Abstract
Human CD38 is a novel multi-functional protein that acts not only as an antigen for B-lymphocyte activation, but also an enzyme catalyzing the synthesis of a Ca2+ messenger molecule, cyclic ADP-ribose, from NAD+. It is well established that this novel Ca2+ signaling enzyme is responsible for regulating a wide range of physiological functions. Based on the crystal structure of the CD38/NAD+ complex, we synthesized a series of simplified N-substituted nicotinamide derivatives (Compound 1–14). A number of these compounds exhibited moderate inhibition of the NAD+ utilizing activity of CD38, with Compound 4 showing the higher potency. The crystal structure of CD38/ Compound 4 complex and computer simulation of Compound 7 docking to CD38 show a significant role of the nicotinamide moiety and the distal aromatic group of the compounds for substrate recognition by the active site of CD38. Biologically, we showed that both Compounds 4 and 7 effectively relaxed the agonist-induced contraction of muscle preparations form rats and guinea pigs. This study is a rational design of inhibitors for CD38 that exhibit important physiological effects, and can serve as a model for future drug development.
Introduction
CD38 is a trans-membrane enzyme, originally identified as a lymphocyte differentiation antigen1. It is now known to be ubiquitously expressed in virtually all mammalian tissues examined2. As a multi-functional protein and a member of ADP-ribosyl cyclase family, CD38 catalyzes the synthesis of cyclic ADP-ribose (cADPR) from NAD+, a cyclic nucleotide messenger mediating Ca2+ release from intracellular stores in a wide range of biological systems from plant to human3 Remarkably, CD38 can also hydrolyze the product, cADPR, and the substrate, NAD+, to produce ADP-ribose4. That CD38 is the naturally occurring enzyme responsible for the synthesis of cADPR has been shown by ablation of the CD38 gene in mice, which results in large reduction in endogenous cADPR in many tissues5,6. The CD38 knockout mice exhibit a variety of defects, establishing the importance of CD38 as a regulator of diverse physiological functions5,6, which include immune cell differentiation7, α-adrenoceptor signaling in aorta8, hormonal signaling in pancreatic acinar cells9, migration of dendritic cell precursors10, bone resorption11, insulin secretion5,12, and social behavior changes13. Clinically, CD38 expression is a negative prognostic marker for chronic lymphocytic leukemia14,15. Moreover, CD38 is responsible for synthesizing yet another ubiquitous Ca2+ messenger, nicotinic acid adenine dinucleotide phosphate (NAADP), from NADP and nicotinic acid via a base-exchange reaction16,17. It should now be a generally accepted fact that CD38 is expressed both in intracellular organelles, such as nucleus, ER, etc., as well as on the surface of some cells, particular the blood cells. It is our belief that internal CD38 may be more relevant for cell signaling.
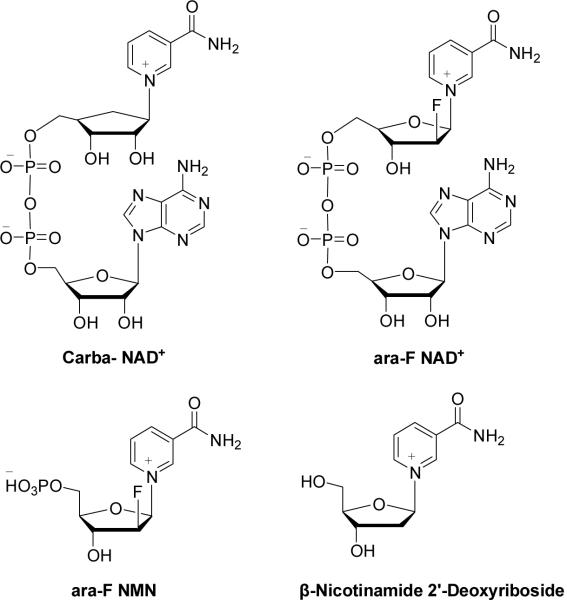
That CD38 plays key roles in physiology provides important impetus for this study to design and synthesize inhibitors of CD38. Inhibitors of the enzymatic activities of CD38 have been described, but none of them have been shown to have physiological effects. Slama et al. synthesized a non-hydrolyzable analog of NAD+, dinucleotide carbanicotinamide adenine dinucleotide (carba-NAD+), as a competitive inhibitor of the NAD+ glycohydrolase activity of CD38 with the IC50 about 100 μM18,19. Inhibitors that form covalent intermediates with the catalytic residues of CD38 have also been described. These inhibitors are mainly NAD+ derivatives with modifications at the nicotinamide ribonucleoside. For example, a series of fluoro-substituted NAD+ derivatives have been produced20,21. The fluoro substitution at the 2′-position of the nicotinamide sugar moiety promotes the formation of a stable covalent bond between the ribose and Glu226, the catalytic residue of CD38, during catalysis. The covalent intermediate has been captured by X-ray crystallography22. Based on the structure of ara-F NAD, Schramm and coworkers reported that ara-F NMN and several β-nicotinamide 2′-deoxyribosides are also potent inhibitors of CD38 with the Ki values at nano-molar range21,23.
These inhibitors, being derivatives of NAD+, are charged molecules with limited permeability to cells and tissues and none has been reported to exhibit biological effects. It is the purpose of this study to develop membrane-permeant CD38 inhibitors as tools for physiological investigations and as potential drug candidates as well. Based on the crystal structure of the CD38/NAD+ complex that we reported previously24, we synthesized a series of simplified N-substituted nicotinamide derivatives (Figure 2, 1–14) and showed that some of them, including Compounds 4 and 7, are good inhibitors of the enzymatic activities of CD38. X-ray crystallography shows that Compound 4 binds inside the catalytic cavity of CD38 and reveals important details of the interacting residues at the active site. Moreover, when applied to guinea pigs tracheal muscle strips, both Compounds 4 and 7 exhibit potent relaxing effect on the agonist -induced tension.
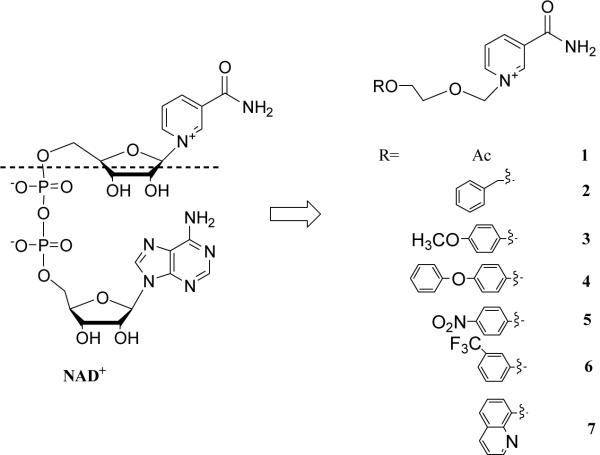
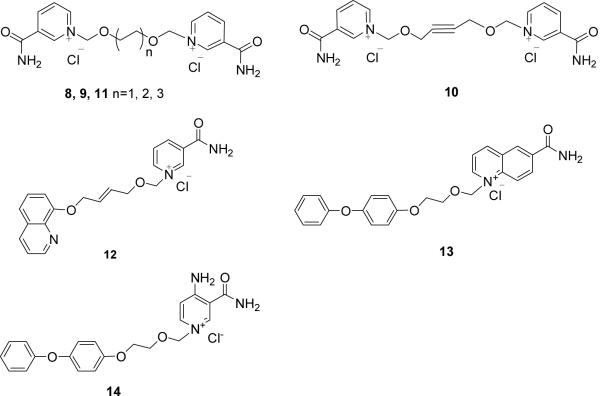
Figure 2.
NAD+ and N-aromatic ether substituted nicotinamides.
