Abstract
Renal vasodilation and sympathoinhibition are recognized responses induced by hypernatremia, but the central neural pathways underlying such responses are not yet entirely understood. Several findings suggest that A2 noradrenergic neurons, which are found in the nucleus of the solitary tract (NTS), play a role in the pathways that contribute to body fluid homeostasis and cardiovascular regulation. The purpose of this study was to determine the effects of selective lesions of A2 neurons on the renal vasodilation and sympathoinhibition induced by hypertonic saline (HS) infusion. Male Wistar rats (280–350 g) received an injection into the NTS of anti-dopamine-beta-hydroxylase-saporin (A2 lesion; 6.3 ng in 60 nl; n = 6) or free saporin (sham; 1.3 ng in 60 nl; n = 7). Two weeks later, the rats were anesthetized (urethane 1.2 g⋅kg−1 b.wt., i.v.) and the blood pressure, renal blood flow (RBF), renal vascular conductance (RVC) and renal sympathetic nerve activity (RSNA) were recorded. In sham rats, the HS infusion (3 M NaCl, 1.8 ml⋅kg−1 b.wt., i.v.) induced transient hypertension (peak at 10 min after HS; 9±2.7 mmHg) and increases in the RBF and RVC (141±7.9% and 140±7.9% of baseline at 60 min after HS, respectively). HS infusion also decreased the RSNA (−45±5.0% at 10 min after HS) throughout the experimental period. In the A2-lesioned rats, the HS infusion induced transient hypertension (6±1.4 mmHg at 10 min after HS), as well as increased RBF and RVC (133±5.2% and 134±6.9% of baseline at 60 min after HS, respectively). However, in these rats, the HS failed to reduce the RSNA (115±3.1% at 10 min after HS). The extent of the catecholaminergic lesions was confirmed by immunocytochemistry. These results suggest that A2 noradrenergic neurons are components of the neural pathways regulating the composition of the extracellular fluid compartment and are selectively involved in hypernatremia-induced sympathoinhibition.
Introduction
Several lines of evidence have found that acute or chronic hypernatremia engages several effector natriuretic mechanisms that remain active until the reestablishment of the plasma sodium concentration. [1]–[8]. Accordingly, previous studies have demonstrated that the intravenous infusion of hypertonic saline (HS) increases arterial blood pressure and renal vascular conductance and induces regionally distinct changes in the sympathetic nerve activity, such as increases in lumbar and decreases in renal and splanchnic nerve discharge [2]–[4], [8], [9]. It is generally accepted that the inhibition of renal sympathetic nerve activity (RSNA) is a key component of these adjustments as it can produce renal vasodilation, decrease renin secretion and reduce renal sodium reabsorption, all mechanisms that, either in isolation or synergistically, will lead to massive sodium loss. However, the central aspects of the neural pathways involved in the activation of these systems remain unclear.
Recent studies have demonstrated that A2 noradrenergic neurons located within intermediate and caudal levels of the nucleus of the solitary tract (NTS) are involved in body fluid homeostasis and cardiovascular regulation [10]–[14]. Changes in the volume and composition of the extracellular compartment stimulate the expression of c-Fos and FosB in A2 neurons, indicating their activation [12], [15]. In addition, Duale et al. [10] demonstrated that the silencing of A2 neurons affected water consumption and urine output.
Neuroanatomical studies have shown that A2 noradrenergic neurons receive sensory afferent input from the cardiovascular and gastrointestinal systems [16], [17]. These noradrenergic neurons project throughout the central nervous system, including areas related to the cardiovascular system and to the regulation of the hydroelectrolytic balance, such as the median preoptic nucleus (MnPO), the subfornical organ (SFO), the paraventricular nucleus (PVN), and the supraoptic nucleus (SON) [18]–[21]. Overall, this evidence suggests an important role of A2 neurons in the maintenance of the circulating volume.
Therefore, in the present study, we tested the hypothesis that the renal sympathoinhibition and vasodilation, induced by acute increases in the plasma sodium levels, depend upon the integrity of A2 neurons. We used anti-dopamine β-hydroxylase (DβH) saporin to induce lesions in the noradrenergic neurons in the NTS and measured the effects of these lesions on the blood pressure pressor, renal vascular conductance, and sympathetic responses induced by acute hypernatremia.
