbiosis verses Pathogenesis: Do Salicylates Have Any Role?
Nature imparts the selective pressure on organisms which is determined by the alteration in prevailing conditions in their niche. This pressure may be a flash of stress or persists for long duration. Struggle for existence may attenuate the defense of two organisms to cooperate for survival discovering new signaling pathways under enhanced rate of spontaneous mutations. The rhizoshpere/rhizoplane or opportunistic microbial intruders seek undue advantage of suboptimal or stressed conditions of their plant hosts through specific airborne or rhizogenic signals.
The epigenomic attenuating regulation gets fixed with the passing generations to strengthen the co-operation shuffling proteome and/or genome vertically or horizontally to stabilize the invasion stress for the genesis of new life organization. In Agrobacterium mediated induction of crown gall; integration of TDNA is facilitated by the early delivery of regulatory proteins in the host cell through pilus.50 This symbiogenesis may domesticate primitive lives endogenously in a mutually beneficial capture (prokaryotes within ciliates, zooxanthalleae in coral reefs, coralloid roots of Cycas etc.), or enwrapped around the macrosymbiont (mycorrhizae and lichens etc.). It is now accepted that evolution of eukaryotic cell has taken place as a chimera of certain prokaryotic even viral parts. Several examples distributed within and among kingdoms where higher and lower eukaryotes and prokaryotes interact negatively or positively with different level of attenuation of either or both interactants.
A keen study of pathogenetic systems suggests different level of class of interactions depending upon virulent attack and/or resistance capacity. The later appears to me more proteomic/epigenetic rather genomic scarcity to defend them. It has been seen that prior mild exposure builds up internal regulatory molecule in host to heighten its response against secondary encounter and resistance thereby. From invader perspective loose genomic scaffold to express and rapid rate of perpetuation favors the chance of acclimated/adapted strain to survive and populate exponentially. In its niche plant appear to invite the edaphic flora, both positive interactants and negative interactants. The Nod and Myc factors have been secreted by plants to invite corresponding reactions, whereas, in several angiosperms strigolactones have been secreted to invite parasitic weeds to interact negatively with host.51
The interaction of plants with symbiotic microorganisms is largely determined by the endogenous salicylic acid levels. Inoculation of transgenic Lotus japonicas and Medicago truncatula overexpressing NahG with Mesorhizobium loti showed increased infection and root nodulation.52 Studies showed direct effect of salicylic acid on microbes viz. Sinorhizobium mililoti (Martinez-Abarca et al.),68 Pseudomonas aeruginosa,53 Staphylococcus aureus54 and in Agrobacterium tumefaeciens.50 The plant defense responses mounted against Agrobacterium are more or less similar to those stimulated by other pathogenic bacteria.55,56 During the later session of encounter the host defense responses were shown to be hacked by virulent strain.55 Salicylic acid dually hinders the invasion of pathogenic bacteria, first, triggering the heightened defense responses of plant and second, inhibiting bacterial growth.
Plants discovered salicylic acid perhaps after their encounter to aerial stressed life where they employed salicylates to burst the ROS. The strategy of working of salicylic acid in both the systems (host and invader) is surprisingly similar. However, the loss of adapted cell for invader is more fatal over host with excessive leakage of electrons to generate negative oxygen (via partial reduction of aerobic oxygen). Nevertheless, environmental attenuation of host and promotion (virulence) of invader enabled them to mild the host hypersensitive response to circumvent the defense. Again the persistent co-living may co-operate later to shuffle the proteomic and up to some extent genomic profile rendered the two to live in captured state forever. The molecular details, biochemical adjustment and signal recognition mechanisms we have unraveled until now is only the beginning of uncovered story. The interaction of Agrobacterium to induce crown gall or excess rooting, or root nodulation of legumes with Rhizobium appear to be the upcoming stories of new symbiotic relation which may fix themselves as new organelles (-nitrosome in case of root nodules) to ensure the constant supply of nitrogen, through hard-to-crack nut of nitrogen fixation.
On the basis of introduction/interaction of plant with microorganism there appear three possible systems. (1) Pathogenic/compatible system (e.g., Biotrophs; Agrobacterium crown gall of stem/ hairy root and Bionecrotrophs; Phytophthora infestans), (2) Nonpathogenic/incompatible system (e.g., Alternative hosts, Puccinia graminis on barberry and other semi resistant hosts (3) Symbiotic systems (e.g., Rhizobium legume symbiosis, Mycorrhization with roots of higher plants and PGPRs.