Results and Discussion
Chemistry
Crystal structure of the CD38/NAD+ complex shows that the nicotinamide group of the bound NAD+ enters the catalytic cavity first and interacts with residues Glu146 and Asp155 at the active site through two hydrogen bonds to its amide. The interaction is further enhanced by the parallel π interactions between its pyridine ring and the Trp189 indole ring24b. It is reasoned that derivatives consisting of either one nicotinamide (Compounds 1–7) or bis-nicotinamide moiety (Compounds 8–11) may have sufficient affinity for the catalytic cavity of CD38 to serve as inhibitors. In addition, a series of derivatives was designed with aromatic ether strands replacing the adenosine-pyrophosphate moiety of the natural substrate, NAD+, so as to improve the membrane permeability of the compounds. Finally, in Compounds 13 and 14, 4-amino-nicotinamide and 6-quinoline carboxylic amide replaced and mimiced the nicotinamide ring, respectively.
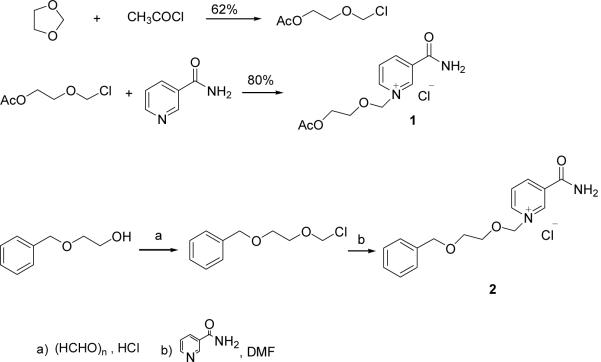
Compounds 1 and 2 were synthesized as depicted in scheme 1.25 Starting from 1,3-dioxolane and acetyl chloride, the chloromethoxyl ethyl acetate was obtained in 62% yield, which was stirred with nicotinamide for 10 hr in DMF at room temperature26 to form compound 1 with high yield (80%). Compound 2 was produced by the condensation of [2-(benzyloxy)ethoxy]methyl chloride27 and nicotinamide in DMF in 60 % yield.
Scheme 1.
Synthesis of analogues 1 and 2.
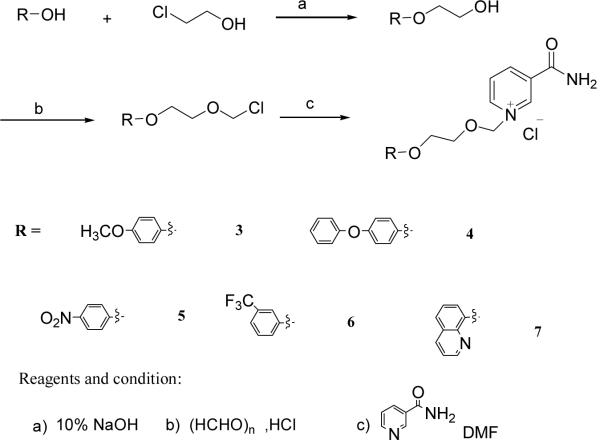
Compounds 3–7 were synthesized by a similar strategy. Substituted phenols or 8-hydroxyl-quinoline were condensed with chloroethanol in a 10% NaOH solution28. The corresponding alcohols were chloromethylated by paraformaldehyde and dry HCl in DCE. The chloromethylation products were used directly to react with nicotinamide to produce Compounds 3–7, respectively, in good yields (Scheme 2).
Scheme 2.
Synthesis of analogues 3–7.
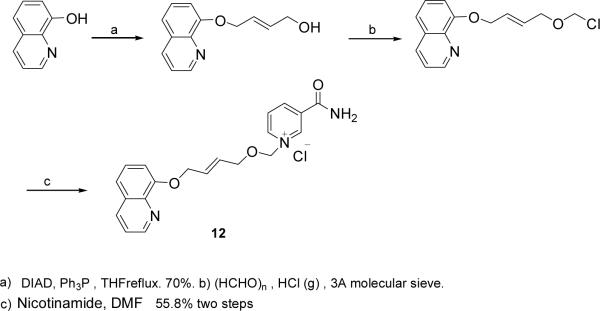
8-OH-qunoline was coupled with 1,4-butenediol by Mitsunobu reaction in 70% yield. The chloromethylation product was obtained as described before and then condensed with nicotinamide to give Compound 12 in 55.8% for two steps. (Scheme 3)
Scheme 3.
Synthesis of analogues 12
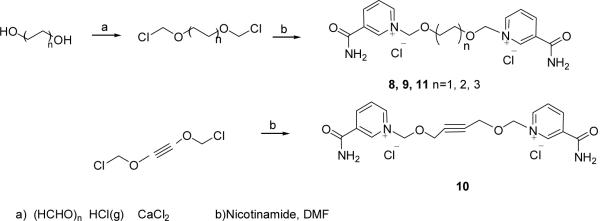
The synthesis of the bis-nicotinamide Compounds 8–11 were completed by the condensation of bis-chloromethylation products with two equivalents of nicotinamide, respectively, in high yields. 1,2-Bis- chloromethoxyethane, 1,4-bis-chloromethoxybutane, 1,4-bis-chloromethoxycrotonylene and 1,6-bis-chloromethoxyhexane29 were prepared from the corresponding dihydroxyl compounds by chloromethylation reaction, respectively, as described before. (Scheme 4)
Scheme 4.
Synthesis of 8–11.
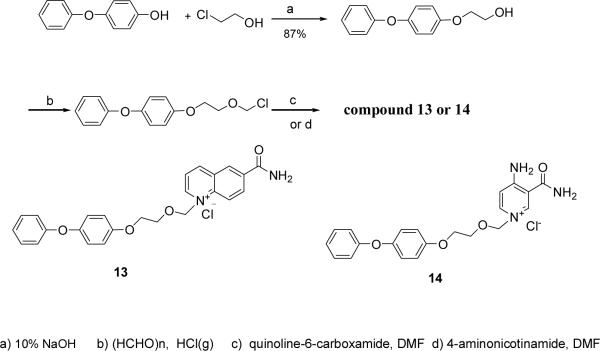
4-Phenoxyphenol was coupled with chloroethanol and then chloromethylated. The product was condensed with quinoline-6-carboxylic amide or 4-amino nicotinamide30 respectively in DMF for 12 h at room temperature to produce Compounds 13 (63%) and 14 (43%) in two steps (Scheme 5).
Scheme 5.
Synthesis of analogues 13 and 14.
Biological Activities
Inhibition of the activity of NADase
The newly synthesized Compounds 1–14 were tested for their inhibitory properties against the NAD-glycohydrolase activity of the recombinant CD38, which was measured using a fluorimetric and highly sensitive coupled enzyme assay as previously described31. As shown in Table 1, compounds 3,4,5,7,12,13 and 14 exhibit weak inhibitory activities. Structurally, compounds with an aromatic group at the distal end from the nicotinamide are more effective in inhibiting CD38. This is likely to be due to the enhanced hydrophobic interactions with the active site. Compound 6 contains a strong electron withdrawing m-CF3-phenol group that should interfere with hydrophobic interaction, and it indeed showed no affinity for CD38. The positively charged bis-nicotinamide moiety in Compounds 8–11 likewise should prohibit hydrophobic interaction and the compounds were, likewise, ineffective in inhibiting CD38. Interestingly, the longer aliphatic chain in Compound 12, as compared to Compound 7, makes this compound to improve its interaction with CD38. Comparing Compounds 13 and 4, replacing the nicotinamide with a quinoline ring, improved the affinity slightly. On the other hand, the additional amino group on the nicotinamide ring in Compound 14 offered no improvement as compared to Compound 4.
Table 1.
Inhibition of the NADase of CD38 by Compounds 1–14.
| Inhibitor | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| IC50(mM) | NM | NM | 11.41 | 3.42 | 5.65 | NM | 10.00 |
| Inhibitor | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| IC50(mM) | NM | NM | NM | NM | 3.45 | 2.12 | 4.80 |
NM=inhibition not measurable.
Physiological effects on muscle preparations from rat and guinea pigs
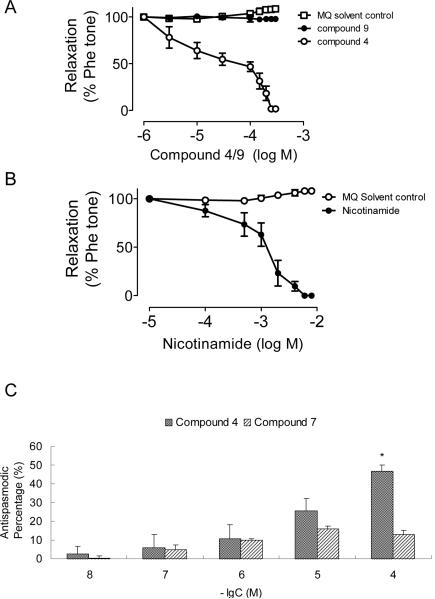
It has previously been shown that CD38 gene ablation attenuates the contraction induced by α-adrenoceptor stimulation in mouse aorta, indicating that the contraction is mediated by the CD38/cADPR-pathway8. We therefore tested the effect of compound 4 on the phenylephrine-induced contraction in rat aortic ring preparations with intact endothelium. As shown in Figure 3A, compound 4 produced concentration-dependent relaxation with a half-maximal effect (pD2) at about 36 μM. The effect is specific because the structurally similar but inactive compound 9 produced no such vascular relaxing effect. Nicotinamide, a commonly used inhibitor of CD38, can effectively attenuate agonist-induced contraction32. Figure 3B shows that it can induce vascular relaxation in a manner similar to compound 4. The half-maximal effect was at about 1 mM.
Figure 3.
Biological effects of compounds 4, 7 and 9 on agonist-induced muscle contraction. A. Concentration–response curves for solvent (MQ water) control, compound 4 (active) and compound 9 (inactive), on the phenylephrine-induced vascular contraction in isolated rat aortic ring preparations. B. Nicotinamide, a commonly used inhibitor of CD38 induced similar vascular relaxation. Results are means±S.E.M. using tissues from three to five rats in each group. C. The relaxing effects of Compounds 4 and 7 on the acetylcholine-induced contraction in isolated tracheal strips of guinea pigs. Data represent the mean ± SEM (n= 7). *P<0.05, statistically significant as compared with Krebs-Henseleit solution group.
We have previously shown that Compound 7, the less potent of the series with an IC50 of 10 mM for the inhibition of the NADase, significantly inhibited the acetylcholine (Ach)-induced Ca2+ increase in PC12 cells. That the effect of Ach is mediated by CD38 is shown by RNAi knockdown of the enzyme, which completely abrogates the ACh-induced production of cADPR33. Airway smooth muscle contraction requires elevation of intracellular Ca2+. Studies indicate that the CD38/cADPR/RyR-Ca2+ signaling pathway plays a vital role in the regulation of Ca2+ homeostasis in the airway smooth muscle cells34. Here we use the newly synthesized inhibitors to illustrate the role of CD38 in mediating the Ach-induced contraction in airway smooth muscle. The isolated tracheal strips of guinea pigs and rats were first contracted by treatment with Ach. The ability of the CD38-inhibitors to relax the contraction was then used as a model for the antispasmodic effects of the compounds. As shown in Figure 3, both Compounds 4 and 7, the best active and lower active compounds in our series, were effective in relaxing the Ach-induced contraction in a dose-dependent manner. Similar results were also observed in the isolated tracheal strips of rat (data not shown). These results are consistent with the known fact that CD38 is important in mediating the Ach-induced contraction in airway smooth muscle34. But more importantly, the results illustrate the usefulness of these new inhibitors for investigating the physiological roles of CD38 in cells and tissues. Although, these inhibitors show only moderate activity in vitro, they are much more effective in vivo. This is likely because of the hydrophobicity of the compounds, allowing them to easily penetrate the cell membrane and accumulate inside cells and tissues. We have calculated the AlogP using Pipeline Pilot V7.5 and the results indicate clearly that compounds 4 and 7 are more lipophilicity than compound 9.(Supporting Information) As is shown below, the compounds bind inside the catalytic pocket of CD38. It is also possible that the structure as well as the residues constituting the active site of rats and guinea pigs CD38 may be somewhat different from those of the human CD38, which was used in all the in vitro assays. Currently, the structures of neither rat nor guinea pigs CD38 have been solved.
Structural study of the binding of Compounds 4 and 7 to CD38
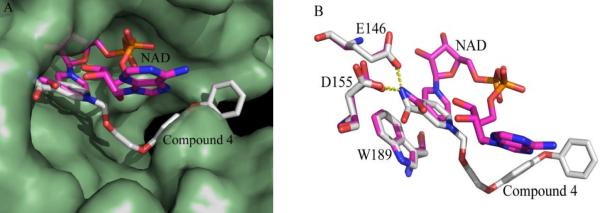
To understand the interactions between CD38 and these inhibitors, we prepared the complexe of Compound 4 with CD38 and analyzed it using X-ray crystallography. Pre-formed crystals of the catalytic domain of CD38 were soaked in the cryoprotectant buffer containing the compound to obtain the complex. We were able to obtain only the complex with Compound 4 (Supporting Information shows the statistics of data collection and structure refinement of the complex). Figure 4A shows that Compound 4 binds inside the catalytic pocket of human CD38. Superimposed in the Figure is the bound NAD previously determined by us24. As can be seen, the nicotinamide groups of both Compound 4 and NAD bind at the same position. They also interact identically with the same residues, forming hydrogen bonds with Glu146 and Asp155, as well as hydrophobic stacking with Trp189 (Figure 4A). The structural results indicate that the inhibitory effect of Compound 4 is likely to be due to its specific binding to the active site. The N-substituted biphenyl ether group in Compound 4 distal to the nicotinamide ring, on the other hand, binds quite differently than the ribose and phosphate groups of NAD, interacting instead mainly with Trp176 through hydrophobic stacking (Fig. 4A).
Figure 4. Structural alignment between CD38-Compound 4 and CD38-NAD complexes.
(A) Surface presentation of the active pocket of CD38 (palegreen). NAD (sticks presentation in magentas) penetrated to the bottom of the active pocket of CD38, while compound 4 (sticks presentation in grey) floated over the active site. (B) The nicotinamide group of both compound 4 (grey) and NAD (magentas) is similarly positioned and stabilized by the interactions with residue Glu146, Asp155 and Trp189 of CD38.
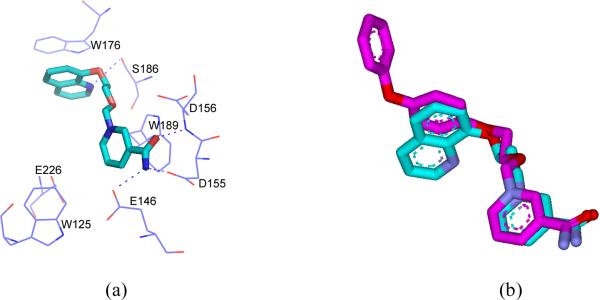
Molecular dynamics simulation allows us to model the interaction of Compound 7 with CD38, even though we were unable to obtain the crystal complex. The stimulation was carried out starting with the crystallographic data obtained from the CD38/Compound 4.
The result is shown in Figure 5a and the superimposition of Compounds 4 and 7 is shown in Figure 5b. The docking study indicated that the positioning of Compound 7 at the active site is approximately the same as Compound 4 as observed in the crystal structure. Beside the same hydrogen bonding of the nicotinamide moieties of Compound 7 and 4 with the active site, interactions between the nitrogen atom of quinoline of the Compound 7 with residue Ser186 and π-stacking interaction between quinoline ring with Trp176 of CD38 were also observed. The similarity of the interactions is consistent with both compounds having inhibitory effect on CD38.
Figure 5.
a) The molecular dynamics simulation binding mode of compound 7 in the active pocket of human CD38. The carbon atoms of 7 are indicated in cyan. All the nitrogen atoms are blue; oxygen atoms are red. Hydrogen bonds are represented by blue dotted lines. Corresponding residues are labeled and shown in blue lines. b) Superimposition the binding poses of compound 4 in the enzyme-inhibitor cyrastal with molecular dynamics simulation result of 7. 4 is shown as magenta stick; 7 is shown as cyan stick.