Effect of Salicylic Acid on Plant Defense Responses Against Pathogen
Salicylic acid is regarded as an endogenous marker of plant disease resistance57 and supposed to be important determinant of pathogenicity. Salicylic acid with its analogs mediates the defense response against a broad spectrum of pathogenic diseases either through exogenous application58 or endogenously.59
The studies on mechanism of salicylic acid action primarily have been focused on plant defense responses.9 The defense response mediated by the expression of PR1 gene induction.60 The evidence in this support came through the study of NahG mutants overexpressing salicylate hydroxylase which break downs the salicylic acid to catechol. The increased susceptibility of mutants to several pathogens was suggested and earlier evidenced due to hindrance in the expression of PR1.15 The expression of salicylic acid induced PR genes and SAR primarily promoted by signaling pathway with the involvement of key transcriptional activator NPR1 indicated in npr1–1 mutant.60 Inhibition/mutation based hampered salicylic acid biosynthesis resulted in the increased intensity of infection. However, exogenous supplementation of salicylic acid restored their resistance16 further confirmed the role of salicylic acid in plant defense.
Studies demonstrate that salicylic acid signaling represses expression of auxin related genes by stabilizing auxin response repressors. Salicylic acid signaling counter the ability of plant pathogens to manipulate host auxin metabolism for disease in a tugwar of resistance verses virulence. Salicylic acid, upon infection triggers localized or systemic acquired resistance in which host gains long lasting immunity against pathogen. Early incompatible pathogenic invasion response involves the induction of local host cell death61 followed by induced resistance protecting plants from subsequent attacks.62 The auxin physiology in Arabidopsis leaves was shown to be modulated in an infection by P. syringae. However, in a compatible pathosystem initial rise of antioxidant system declines subsequently40 to overwhelm oxidative local death. Amount of free auxin was shown to suppress the host defense against pathogens63,64 and is associated to disease development.65 In plant–microbe interactions cytokinins have also emerged as a major candidate during nodule organogenesis and pathogenesis. Cytokinins prompt the salicylic acid accumulation which in turn orchestrates the plant immune response. Choi et al.3 recently reviewed how pathogen derived cytokinins interferes the plant derived immunity responses checking the auxin growth responses and salicylic acid signaled plant immunity. Although there is no clearcut answer available whether NO induced stomatal opening and bypassed (preventive) cell death during infection is directly modulated by pathogenic factors.
Salicylic Acid Affects Symbiotic Relationships
The still debatable question is how plant recognizes the invader, whether it is a pathogen or a beneficial symbiont? Symbiosis appears to be regulated by clear recognition and precise systematic regulation of host genes in response to initial confirmation of host at least at the proteomic level. The early stages of Rhizobium-legume symbiosis development is reported to be affected by exogenous salicylic acid application.66 Rhizobia colonizing the roots release the nod factors in response to flavanoids exuded by legumes in rhizosphere, change endogenous salicylic acid content of the host during early nodulation stage. Exogenous salicylic acid inhibit the growth of Rhizobia and the production of nod factors by them and also delay the nodule formation, thereby decreasing the number of nodules per plan.67 Inoculation of Medicago sativa with incompatible strains of Rhizobia resulted in marked increase of endogenous salicylic acid in roots. Contrarily, the compatible strains suppressed salicylic acid accumulation in host roots. It was concluded that later produces certain signals (specific Nod factors) in response, to suppress salicylic acid accumulation.68 The higher concentration of salicylic acid application (5 and 1 mM) inhibited nodulation and markedly decreased nodule number and their dry mass in assistance with lowered nitrogen fixation and photosynthesis.69 Also, the lower concentration of exogenous salicylic acid strongly inhibited indeterminate nodulation in Vicia sativa and pea thereby decreased nodulation, nitrogen fixation and ultimately growth of plants. Interestingly, the same concentration of salicylic acid when sprayed on Phaseolus vulgaris, Lotus japonicas and soybean; formed determinate nodules.