Conclusion
There is a growing tendency to develop non-nucleotide compound to mimic such signaling molecules.35 This study represents a rational design of a series of inhibitors of CD38, one of the key enzyme in cellular Ca2+ signaling. The design was based on the crystal structure of NAD binding to the active site of CD38 and takes into account the need for membrane permeability. We targeted the nicotinamide portion of the NAD because it interacts strongly with the active site via not only hydrogen bonding but also hydrophobic stacking. The reasoning was substantiated by the crystal structure, showing the nicotinamide portion of the Compound 4 binds identically as NAD. The replacement of the rest of the highly charged moities of the NAD with aromantic groups ensures membrane permeability and contributes to the greatly increased in vivo potency of the inhibitors. The potency increase is observed both in cultured PC12 cells as we have previously reported33 and muscle preparations from rats and guinea pigs described here, verifying the general applicability of the new inhibitors. Structure-activity comparison of all the compounds in this series provides clues for next iteration to produce even more effective inhibitors and set the stage toward developing drug candidates for CD38-related diseases.
Experiment Section
Chemistry
All solvents and reagents were obtained from commercial sources and used without further purification unless otherwise stated. HR-ESI-MS (electrospray ionization) were performed with Bruker BIFLEX III. 1H NMR and 13C NMR were recorded with a Bruker AVANCE III 400 or a JEOL AL300 spectrometer. CD3OD, DMSO-d6 or D2O were used as a solvent. Chemical shifts are reported in parts per million downfield from TMS (1H and 13C). 19F-NMR spectra were recorded on a Varian VXR-500 spectrometer. Chemical shifts of 19F-NMR are reported in ppm with reference to CF3COOH as an external standard. Anhydrous solvents were obtained as follows: DMF was dried with CaH2 at room temperature before distilled in vacuo; Ethyl acetate was dried with P2O5 before distill. 1,2-dichlorethane, ethanol and methanol were distilled over CaH2.
1-[(2-acetoxyethoxy)methyl]-3-(aminocarbonyl)-pyridinium chloride (Compound 1)
Dry nicotinamide (122mg, 1 mmol) was dissolved in dry DMF (3 mL), chloromethoxyl ethyl acetate (0.14 mL, 1 mmol) was added under N2. The mixture was stirred at room temperature for 1h and a large amount of solid appeared. Dry ethyl acetate (3ml) was added and the mixture was stirred for 30 mins. The white precipitates were collected by filtration and washed with dry ethyl acetate, then recrystallized from EtOH/EA to give the desired Compound 1 (221 mg, 80%) as a white solid. 1H NMR (300 MHz, CD3OD) δ 2.03 (s, 3H), 3.98 (m, 2H), 4.25 (m, 2H), 6.06 (s, 2H), 8.30 (dd, 1H, J =7.2, 5.1 Hz), 9.07 (d, 1H, J =7.2 Hz), 9.21 (d, 1H, J =5.1 Hz), 9.53 (s, 1H).13C NMR (75 MHz, CD3OD) δ 20.7, 63.9, 70.8, 90.7, 129.3, 135.8, 144.2, 145.8, 146.5, 165.0, 172.4. ESI-MS: [M-Cl]+: 239.1. Anal. (C11H15ClN2O4·0.3H2O) C, H, N.
1-[(2-benzyloxyethoxy)methyl]-3-(aminocarbonyl)-pyridinium chloride (Compound 2)
To a solution of 2-(benzyloxy) ethanol (0.85ml, 6 mmol) in dry 1,2-dichloroethane (8ml), paraformaldehyde (0.18g, 6mmol) was added. Dry HCl gas, obtained in situ from H2SO4 and NaCl, was bubbled through the reaction mixture over 10 h at 0°C. The solution was then dried with anhydrous Na2SO4, filtered and concentrated under reduced pressure to give a yellow oily residue. The residue was added to a solution of nicotinamide (0.73g, 6 mmol) in DMF (15mml). The mixture was stirred at room temperature for 4h. The white precipitates were collected by filtration and washed with dry ethyl acetate and ethanol, then recrystallized from EtOH by freezing to give the desired Compound 2 (1.25g, 65% for two steps) as a white solid. 1H NMR (300 MHz, CD3OD) δ 3.64 (m, 2H), 3.99 (m, 2H), 4.36 (s, 2H), 6.01 (s, 2H), 7.18–7.32 (m, 5H), 8.05 (dd, 1H, J =8.1, 6.3 Hz), 8.82 (d, 1H, J =8.1 Hz), 9.12 (d, 1H, J =6.3 Hz), 9.45 (s, 1H).13C NMR (75 MHz, CD3OD) δ 165.0, 146.0, 145.7, 144.1, 139.0, 135.3, 129.5, 129.1, 129.0, 128.9, 128.8, 91.4, 74.2, 73.3, 70.3. ESI-MS: [M-Cl]+ 287.1. Anal. (C16H19ClN2O3) C, H, N.
1-{[2-(4-methoxy-phenoxy)ethoxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 3)
4-methoxyphenol (0.56g, 4.5mmol) was dissolved in 10% sodium hydroxide solution (16ml, 40mmol). 2-chloroethanol (2.68 ml, 40mmol) was then added and the mixture stirred for 24 h at room temperature. The solution was extracted with CH2Cl2. The extract was washed with water three times then dried by evaporation. The product obtained, 2-(4-methoxyphenoxy) ethanol (0.68g, 4.1mmol, 90%), was used in next step directly and dissolved in 1,2-dichloroethane (10ml). Paraformaldehyde (0.13g, 4.1mmol) was added and dry HCl gas was bubbled through the reaction mixture for 10 h at 0°C. The solution was then treated as in the synthesis of Compound 2 and reacted with nicotinamide (0.5g, 4.1 mmol) in DMF (8mml). The mixture was stirred at room temperature for 8h. The white precipitates were collected by filtration and washed with dry ethyl acetate and ethanol. The crude product was recrystallized from EtOH/EA to obtain the desired Compound 3 (1.14g, 82% for two steps) as a white solid. 1H NMR (300 MHz, CD3OD) δ 3.72 (s, 3H), 4.11 (m, 4H), 6.11 (s, 2H), 6.72–6.83 (m, 4H), 8.23 (dd, 1H, J =8.4, 6.0 Hz), 8.99 (dt, 1H, J =8.4, 1.5 Hz), 9.22 (d, 1H, J =6.0 Hz), 9.55 (s, 1H).13C NMR (75 MHz, CD3OD) δ 165.0, 155.8, 153.5, 146.3, 145.7, 144.2, 135.5, 129.1, 91.2, 72.3, 68.5, 56.0. ESI-MS: [M-Cl]+ 303.1. Anal. (C16H19ClN2O4) C, H, N.
1-{[2-(4-phenoxy-phenoxy)ethoxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 4)
The procedure used was similar to that of the synthesis of Compound 3. 4-phenoxyphenol was condensed with 2-chloroethanol in 83% yield. The alcohol obtained was chloromethylated and reacted with nicotinamide successively to yield the Compound 4 (71.5% for two steps). 1H NMR (300 MHz, CD3OD) δ 4.07–4.15 (m, 4H), 6.13 (s, 2H), 6.82–6.93 (m, 6H), 7.04 (t, 1H, J =7.5 Hz) 7.29 (dd, 2H, J =8.5, 7.5 Hz) 8.26 (dd, 1H, J =8.1, 6.0 Hz), 9.02 (d, 1H, J =8.1 Hz), 9.24 (d, 1H, J =6.0 Hz), 9.58 (s, 1H).13C NMR (75 MHz, CD3OD) δ 165.0, 159.7, 155.7, 152.2, 146.3, 145.7, 144.2, 135.6, 130.8, 129.1, 123.8, 121.6, 118.8, 116.7, 91.1, 72.2, 68.5. ESI-MS: [M-Cl]+ 365.1. Anal. (C21H21ClN2O4) C, H, N.
1-{[2-(4-nitro-phenoxy)ethoxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 5)
The procedure used was similar to that of the synthesis of Compound 3. 4-nitrophenol was condensed with 2-chloroethanol in 87% yield. The alcohol obtained was chloromethylated and reacted with nicotinamide successively to yield the Compound 5 (82% for two steps) 1H NMR (300 MHz, CD3OD) δ 4.19 (m, 2H), 4.32 (m,2H), 6.14 (s, 2H), 7.05 (d, JAB =9.3 Hz,2H,A of aryl A2B2), 8.18 (d, JAB =9.3 Hz,2H,B of aryl A2B2), 8.30 (dd, 1H, J =8.1, 6.0 Hz), 9.05 (d, 1H, J =8.1 Hz), 9.25 (d, 1H, J =6.0 Hz), 9.58 (s, 1H).13C NMR (75 MHz, CD3OD) δ 165.0, 164.8, 146.5, 145.8, 144.2, 143.1, 135.7, 129.3, 129.2, 126.8, 115.8, 90.9, 71.4, 68.8. ESI-MS: [M-Cl]+ 318.1. Anal. (C15H16ClN3O5) C, H, N.
1-{[2-(3-trifluoromethyl-phenoxy)ethoxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 6)
The procedure follows the synthesis of Compound 3. 3-trifluoromethylphenol was condensed with 2-chloroethanol in 95% yield. The alcohol obtained was chloromethylated and reacted with nicotinamide successively to yield the Compound 6 (40% for two steps). 1H NMR (300 MHz, CD3OD) δ 4.16–4.25 (m, 4H), 6.13 (s, 2H), 7.12–7.25 (m, 3H), 7.45 (m, 1H) 8.27 (dd, 1H, J =8.1, 6.0 Hz), 9.03 (dt, 1H, J =8.1, 1.5 Hz), 9.24 (d, 1H, J =6.0 Hz), 9.59 (s, 1H). 13C NMR (100 MHz, CD3OD) δ 164.9, 160.0, 146.4, 145.8, 144.2, 135.7, 132.9 (q, JCF = 32 Hz), 131.6, 129.2, 125.4 (q, 1JCF = 270 Hz), 119.3, 118.8 (q, JCF = 4 Hz), 112.4 (q, JCF = 4 Hz), 91.0, 71.8, 68.4. 19F NMR (470 MHz, CD3OD) δ 21.3. ESI-MS: [M-Cl]+ 341.1. Anal. (C16H16ClF3N2O3) C, H, N.
1-{[2-(8′-quinolyloxy)ethoxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 7)
The procedure follows the synthesis of Compound 3. 8-hydroxyqunoline was condensed with 2-chloroethanol in 65% yield. A suspension of 2-(8′-quinolinoxy)ethanol (1.28g, 6.77mmol), paraformaldehyde (0.2g, 6.77 mmol) and 1.5g 3Å MS in 10 ml 1,2-dichloroethan was bubbled with HCl gas for 10 h at 0°C. The molecular sieve was filtered and washed with DCE, the filtrate was concentrated under reduced pressure and treated with nicotinamide as before. After recrystallized from MeOH/EA, Compound 7 was obtained as a yellow solid.(78% for two steps). 1H NMR (300 MHz, CD3OD) δ 4.36 (t, 2H, J =4.2 Hz), 4.67 (m, 2H, J =4.2 Hz), 6.19 (s, 2H), 7.70 (dd, 1H, J =6.0, 2.7 Hz), 7.91 (m, 2H), 8.16 (dd, 1H, J =8.4, 5.7 Hz), 8.25 (dd, 1H, J =8.1, 6.0 Hz), 9.00 (d, 1H, J =8.1 Hz), 9.18–9.27 (m, 3H), 9.58 (s, 1H).13C NMR (75 MHz, CD3OD) δ 164.9, 150.0, 148.5, 146.2, 145.9, 145.5, 144.1, 135.7, 131.8, 131.7, 131.0, 129.3, 123.8, 122.0, 115.3, 90.7, 71.1, 70.0. ESI-MS: [M-Cl]+ 324.2. Anal. (C18H18ClN3O3·3H2O) C, H, N.
1,2-dimethoxy-ethylene-bis-N,N′-3-(aminocarbonyl)-pyridinium dichloride (Compound 8)
1,2-Bis-chloromethoxy-ethane was prepared as the literature29. The bis- chloromethylation product (0.78 ml, 5 mmol) was added to a solution of nicotinamide (1.34 g, 11 mmol) in 20ml DMF. The mixture was stirred for 12 h at room temperature. Dry ethyl acetate (10ml) was added and the mixture was stirred a further 30mins. The white precipitate was collected by filtration and washed with dry ethyl acetate and ethanol to give the desired Compound 8 (1.71g, 85%) as a white solid. 1H NMR (300 MHz, D2O) δ 3.80 (s, 4H), 5.87 (s, 4H), 8.11 (dd, 2H, J =8.1, 6.3 Hz), 8.84 (d, 2H, J =8.1Hz), 8.96 (d, 2H, J =6.3 Hz), 9.26 (s, 2H). 13C NMR (75 MHz, CD3OD) δ 165.5, 146.5, 146.0, 144.4, 135.9, 129.4, 90.7, 71.4. ESI-MS: [M-Cl]+ 367.1. Anal. (C16H20Cl2N4O4·2H2O) C, H, N.
1,4-dimethoxy-butylene-bis-N,N′-3-(aminocarbonyl)-pyridinium dichloride (Compound 9)
The same procedure of Compound 8 was followed to afford the desired Compound 9 (52.7% for two steps). 1H NMR (300 MHz, D2O) δ 1.57 (s, 4H), 3.58 (s, 4H), 5.86 (s, 4H), 8.14 (dd, 2H, J =8.4, 6.0 Hz), 8.86 (d, 2H, J =8.4Hz), 8.99 (d, 2H, J =6.0 Hz), 9.26 (s, 2H). 13C NMR (125 MHz, CD3OD) δ 165.1, 146.5, 145.8, 144.2, 135.8, 129.4, 91.0, 72.4, 26.8. ESI-MS: [M-Cl]+ 395.1. Anal. (C18H24Cl2N4O4·1.4H2O) C, H, N
1,4-dimethoxy-butyne-bis-N,N′-3-(aminocarbonyl)-pyridinium dichloride (Compound 10)
The same procedure of Compound 8 was followed to afford the desired Compound 10 (46% for two steps). 1H NMR (300 MHz, CD3OD) δ 4.48 (s, 4H), 6.10 (s, 4H), 8.31 (dd, 2H, J =8.1, 6.3 Hz), 9.09 (dt, 2H, J =8.1, 1.5 Hz), 9.25 (d, 2H, J =6.3 Hz), 9.61 (s, 2H). 13C NMR (75 MHz, CD3OD) δ 165.1, 146.8, 146.6, 145.0, 135.7, 129.3, 90.0, 83.7, 59.8. ESI-MS: [M-Cl]+ 391.1. Anal. (C18H20Cl2N4O4·0.8H2O) C, H, N.
1,4-dimethoxy-hexamethylene-bis-N,N′-3-(aminocarbonyl)-pyridinium dichloride (Compound 11)
The same procedure of Compound 8 was followed to afford the desired Compound 11 (52% for two steps). 1H NMR (300 MHz, CD3OD) δ 1.40 (m, 4H), 1.67 (m, 4H), 3.69 (t, 4H, J =6.3 Hz), 6.01 (s, 4H), 8.29 (dd, 2H, J =8.4, 6.3 Hz), 9.07 (dt, 2H, J =8.4, 1.5 Hz), 9.20 (d, 2H, J =6.3 Hz), 9.50 (s, 2H). 13C NMR (75 MHz, CD3OD) δ 165.1, 146.5, 145.8, 144.2, 135.8, 129.3, 91.0, 72.6, 30.2, 26.6. ESI-MS: [M-Cl]+ 423.2. Anal. (C20H28Cl2N4O4) C, H, N.