It is very likely that the structure of Nod factor is the key regulator of their bioactivity to be recognized by the receptors of the host. The Nod factors Different Rhizobium species share a common basic structure of lipochitosaccharide; a four to five unit backbone of β1,4linked N-acyl-D-glucosamine, containing a fatty acid at the non-reducing terminal sugar.70 Biochemical studies have shown few putative Nod-factor binding proteins, but these are yet not clearly confirmed as Nodfactor receptors.71
Another important symbiont of plant roots are arbuscular mycorrhizal (AM) fungi, the member of order Glomales colonizing roots of higher plants symbiotically, improving plant nutrition and water use efficiency, protecting against biotic and metal stress particularly.72 During early root colonization of AMF defense responses are activated and suppressed subsequently.59,67 Comparative transcriptome analyses of rice provided an overlap in the response to mycorrhizal fungi and fungal pathogens73 including some responses associated with defense while some other pointed to common compatibility responses to microbes with diverse lifestyles. Liu and coworkers29 marked the induction of cysteine rich proteins sharing identity with defensing domain. Studies performed by Yang and colleagues74 showed induction of nodulin (TC111056), a respiratory burst oxidase (TC100875) and Ntdin (TC107460), a chloroplast protein involved in the synthesis of molybdenum co-factor. Exogenous application of salicylic acid to roots of rice inoculated with AMF reduced inoculation initially during interaction, but on appressoria formation showed no effect. This excludes a direct inhibitory effect of the compound on AMF.45
Interestingly, mutants of Nod- (absence of nodule formation) were also Myc- (absence of mycorrhization).75,76 The sym30 gene mutated in these (P2) plants75,77 was implicated to share a common pathway suppressing salicylic acid dependent defense in plant against these symbionts.78 Nod factor suggested to inhibit the salicylic acid mediated defense in alfalfa roots.68 Gianinazzi-Pearson and coworkers79 reported at least five separate loci involved in both nodulation and AM formation.
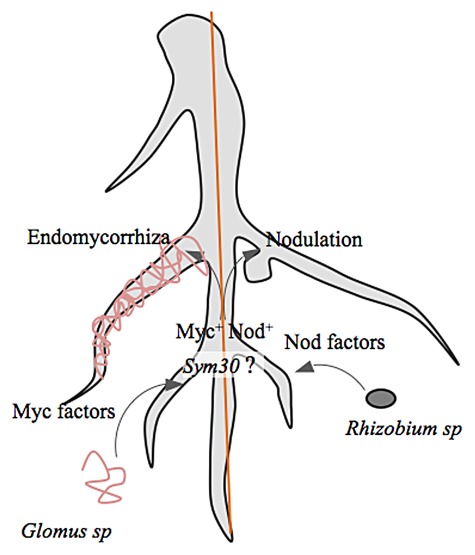
The increasing accumulation of salicylic acid with time in symbiosis-resistant (P2) pea mutants (Nod-, Myc-) for Rhizobium leguminoserum and Glomus mosseae suggested that salicylic acid accumulation in roots of plants depends upon the inability of bacterium to elicit Nod factors. Furthermore, the two unrelated endosymbionts with drastically different host specificities and symbiotic structures may show convergence of molecular and genetic mechanisms80,81 (Fig. 3).
Figure 3. Two most important microbial interactants of plants i.e., root noduling rhizobium and VAM fungi shares the signaling pathways converging at key factors.
Molecular and genetic studies show that the infection processes are strikingly similar. Several genes have been identified which are induced during both symbiotic interactions, e.g., the early nodulin genes ENOD2, ENOD40,82 ENOD5, ENOD12,83 the leg-hemoglobin gene VFLb2984 and the aquaporin encoding gene NOD26.85 However, the most convincing evidence that the infection processes used by either of micro-symbionts involve common steps came from studies with legume mutants which have lost the ability to form nodules. A large proportion of the nodulation-resistant mutants were also completely resistant to AM fungi, while their interaction with soil pathogens was not affected.86,87
In pea, four genes have been identified that are essential for early steps of both the rhizobial and mycorrhizal interactions. Mutants of three of these genes, sym8, sym9 and sym19, have been studied in more detail at a cytological level. These mutants are unable to form an infection thread88 and although AM fungi still can form appressoria on these Nod-/Myc- mutants, they fail to develop intercellular hyphae.86 This shows that rhizobial and mycorrhizal infection involves common mechanisms. The gene ENOD12 is induced in cells involved in, or getting prepared for infection by rhizobia89,90 and is activated when the fungus infects the roots.83
Induction of Biosynthetic Genes and Salicylic Acid Mediated Gene Expression: A Regulatory Affair
The salicylic acid regulates specific kinases and phosphatases to de/activate enzymes (and flux) of specific pathway and their key branching points, the activity of antioxidative enzymes and molecules, the upregulation enzymes of specific pathways as phenylpropanoid pathway (PAL,91 ICS,92 polyamine biosynthesis; P5C) and for other osmolytes and ultimately at the expression level the sensors/receptors and specific transcription factors etc. Possibly salicylic acid inhibits pathogen growth repressing the auxin signaling pathways in plants.65 Evidence shows the manipulated host auxin-biosynthesis by pathogen interfere normal developmental process.92,93 Plant probably themselves represses auxin signaling to come up with defense strategy during infection, though many pathogens can themselves produce auxins.94 Wang and coworkers95 extensively studied the regulatory nodes of SAR at transcriptional level in Arabidopsis thaliana. The involvement of NPR1dependent salicylic acid signaling with WRKY60 and MYB96 transcription factors, SABP2,97 MRPtype ABC transporters8 and NPR1TGA protein complex in activation of salicylic acid responsive elements of PR genes have been recently recognized components of salicylic acid based gene expression regulation and signal transduction cascade.