(E)-1-{[4-(8′-quinolyloxy)but-2-enyloxy]methyl}-3-(aminocarbonyl)-pyridinium chloride (Compound 12)
To a solution of 1,4-butendinol (0.88g, 10mmol), 8-hydroxyqunoline (0.725g, 5mmol) and triphenylphosphine (PPh3) (1.57g, 6mmol) in THF (25ml), diisopropyl azodicarboxylate (1.21g, 6mmol) was added dropwise. The reaction was stirred for 4 h at room temperature and refluxed for 12 h. The solution was concentrated under reduce pressure. The crude mixture was purified by flash column chromatography (2:1–3:1 hexanes:ethyl acetate eluent) to furnish pure alcohol (0.72g, 70%). The alcohol was chloromethylated and treated with nicotinamide as in the synthesis of Compound 7. After recrystallized from MeOH/EA, Compound 12 was obtained as a yellow-green solid.(78% for two steps). 1H NMR (500 MHz, D2O) δ 4.50 (d, 2H, J =7.0 Hz), 4.94 (d, 2H, J =6.5 Hz), 5.99 (m, 1H), 6.09 (s, 2H), 7.50 (d, 1H, J =6.5 Hz), 7.86 (m, 2H), 8.11 (dd, 1H, J =8.0, 5.5 Hz), 8.25 (t, 1H, J =7.0 Hz), 8.91 (d, 1H, J =8.5 Hz), 9.03 (d, 1H, J =5.0 Hz), 9.12 (d, 1H, J =8.5 Hz), 9.19 (d, H, J =6.5 Hz), 9.40 (s, 1H). 13C NMR (125 MHz, D2O) δ 165.8, 148.6, 148.0, 146.3, 145.6, 143.9, 143.4, 134.4, 131.2, 130.6, 130.1, 130.0, 129.3, 128.8, 123.1, 121.3, 114.9, 89.6, 67.2, 66.0. ESI-MS: [M-Cl]+ 350.2. Anal. (C20H20ClN3O3·3H2O) C, H, N.
1-{[2-(4-phenoxy-phenoxy)ethoxy]methyl}-6-(aminocarbonyl)-quinolinium chloride (Compound 13)
The corresponding alcohol was chloromethylated as in Compound 4 and reacted with quinoline-6- carboxamide successively to yield Compound 13 (63% for two steps).1H NMR (300 MHz, DMSO) δ 4.07 (s, 4H), 5.77 (s, 2H), 6.54 (s, 2H), 6.73–7.38 (m, 9H) 7.93 (s, 1H), 8.31 (dd, 1H, J =8.4, 5.7 Hz), 8.53 (s, 1H), 8.65 (s, 2H), 9.00 (s, 1H), 9.44 (d, 1H, J =8.4 Hz), 9.78 (s, 1H). 13C NMR (125 MHz, D2O) δ 169.8, 160.1, 155.6, 152.3, 151.5, 150.5, 141.3, 136.8, 135.3, 131.4, 131.3, 130.8, 123.8, 123.5, 121.5, 121.0, 118.8, 116.6, 89.2, 71.7, 68.6. ESI-MS: [M-Cl]+ 415.2. Anal. (C25H23ClN2O4·1.4H2O) C, H, N.
1-{[2-(4-phenoxy-phenoxy)ethoxy]methyl}-3-(aminocarbonyl)-4-amino-pyridiniu m chloride (Compound 14)
The corresponding alcohol was chloromethylated as in Compound 4 and reacted with 4-aminonicotinamide successively to yield the Compound 14 (43% for two steps). 1H NMR (300 MHz, DMSO) δ 3.92 (s, 2H), 4.10 (s, 2H), 5.55 (s, 2H), 6.90–7.10 (m, 8H), 7.35 (t, 2H, J =7.6 Hz), 7.91 (s, 1H), 8.30 (d, 2H, J =6.9 Hz), 8.43 (bs, 1H), 9.11 (m, 3H). 13C NMR (100 MHz, DMSO) δ 166.5, 158.6, 157.9, 154.4, 149.6, 143.9, 141.6, 129.9, 122.7, 120.6, 117.3, 115.7, 112.0, 110.3, 85.7, 68.0, 66.9. ESI-MS: [M-Cl]+ 380.2. Anal. (C21H22ClN3O4·0.5H2O) C, H, N.
Experimental details of Antagonist Assay
Recombinant CD38 was prepared by a yeast expression system as described36. Inhibitors were dissolved in 50 mM Hepes buffer (pH 7), except Compound 5, which was dissolved in 50% DMSO (v/v). Recombinant CD38 (0.04 μg/ml) and BSA (50 μg/ml) were mixed different concentrations of the inhibitors and incubated for 1 hours at room temperature. NAD (2 μM) was added to initiate the reactions and aliquots of were collected at T = 0, 4, 8 and 12 mins, respectively. The aliquots were immediately stopped with equal volume of 0.6M HCl followed by neutralization with two volumes of 0.5M sodium phosphate buffer (pH8). The amounts of NAD in the samples were measured by the coupled enzyme assay31. The percentages of inhibition of the NADase activity as compared to the control without the inhibitor were plotted against various concentrations of the inhibitor to obtain the IC50 value.
Biological effects on muscle preparations from rat and guinea pigs
1. Materials and methods
Animals: Male Sprague-Dawley rats weighing 250–300 g were supplied from the Laboratory Animal Unit of the University of Hong Kong. Some male Sprague-Dawley rats were also purchased from the Laboratory Animal Center of Peking University Health Science Center (Beijing, China). Rats were anesthetized by pentobarbitone sodium (50 mg/kg, by intraperitoneal injection) and then sacrificed by cervical dislocation. All experiments performed in this study were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong and by the Beijing animal committee with the confirmation number: SCXK (Jing) 2006–0008.
Male Hartley guinea pigs were purchased from Beijing Fangyuanyuan Laboratory Animal Company and approved by the local animal committee with the confirmation number: SCXK (Jing) 2009–0014. Animals were housed under standard conditions (temperature 22±2°C, relative humidity 55±5%, 12-h-light/dark cycle) with food and water available ad libitum. In the present study, all experiments were performed under the guidelines of the Experimental Laboratory Animal Committee of Peking University Health Science Center and were in strict accordance with the principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Reagents: phenylephrine (Phe) and acetylcholine chloride (ACh) was purchased from Sigma Chemicals (St. Louis, MO, USA). All chemicals (including compound 4, 7 and compound 9) were dissolved in miliQ water.
2. Tension measurement on isolated rat aortic ring preparations
The thoracic aorta was excised. After the surrounding connective tissue had been carefully cleaned off, four 3 mm-wide ring segments were prepared from each aorta. Each was dispensed between two stainless wire hooks in a 5-mL organ bath. The upper wire was connected to a force-displacement transducer (RM6240 system, Chengou Instrument Factory.) and the lower one was fixed at the bottom of the organ bath. The organ bath was filled with Krebs solution of the following composition (in mM): 119 NaCl, 4.7 KCl, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, 1.2 KH2PO4, and 11 D-glucose. The bathing solution was gassed with 95% O2-5% CO2 at 37°(pH≈7.4). The rings were placed under an optimal basal tone of 15 mN, determined from previous length–tension experiments. Changes in isometric tension were measured with a Grass force transducer and stored on RM6240 software for later data analysis. Twenty minutes after mounting in organ baths, the rings were first contracted with 0.3 μM phenylephrine (Phe) to test the contractility and then relaxed by 1 μM ACh. They were rinsed several times until baseline tone was restored. The rings were thereafter allowed to equilibrate for 60 min. Baseline tone was readjusted to 15 mN when necessary. Each set of experiments was performed on rings prepared from different rats. The use of laboratory animals was approved by the Animal Research Ethical Committee of the University of Hong Kong.
In the set of experiments, relaxation of Phe (1 μM)-contracted endothelium-intact rings was induced by compound 4 (30 – 300 μM) or compound 9 (30 – 300 μM) or nicotinamide (0.01 – 6 mM).
The relaxant effects of the vasodilators were expressed as 100 minus percentage reduction from the Phe-induced contractile response. Non-linear regression curve fitting was performed on individual cumulative concentration–response curves (GraphPad software, Version 5.0). pD2 values (IC50) were calculated as negative log molar of dilator that induced 50% of the maximal relaxation. All data were shown as means±SEM. Statistical significance was determined by two-tailed Student's t-test or one-way ANOVA followed by the Newman–Keuls test when more than two treatments were compared. A P value of less than 0.05 was regarded to be significant.