Future Perspectives
Pests involve plethora of crop feeding pathogens, insects, worms, herbivores and the entire similar category that curtails human consumptionable plants or plant parts. Integrative pest management (IPM) emphasizes to use the term ‘management’ over ‘control’ for pests. We can only manage pests below threshold level to reduce the economic loss as complete elimination of pests is impossible. Life systems are quite intelligent98 to incorporate acclimation (the temporary adjustment within a generation) and adaptation (permanent modification over generations) to survive and evolve further. Under hostile conditions struggling living systems may intensify the struggle to either totally discard another or live together temporarily or permanently. Nevertheless, crops are grown keeping in mind interests of human beings to reduce negative effects of abiotic and biotic stressors maximally. However, human grown crops are homogenous populations of nutritionally important plants, which invite pests inevitably as an easy, safe and sustained substrate to feed over. Salicylic acid has been evolved as a secondary metabolite to ascertain the integrity of system to resist the external trespass. The recruitment of auxins is more common and economic99 in plants allowing the systemic integration. The evolution of these interactants appears to follow the history of prekaryotic invasion of proterobacteria as during the evolution of chloroplasts and mitochondria. This new frontier of research may open the new door in assistance with gene-tailoring and their invitro stabilization into plants to favor the plant growth yield and quality of produce.
Acknowledgments
The breaking and making the boundaries of concepts is the real fuel of science itself. This writing of article is encouraged by the thoughts and ideas of Dr. Clinton Richard Dawkins, University of California, Berkeley, University of Oxford; who influenced the way of thinking through his two popular books; one The selfish genes in 1976 and another in 1982, The extended phenotype. The authors implemented their concepts to correlate communication of messages among plant and microbial kingdom to establish evolving relationships through biochemical cross talk. The authors greatly thank the King Saud University, Deanship of Science, Scientific Research, College of Science Research Center, Riyadh for their financial support.
Glossary
Abbreviations:
- APX
ascorbate peroxidase
- AsA
ascorbic acid
- CAT
catalase
- GR
glutathione reductase
- GSH
glutathione (reduced)
- HR
hypersensitive response
- JA
jasmonic acid
- MeSA
methyl salicylic acid
- NO
nitric acid
- NPR1
non-expresser of PR1
- PCD
programmed cell death
- PGPRs
plant growth promoting rhizobacteria
- POX
peroxidase
- PR1
pathogenesis related (gene) 1
- ROS
reactive oxygen species
- SABP
salicylic acid binding protein
- SAMT1
salicylic acid carboxyl methyl-transferase 1
- SAR
systemic acquired resistance
- SOD
superoxide dismutase
- TMV
tobacco mosaic virus
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18620
References
- 1.van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ. Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2000;97:8711–6. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaler JS, Owen B, Higgins VJ. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 2004;135:530–8. doi: 10.1104/pp.104.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J, Choi D, Lee S, Ryu C-M, Hwang I. Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci. 2011;16:388–94. doi: 10.1016/j.tplants.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Robert-Seilaniantz A, Grant M, Jones JDJ. Hormone crosstalk in plant disease and defense: More than just JASMONATE-SALICYLATE antagonism. Annu Rev Phytopathol. 2011;49:317–43. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 5.Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–63. doi: 10.1146/annurev.pp.43.060192.002255. [DOI] [Google Scholar]
- 6.Hayat S, Fariduddin Q, Ali B, Ahmad A. Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron Hung. 2005;53:433–7. doi: 10.1556/AAgr.53.2005.4.9. [DOI] [Google Scholar]
- 7.Hayat S, Ali B, Ahmad A. Salicylic acid: Biosynthesis, metabolism and physiological role in plants In: Hayat S and Ahmad A eds. Salicylic acid: A plant hormone. Springer, Dordrecht; 2007:1-14. [Google Scholar]