3. Tension measurement on isolated tracheal strips of guinea pigs
Guinea pigs weighing 250–350g were sacrificed by an overdose of sodium pentobarbital (75 mg/kg intraperitoneally). The tracheas were removed and placed in ice-cold Krebs-Henseleit solution bubbled through with 95%O2 / 5%CO2. The trachea was then isolated from surrounding connective tissue and cut spirally into two strips 3mm wide and 15mm long. The composition of Krebs-Henseleit solution was (in mM): NaCl 118.00, KCl 4.70, CaCl2 2.50, MgSO4.7H2O 1.20, KH2PO4, 1.20, NaHCO3 25.00, and glucose 11.00. The ends of each tracheal strip were then fixed, via two small clips, to the bottom of the chamber and to a force displacement transducer for recording tension with a polygraph. The chamber (50-mL capacity) was filled with Krebs-Henseleit solution at 37°C and bubbled through with 95% O2 /5% CO2. Each strip was subjected to a load of 2g for at least 1 h, with frequent changes of the bath fluid until a stable baseline tension was obtained. 10 μM acetylcholine chloride was added into the Krebs-Henseleit solution to induce contraction of tracheal strips. After the tension become stable, compound 4 and compound 7 of 10−8, 10−7, 10−6, 10−5 and 10−4 M were added every 10 minutes in sequence, respectively. The tension of tracheal strips were recorded by MedLab-U4C501H bio-signals collecting-processing system in the whole experiment37.
4. Statistical evaluation
Antispasmodic Percentage = (Tension before addition of compounds- Tension after addition of compounds)/ (Tension before given compounds - Basal tension) × 100%. Tension changes of tracheal strips obtained from administration groups and blank group were compared using Student's t test. All values are represented as mean ± SEM. Two means were considered significantly different when P value was < 0.05 or <0.01.
Protein Crystallography
The catalytic domain of human CD38 was expressed in a yeast expression system and purified as reported previously36. Using the hanging vapor diffusion method, CD38 crystals were obtained by mixing 1 μl 10 mg/ml protein with 1 μl crystallization solution containing 100 mM MES, pH 6.0, 10% PEG4000 at room temperature. To obtain CD38-Compound 4 complex, native CD38 crystals were soaked for several minutes at room temperature in the crystallization mother liquid containing 40 mM Compound 4 and 30% glycerol.
Data collection, Reduction and Structure Refinement
All X-ray diffraction data were collected at the Cornell High-Energy Synchrotron Source (CHESS) A1 station under cryo-protection at 100 K with a fixed wavelength of 0.976 Å. A total of 360 images with an oscillation angle of 1° each were collected for each crystal using a Quantum Q-210 CCD detector. The complete data sets were processed using the program package DENZO/SCALEPACK38. The crystallographic statistics are listed in Table1. The shCD38 apo structure was served as the initial model for structure solution with the method of molecular replacement. Subsequent crystallographic refinements were done with the program REFMAC539. All substrates and products were built using the Program COOT38.
Computer simulation of compound 7 for the docking to CD38
The structure of Compound 7 first was constructed based upon the crystal coordinates of Compound 4 followed by energy minimization using Discovery Studio 2.1. It was then docked into the binding pocket of CD38 using the AutoDock 3.0.540 program. Considering the interactions with the key residues in CD38, one conformation with a relatively low energy was selected as the starting conformation for the subsequent molecular dynamics simulation. The molecular topology file for Compound 7 was generated by the PRODRG241 server (http://davapc1.bioch.dundee.ac.uk/prodrg/). The partial atomic charges of the compound were calculated by Gaussian03 program42 at the level of HF/6–31G*. The simulations were performed with the GROMACS43 (version: 3.3.1) software and the force field GROMOS9644 43a1 was applied for the protein. The complex was put into a cubic periodic box with edge approximately 10 Å from the system's periphery in each dimension. Then 19,974 SPC water molecules were added into the box and 2 Cl− were also added in order to ensure the charge neutrality of the system. The final system contains 62,601 atoms. During the entire simulation, all bond lengths were constrained by the LINCS algorithm45. Long-range electrostatic interactions were calculated using the PME method46. The Berendsen thermostat47 was applied using a coupling time of 0.1 ps to maintain the systems at a constant temperature of 300 K and the pressure was also maintained by coupling to a reference pressure of 1 bar by Berendsen thermostat. The simulations began with 2000 step of steepest-descents algorithm to reach the tolerance. Then, the solvent equilibration was performed in 50 ps with the protein and the ligand fixed. Following that, a second 50 ps simulation was carried out with the main chain and the ligand fixed. Another 20 ps simulation was used to relax the whole system except for the Cα atoms and the ligand. The equilibration was completed after the 10 ps relaxation for the ligand. Finally, the production simulation of 5 ns was performed on the whole system. The system was equilibrated after about 2 ns and the average structure was obtained and minimized, which was considered as the stable binding mode of Compound 7.
Supplementary Material
Figure 1.
Inhibitors of CD38
Acknowledgements
This study was supported by grants from the National Natural Sciences Foundation of China to LH Zhang (NSFC-RGC 20831160506), and the NSFC/RGC grant N_HKU 722/08 and General Research Fund of Hong Kong: 769107, 768408, 769309, 770610 (to H.C.Lee and Q Hao).
References
- 1.Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HC. Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 2000;75:39–59. doi: 10.1159/000058774. [DOI] [PubMed] [Google Scholar]
- 3.a) Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Ann. Rev. Pharmacol. Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]; b) Lee HC, Aarhus R, Levitt D. The crystal structure of cyclic ADP-ribose. Nat. Struct. Biol. 1994;1:143–144. doi: 10.1038/nsb0394-143. [DOI] [PubMed] [Google Scholar]; c) Lee HC, Walseth TF, Bratt GT, et al. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989;264:1608–15. [PubMed] [Google Scholar]
- 4.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 5.Kato I, Yamamoto Y, Fujimura M, Noguchi N, Takasawa S, Okamoto H. CD38 disruption impairs glucose-induced increases in Cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999;274:1869–1872. doi: 10.1074/jbc.274.4.1869. [DOI] [PubMed] [Google Scholar]
- 6.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, Lund FE. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 7.Shubinsky G, Schlesinger M. The CD38 lymphocyte differentiation marker: new insight into its ectoenzymatic activity and its role as a signal transducer. Immunity. 1997;7:315–324. doi: 10.1016/s1074-7613(00)80353-2. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui-Saito M, Kato I, Takasawa S, Okamoto H, Yanagisawa T. CD38 gene disruption inhibits the contraction induced by alphaadrenoceptor stimulation in mouse aorta. J. Vet. Med. Sci. 2003;65:1325–1330. doi: 10.1292/jvms.65.1325. [DOI] [PubMed] [Google Scholar]
- 9.Fukushi Y, Kato I, Takasawa S, Sasaki T, Ong BH, Sato M, Ohsaga A, Sato K, Shirato K, Okamoto H, Maruyama Y. Identification of cyclic ADP-ribose-dependent mechanisms in pancreatic muscarinic Ca2+ signaling using CD38 knockout mice. J. Biol. Chem. 2001;276:649–655. doi: 10.1074/jbc.M004469200. [DOI] [PubMed] [Google Scholar]