- 8.Yazaki K. Transporters of secondary metabolites. Curr Opin Plant Biol. 2005;8:301–7. doi: 10.1016/j.pbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 10.Dawkins R. The Extended Phenotype. Oxford: Oxford University Press. ISBN 0-19-28805; 1989:1-9 p xiii. [Google Scholar]
- 11.Krouk G, Ruffel S, Gutierrez RA, Gojon A, Crawford NM, Coruzzi GM, et al. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011;16:178–82. doi: 10.1016/j.tplants.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 12.White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–2. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- 13.Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–19. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malamy J, Klessig DF. Salicylic acid and plant disease resistance. Plant J. 1992;2:643–54. doi: 10.1111/j.1365-313X.1992.tb00133.x. [DOI] [Google Scholar]
- 15.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–50. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 16.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–5. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 17.Catinot J, Buchala A, Mansour EA, Metraux JP. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana, (Sussman M.R ed.) FEBS Lett. 2008;582:473–8. doi: 10.1016/j.febslet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Morris K, Mackerness SA-H, Page T, John CF, Murphy AM, Carr JP, et al. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000;23:677–85. doi: 10.1046/j.1365-313x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang WE, Huang L, Preston GM, Martin N, Carr JP, Yanhong L, et al. Quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter sp. ADP1. Plant J. 2006;46:1073–83. doi: 10.1111/j.1365-313X.2006.02758.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaerle L, van Caeneghem W, Messens E, Lambers H, van Montagu M, van der Straeten D. Pre-symptomatic visualization of plant-virus interactions by thermography. Nat Biotechnol. 1999;17:813–6. doi: 10.1038/11765. [DOI] [PubMed] [Google Scholar]
- 21.Chaerle L, Hagenbeek D, De Bruyne E, Valcke R, van der Straeten D. Thermal and chlorophyll-fluorescence imaging distinguish plant–pathogen interactions at an early stage. Plant Cell Physiol. 2004;45:887–96. doi: 10.1093/pcp/pch097. [DOI] [PubMed] [Google Scholar]
- 22.Pancheva TV, Popova LP, Uzunova AM. Effect of salicylic acid on growth and photosynthesis in barley plants. J Plant Physiol. 1996;149:57–63. [Google Scholar]
- 23.Ghai N, Setia RC. Effect of paclobutrazol and salicylic acid on chlorophyll content, hill activity and yield components in Brassica napus L. (cv. GSL-1) Phyomorphol. 2002;52:83–7. [Google Scholar]
- 24.Moharekar ST, Lokhande SD, Hara T, Tanaka R, Tanaka A, Chavan PD. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica. 2003;41:315–7. doi: 10.1023/B:PHOT.0000011970.62172.15. [DOI] [Google Scholar]
- 25.Kumar P, Lakshmi NJ, Mani VP. Interactive effects of salicylic acid and phytohormones on photosynthesis and grain yield of soybean (Glycine max L. Merrill) Physiol Mol Biol Plants. 2000;6:179–86. [Google Scholar]
- 26.Fariduddin Q, Hayat S, Ahmad A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica. 2003;41:281–4. doi: 10.1023/B:PHOT.0000011962.05991.6c. [DOI] [Google Scholar]
- 27.Khan W, Prithviraj B, Smith DL. Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol. 2003;160:485–92. doi: 10.1078/0176-1617-00865. [DOI] [PubMed] [Google Scholar]
- 28.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007;50:529–44. doi: 10.1111/j.1365-313X.2007.03069.x. [DOI] [PubMed] [Google Scholar]
- 30.Heil M, Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–72. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, et al. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell. 1994;6:959–65. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–6. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 33.Park S-W, Liu P, Forouhar F, Vlot AC, Tong L, Tietjen K, et al. Use of a synthetic salicylic acid analog to investigate the roles of methyl salicylate and its esterases in plant disease resistance. J Biol Chem. 2009;284:7307–17. doi: 10.1074/jbc.M807968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Attaran E, Zeier TE, Griebel T, Zeier J. Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell. 2009;21:954–71. doi: 10.1105/tpc.108.063164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorpe MR, Ferrieri AP, Herth MH, Ferrieri RA. 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photo-assimilate even after proton transport is decoupled. Planta. 2007;226:541–51. doi: 10.1007/s00425-007-0503-5. [DOI] [PubMed] [Google Scholar]
- 36.Gutie´rrez-Luna FM, López-Bucio J, Altamirano-Herńndez J, Valencia-Cantero E, Reyes de la Cruz H, Maci´as-Rodri´guez L. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis. 2010;51:75–83. doi: 10.1007/s13199-010-0066-2. [DOI] [Google Scholar]
- 37.Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA. 2007;104:1075–80. doi: 10.1073/pnas.0605423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishina TE, Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 2007;50:500–13. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 39.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Kuzniak E, Sklodowska M. Fungal pathogen-induced changes in the antioxidant systems of leaf peroxisomes from infected tomato plants. Planta. 2005;222:192–200. doi: 10.1007/s00425-005-1514-8. [DOI] [PubMed] [Google Scholar]
- 41.Overmyer K, Brosche M, Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–42. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 42.Pasqualini S, Della Torre G, Ferranti F, Ederli L, Piccioni C, Reale L, et al. Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Physiol Plant. 2002;115:204–12. doi: 10.1034/j.1399-3054.2002.1150205.x. [DOI] [PubMed] [Google Scholar]
- 43.Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–6. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- 44.Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–37. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- 45.Blilou I, Ocampo JA, Garcı’a Garrido JM. Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J Exp Bot. 2000;51:1969–77. doi: 10.1093/jexbot/51.353.1969. b. [DOI] [PubMed] [Google Scholar]
- 46.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–75. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 47.Dangl JL, Jones JDG. Plant pathogens and integrated defense responses to infection. Nature. 2001;411:826–33. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 48.Anderson MD, Chen Z, Klessig DF. Possible involvement of lipid peroxidation in salicylic acid-mediated induction of PR1 gene expression. Phytochem. 1998;47:555–66. doi: 10.1016/S0031-9422(97)00726-7. [DOI] [Google Scholar]
- 49.Orti´z-Castro R, Contreras-Cornejo HA, Maci´as-Rodri´guez L, López-Bucio J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009;4:701–12. doi: 10.4161/psb.4.8.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anand A, Krichevsky A, Schornack S, Lahaye T, Tzfira T, Tang Y, et al. Arabidopsis VirE2 interacting protein2 is required for Agrobacterium T-DNA integration in plants. Plant Cell. 2007;19:1695–708. doi: 10.1105/tpc.106.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuchiya Y, McCourt P. Strigolactones: A new hormone with a past. Curr Opin Plant Biol. 2009;12:556–61. doi: 10.1016/j.pbi.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Stacey G, McAlvin CB, Kim SY, Olivares J, Soto MJ. Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 2006;141:1473–81. doi: 10.1104/pp.106.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prithiviraj B, Bais HP, Jha AK, Vivanco JM. Staphylococcus aureus pathogenicity on Arabidopsis thaliana is mediated either by a direct effect of salicylic acid on the pathogen or by salicylic acid-dependent, NPR1-independent host responses. Plant J. 2005;42:417–32. doi: 10.1111/j.1365-313X.2005.02385.x. a. [DOI] [PubMed] [Google Scholar]
- 54.Prithiviraj B, Bais HP, Weir T, Suresh B, Najarro EH, Dayakar BV, et al. Down regulation of virulence factors of Pseudomonas aeruginosa by salicylic acid attenuates its virulence on Arabidopsis thaliana and Caenorhabditis elegans. Infect Immun. 2005;73:5319–28. doi: 10.1128/IAI.73.9.5319-5328.2005. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veena, Jiang H, Doerge RW, Gelvin SB. Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J. 2003;35:219–36. doi: 10.1046/j.1365-313X.2003.01796.x. [DOI] [PubMed] [Google Scholar]