- 10.Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of dendritic cell trafficking by the ADPribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004;20:279–291. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Iqbal J, Dolgilevich S, Yuen T, Wu XB, Moonga BS, Adebanjo OA, Bevis PJ, Lund F, Huang CL, Blair HC, Zaidi M. Disordered osteoclast formation and function in a CD38 (ADP-ribosyl cyclase)-deficient mouse establishes an essential role for CD38 in bone resorption. FASEB J. 2003;17:369–375. doi: 10.1096/fj.02-0205com. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JD, Ford EL, Bernal-Mizrachi E, Kusser KL, Luciani DS, Han Z, Tran H, Randall TD, Lund FE, Polonsky KS. Suppressed insulin signaling and increased apoptosis in CD38-null islets. Diabetes. 2006;55:2737–2746. doi: 10.2337/db05-1455. [DOI] [PubMed] [Google Scholar]
- 13.Jin D, Liu HX, Hirai H, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 14.Deaglio S, Vaisitti T, Aydin S, Ferrero E, Malavasi F. In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood. 2006;108:1135–1144. doi: 10.1182/blood-2006-01-013003. [DOI] [PubMed] [Google Scholar]
- 15.Morabito F, Damle RN, Deaglio S, Keating M, Ferrarini M, Chiorazzi N. The CD38 ectoenzyme family: advances in basic science and clinical practice. Mol. Med. 2006;12:342–344. doi: 10.2119/2006-00110.Morabito. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HC. Mechanisms of calcium signaling by cyclic ADPribose and NAADP. Physiol. Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 17.Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J. Biol. Chem. 2006;281:28951–28957. doi: 10.1074/jbc.M604370200. [DOI] [PubMed] [Google Scholar]
- 18.Slama JT, Simmons AM. Carbanicotinamide adenine dinucleotide: synthesis and enzymological properties of a carbocyclic analogue of oxidized nicotinamide adenine dinucleotide. Biochemistry. 1988;271:183–193. doi: 10.1021/bi00401a028. [DOI] [PubMed] [Google Scholar]
- 19.Wall KA, Klis M, Kornet J, Coyle D, Amé JC, Jacobson MK, Slama JT. Inhibition of the intrinsic NAD+ glycohydrolase activity of CD38 by carbocyclic NAD analogues. Biochem. J. 1998;335:631–636. doi: 10.1042/bj3350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller-Steffner HM, Malver O, Hosie L, Oppenheimer NJ, Schuber F. Slow-binding Inhibition of NAD+ Glycohydrolase by Arabino Analogues of α-NAD. J. Biol. Chem. 1992;267:9606–9611. [PubMed] [Google Scholar]
- 21.Sauve AA, Deng HT, Angeletti RH, Schramm VL. A Covalent Intermediate in CD38 Is Responsible for ADP-Ribosylation and Cyclization Reactions. J. Am. Chem. Soc. 2000;122:7855–7859. [Google Scholar]
- 22.a) Liu Q, Graeff R, Kriksunov IA, et al. Structural basis for enzymatic evolution from a dedicated ADP-ribosyl cyclase to a multi-functional NAD hydrolase. J. Biol. Chem. 2009;284:27637–27645. doi: 10.1074/jbc.M109.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Q, Kriksunov IA, Jiang H, Graeff R, Lin H, Lee HC, Hao Q. Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38. Chemistry & Biology. 2008;15:1068–1078. doi: 10.1016/j.chembiol.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauve AA, Schramm VL. Mechanism-Based Inhibitors of CD38: A Mammalian Cyclic ADP-Ribose Synthetase. Biochemistry. 2002;41:8455–8463. doi: 10.1021/bi0258795. [DOI] [PubMed] [Google Scholar]
- 24.a) Liu Q, Kriksunov IA, Graeff R, et al. Crystal structure of human CD38 extracellular domain. Structure. 2005;13:1331–1339. doi: 10.1016/j.str.2005.05.012. [DOI] [PubMed] [Google Scholar]; b) Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q. Structural basis for the mechanistic understanding of human CD38-controlled multiple catalysis. J. Biol. Chem. 2006;281:32861–32869. doi: 10.1074/jbc.M606365200. [DOI] [PubMed] [Google Scholar]; c) Liu Q, Kriksunov IA, Moreau C, Graeff R, Potter BV, Lee H,C, Hao Q. Catalysis-associated conformational changes revealed by human CD38 complexed with a non-hydrolyzable substrate analog. J Biol Chem. 2007;282:24825–24832. doi: 10.1074/jbc.M701653200. [DOI] [PubMed] [Google Scholar]
- 25.Broussy S, Coppel Y, Nguyen M, Bernadou J, Meunier B. 1H and 13C NMR characterization of hemiamidal isoniazid-NAD(H) adducts as possible inhibitors of InhA reductase of mycobacterium tuberculosis. Chem. Eur. J. 2003;9:2034–2038. doi: 10.1002/chem.200204637. [DOI] [PubMed] [Google Scholar]
- 26.Pernak J, Kalewska J, Ksycinska H, Cybulski J. Synthesis and anti-microbial activities od some pyridinium salts with alkoxymethyl hydrophobic group. Eur. J. Med. Chem. 2001;36:899–907. [PubMed] [Google Scholar]
- 27.Saluja S, Zou R, Drach JC, Townsend LB. Structure-Activity Relationships among 2-Substituted 5,6-Dichloro-,4,6-Dichloro-,and 4,5-Dichloro-1-[(2-hydroxyethoxy)methyl]-and-1-[(1,3-dihydroxy-2-propoxy)methyl] benzimidazoles. J. Med. Chem. 1996;39:881–891. doi: 10.1021/jm950556a. [DOI] [PubMed] [Google Scholar]
- 28.Inokuma S, Kimura K, Funaki T, Nishimura J. Synthesis of pyridinecrown ophanes exhibiting high Ag+-affinity. Heterocycles. 2001;54:123–130. [Google Scholar]
- 29.Yang GY, Oh KA, Park NJ, Jung YS. New oxime reactivators connected with CH2O(CH2)nOCH2 linker and their reactivation potency for organophosphorus agents-inhibited acetylcholinesterase. Bioorg. Med. Chem. 2007;15:7704–7710. doi: 10.1016/j.bmc.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Jang MY, Jonghe S. De., Gao LJ, Herdewijn P. Regioselective cross-coupling reactions and nucleophilic aromatic substitutions on a 5,7-dichloropyrido[4,3-d]pyrimidine scaffold. Tetrahedron Lett. 2006;47:8917–8920. [Google Scholar]
- 31.Graeff R, Lee HC. A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem J. 2002;361:379–384. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge ZD, Zhang DX, Chen YF, Yi FX, Zou AP, Campbell WB, Li PL. Cyclic ADP-ribose contributes to contraction and Ca2+ Release by M1 muscarinic receptor activation in coronary arterial smooth muscle. J. Vasc. Res. 2003;40:28–36. doi: 10.1159/000068936. [DOI] [PubMed] [Google Scholar]
- 33.Yue J, Wei W, Lam CMC, Zhao YJ, Dong M, Zhang LR, Zhang LH, Lee HC. The CD38/cADPR/Ca2+-pathway promotes cell proliferation and delays NGF-induced differentiation in PC12 cells. J. Biol. Chem. 2009;284:29335–29242. doi: 10.1074/jbc.M109.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.a) Deepak A, Deshpande TA, White SD, et al. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:773–788. doi: 10.1152/ajplung.00217.2004. [DOI] [PubMed] [Google Scholar]; b) Stevenson GT. CD38 as a therapeutic target. Mol. Med. 2006;12:345–346. doi: 10.2119/2006-00082.Stevenson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nature Chemical Biology. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HC, Munshi CB. Large scale production of human CD38 in yeast by fermentation. Meth. Enzymol. 1997;280:230–241. doi: 10.1016/s0076-6879(97)80123-1. [DOI] [PubMed] [Google Scholar]
- 37.Hirota K, Hashiba E, Yoshioka H, Kabara S, Matsuki A. Effects of three different L-type Ca2+ entry blockers on airway constriction induced by muscarinic receptor stimulation. Brit. J. Anaesth. 2003;90:671–675. doi: 10.1093/bja/aeg118. [DOI] [PubMed] [Google Scholar]
- 38.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Collaborative Computational Project, Number 4 The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 40.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
- 41.Schuettelkopf AW, van. Aalten DMF. PRODRG-a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. 2004;D60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 42.Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian, Inc. Pittsburgh PA: 2003. [Google Scholar]
- 43.Lindahl E, Hess B, van der Spoel D. Gromacs 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Mod. 2001;7:306–317. [Google Scholar]
- 44.van Gunsteren WF, Billeter SR, Eising AA, Hunenberger PH, Kruger P, Mark AE, Scott WRP, Tironi IG. Biomolecular Simulation: The GROMOS96 manual and user guide. Hochschulverlag AG an der ETH Zürich; Zurich, Switzerland: 1996. [Google Scholar]
- 45.Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: A linear constraint solver for molecular simulations. J. Comp. Chem. 1997;18:1463–1472. [Google Scholar]
- 46.Darden T, York D, Pedersen L. Particle mesh Ewald: An N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 47.Berendsen HJC, Postma JPM, van Gunsteren WF, Dinola A, Haak JR. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.