- 56.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–60. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994;26:1439–58. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- 58.Vallad GE, Goodman RM. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004;44:1920–34. doi: 10.2135/cropsci2004.1920. [DOI] [Google Scholar]
- 59.Marti´nez-Medina A, Roldán A, Albacete A, Pascual JA. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry. 2011;72:223–9. doi: 10.1016/j.phytochem.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Chaturvedi R, Shah J. Salicylic acid in plant disease resistance In: Hayat S, Ahmad A eds. Salicylic Acid: A Plant Hormone. Springer, Dordrecht, 2006:335-370. [Google Scholar]
- 61.Ross AF. Systemic effects of local lesion formation. In: Beemster ABR, Dijkstra J eds. Viruses of Plants. Amsterdam, North-Holland. 1966:127-150. [Google Scholar]
- 62.McIntyre JL, Dodds JA, Hare D. Effects of localized infections of Nicotiana tabacum by tobacco mosaic virus on systemic resistance against diverse pathogens and an insect. Phytopathology. 1981;71:297–301. doi: 10.1094/Phyto-71-297. [DOI] [Google Scholar]
- 63.Chen Z, Agnew JL, Cohen JD, He P, Shan L, Sheen J, et al. Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc Natl Acad Sci USA. 2007;104:20131–6. doi: 10.1073/pnas.0704901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang D, Mukhtar KP, Culler AH, Dong X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol. 2007;17:1784–90. doi: 10.1016/j.cub.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, et al. Activation of the indole-3-acetic acid-amido synthetase GH3–8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell. 2008;20:228–40. doi: 10.1105/tpc.107.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blilou I, Bueno P, Ocampo JA, Garcı’a Garrido JM. Induction of catalase and ascorbate peroxidase activities in tobacco roots inoculated with arbuscular mycorrhizal Glomus mosseae. Mycol Res. 2000;104:722–5. doi: 10.1017/S095375629900204X. a. [DOI] [Google Scholar]
- 67.Mabood F, Smith D. The role of salicylates in rhizobium-legume symbiosis and abiotic stresses in higher plants. In: Hayat S, Ahmad A eds. Salicylic acid: A Plant Hormone. Springer, Dordrecht, Netherlands, 2007:151-162. [Google Scholar]
- 68.Marti´nez-Abarca F, Herrera J, Bueno P, Sanjuan J, Bisseling T, Olivares J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol Plant Microbe Interact. 1998;11:153–5. doi: 10.1094/MPMI.1998.11.2.153. [DOI] [Google Scholar]
- 69.van Spronsen PC, Tak T, Rood AMM, van Brussel ANN, Kijne JW, Boot KJM. Salicylic acid inhibits indeterminate-type nodulation but not determinate-type nodulation. Mol Plant Microbe Interact. 2003;16:83–91. doi: 10.1094/MPMI.2003.16.1.83. [DOI] [PubMed] [Google Scholar]
- 70.Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–98. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niebel A, Bono JJ, Ranjeva R, Cullimore JV. Identification of a high affinity binding site for lipooligosaccharidic NodRm factors in microsomal fraction of Medicago cell suspension cultures. Mol Plant Microbe Interact. 1997;10:132–4. doi: 10.1094/MPMI.1997.10.1.132. [DOI] [Google Scholar]
- 72.Linderman RG. Role of VAM in biocontrol. In: Pfleger FL, Linderman RG eds. Mycorrhizae and Plant Health. APS Press, 1994:1-25. [Google Scholar]
- 73.Güimil S, Chang H-S, Zhu T, Sesma A, Osbourn A, Roux C, et al. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc Natl Acad Sci USA. 2005;102:8066–70. doi: 10.1073/pnas.0502999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang SH, Berberich T, Miyazaki A, Sano H, Kusano T. Ntdin, a tobacco senescence-associated gene, is involved in molybdenum cofactor biosynthesis. Plant Cell Physiol. 2003;44:1037–44. doi: 10.1093/pcp/pcg122. [DOI] [PubMed] [Google Scholar]
- 75.Duc G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S. First report of non-mycorrhizal plant mutants (Myc-) obtained in pea (Pisum sativum) and Faba bean (Vicia Faba L.) Plant Sci. 1989;60:215–22. doi: 10.1016/0168-9452(89)90169-6. [DOI] [Google Scholar]
- 76.Bradbury SM, Peterson RL, Bowley SR. Interactions between three alfalfa nodulation genotypes and two Glomus species. New Phytol. 1993;124:605–73. doi: 10.1111/j.1469-8137.1991.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 77.Sagan M. Analyse de mutants symbiotiques de pois. Pisum sativum The`se de l'Univertite´ de Paris Sud-Orsay, 1992.
- 78.López-R´ez JA, Verhage A, Ferńndez I, Garci´a JM, Azcón-Aguilar C, Flors V, et al. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot. 2010;61:2589–601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gianinazzi-Pearson V, Gianinazzi S, Guillemin GP, Trouvelot A, Duc G. Genetic and cellular analysis of resistance to vesicular (VA) mycorrhizal fungi in pea mutants. In: Hennecke H, Verma DPS eds. Advances in molecular genetics and plant–microbe interactions. Kluwer, Dordrecht, 1991:336– 342. [Google Scholar]
- 80.Gianinazzi-Pearson V. Biological fixation of nitrogen for ecology and sustainable agriculture, Legocki A, Bothe H, Pühler, A eds. Springer, Heidelberg, 1997. [Google Scholar]
- 81.Hirsch AM, Kapulnik Y. Signal transduction pathways in mycorrhizal associations: Comparisons with the Rhizobium–legume symbiosis. Fungal Genet Biol. 1998;23:205–12. doi: 10.1006/fgbi.1998.1046. [DOI] [PubMed] [Google Scholar]
- 82.van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–42. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albrecht C, Geurts R, Lapeyrie F, Bisseling T. Endomycorrhizae and rhizobial Nod factors both require SYM8 to induce the expression of the early nodulin genes PsENOD5 and PsENOD12A. Plant J. 1998;15:605–14. doi: 10.1046/j.1365-313x.1998.00228.x. [DOI] [PubMed] [Google Scholar]
- 84.Frühling M, Roussel H, Gianinazzi-Pearson V, Puhler A, Perlick AM. The Vicia faba leghemoglobin gene VfLb29 is induced in root nodules and in roots colonized by the arbuscular mycorrhizal fungus Glomus fasciculatum. Mol Plant Microbe Interact. 1997;10:124–31. doi: 10.1094/MPMI.1997.10.1.124. [DOI] [PubMed] [Google Scholar]
- 85.Wyss P, Mellor RB, Wiemken A. Vesicular–arbuscular mycorrhizas of wild-type soybean and non-nodulating mutants with Glomus mossae contain symbiosis-specific polypeptides (mycorrhizins), immunologically cross-reactive with nodulins. Planta. 1990;182:22–6. doi: 10.1007/BF00239978. [DOI] [PubMed] [Google Scholar]
- 86.Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell. 1996;8:1871–83. doi: 10.1105/tpc.8.10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrison MJ. The arbuscular mycorrhizal symbiosis: an underground association. Trends Plant Sci. 1997;2:54–60. doi: 10.1016/S1360-1385(97)82563-0. [DOI] [Google Scholar]
- 88.LaRue TA, Weeden NF. The symbiosis genes of the host. In: Kiss GB, Endre G. Proceedings of the 1st European Nitrogen Fixation Conference eds. Officina Press, Szeged, Hungary, 1994:147–151. [Google Scholar]
- 89.Scheres B, Van Engelen F, Van der Knaap E, Van de Wiel C, Van Kammen A, Bisseling T. Sequential induction of nodulin gene expression in the developing pea nodule. Plant Cell. 1990;2:687–700. doi: 10.1105/tpc.2.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Journet EP, Pichon M, Dedieu A, De Billy F, Truchet G, Barker DG. Rhizobium meliloti Nod factors elicit cell specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J. 1994;6:241–9. doi: 10.1046/j.1365-313X.1994.6020241.x. [DOI] [PubMed] [Google Scholar]
- 91.Maslenkova V, Stojnova PZ, Popova L. Salicylic acid-induced changes in photosystem II reactions in barley plants. Biotechnol and Biotechnol 2009; 23:EQ.23/2009/SE Special edition/On-line.
- 92.Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9:109–19. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 93.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–51. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 94.Maor R, Haskin S, Levi-Kedmi H, Sharon A. In planta production of indole-3-acetic acid by Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 2004;70:1852–4. doi: 10.1128/AEM.70.3.1852-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang D, Amornsiripanitch N, Dong X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006;2:e123. doi: 10.1371/journal.ppat.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galis I, Matsuoka K. Transcriptomic analysis of salicylic acid-responsive genes in tobacco BY-2 cells. In: Hayat S, Ahmad A eds. Salicylic acid-A plant hormone, Springer, Dordrecht, The Netherlands, 2007; 371-396. [Google Scholar]
- 97.Anand A, Uppalapati SR, Ryu C-M, Allen SN, Kang L, Tang Y, et al. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008;146:703–15. doi: 10.1104/pp.107.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trewavas A. Aspects of plant intelligence. Ann Bot. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stewart JL, Nemhauser JL. Do Trees Grow on Money? Auxin as the currency of the cellular economy. Cold Spring Harb Perspect Biol. 2010;2:a001420. doi: 10.1101/cshperspect.a001420. [DOI] [PMC free article] [PubMed] [Google Scholar]