Results
Lesion of the A2 noradrenergic neurons with nanoinjections of anti-DβH–saporin into the NTS
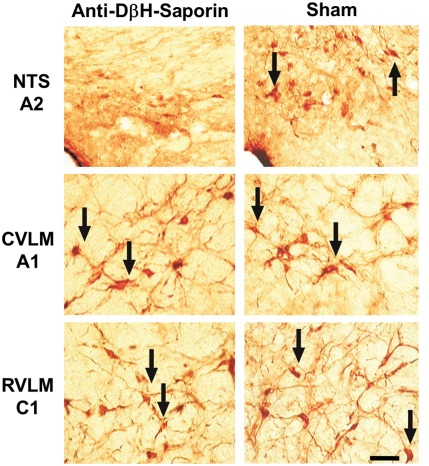
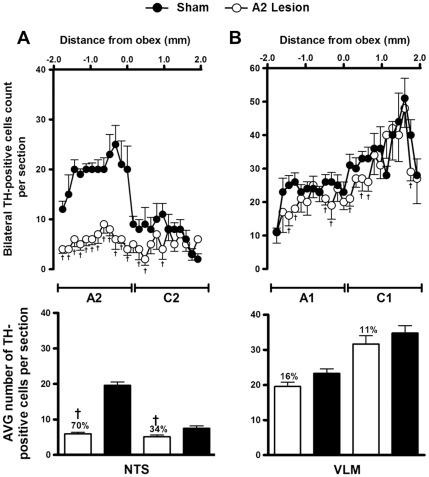
Tyrosine hydroxylase (TH)-positive neurons were found within the ventrolateral medulla (VLM) and the NTS from positions approximately 1800 µm caudal to 1900 µm rostral to the obex (Figures 1 and 2). In the saporin-treated animals (sham; n = 7), TH-positive neurons in the region between the obex and 1800 µm caudal to the obex averaged approximately 20 cells per section (A2 neurons). In rats treated with bilateral nanoinjections of anti-DβH-saporin into the NTS (n = 6), the number of TH-positive neurons caudal to the obex was reduced to approximately six cells per section. Overall in this region, which encompasses the area of the A2 noradrenergic neurons, the number of TH-positive neurons was reduced by 70% (range 66 to 88%) compared to the saporin-treated animals (Figures 1 and 2).
Figure 1. Medullary catecholaminergic neurons.
Photomicrographs taken at 3 levels of the medulla showing TH-immunoreactive cells in the NTS (A2 noradrenergic neurons), CVLM (A1 noradrenergic neurons) and RVLM (C1 adrenergic neurons) in rats nanoinjected with unconjugated saporin (sham) or anti-DβH-saporin. Arrows indicate TH-positive cells. Scale bar 50 µm.
Figure 2. Lesion of A2 noradrenergic neurons with nanoinjections of anti-DβH-saporin into the NTS.
Number and average (mean ± S.E.M.) of TH-positive cells in 40-µm-thick sections from the dorsal (A) and the ventrolateral medulla (B). Sections were taken from 1.9 mm rostral to the obex to 1.9 mm caudal to the obex in animals submitted to A2 or sham lesions. Bilateral nanoinjections of anti-DβH-saporin into the NTS produced a loss of TH-containing neurons in this area (A2 group, loss = 70%; C2 group, loss = 34%), in the RVLM (C1 group, loss = 11%) and in the CVLM (A1 group, loss = 16%). † p<0.05 compared with sham lesion.
The nanoinjection of anti-DβH-saporin into the NTS reduced the number of TH-positive neurons in the C2 adrenergic cell group. Within the region located from the obex up to 1900 µm rostral (the region encompassing the C2 cells), a reduction of approximately 34% in the number of TH immunopositive neurons was observed (Figures 1 and 2). Slight reductions in the total number of TH-positive neurons were also observed in the A1 and C1 cell groups in the VLM, although they did not reach statistical significance. In the region from 1800 to 300 µm caudal to the obex, A1 noradrenergic neurons were reduced by 16% (p = 0.0591; Figures 1 and 2). Similarly, in the region from 300 µm caudal to 1900 µm rostral to the obex, C1 adrenergic neurons were reduced by 11% (p = 0.3296; Figures 1 and 2).
Effects of HS infusion on plasma sodium and blood hemoglobin concentrations
The plasma sodium and hemoglobin concentration changes that were induced by HS infusion were determined in sham and A2-lesioned rats. The pre-infusion plasma sodium concentrations were similar in sham (141±1.2 mM; n = 4) and A2-lesioned rats (141±2.2 mM; n = 4). The plasma sodium levels increased similarly after HS infusion in both groups (150±1.4 mM and 149±1.2 mM in sham and A2-lesioned rats, respectively, 10 min after infusion) and remained at these levels throughout the experimental period (148±1.2 mM and 150±1.7 mM 60 min after HS infusion). Ten minutes after HS infusion, there were no changes in the blood hemoglobin concentrations in sham (−1±1.0%) and A2-lesioned rats (−1±0.9%). The blood hemoglobin concentration remained at these levels throughout the experimental period (−1±0.6% and −1±0.8%, respectively, 60 min after HS infusion).
Effects of A2 noradrenergic neuron lesions on cardiovascular and RSNA responses induced by HS infusion
The body weight and baseline MAP, HR, RBF, RVC and RSNA are shown in Table 1. These variables were similar in sham rats and A2-lesioned rats.
Table 1. Baseline values for body weight, MAP, HR, RBF, RVC and RSNA in sham and A2 lesioned rats.
| Group | b.w.g | MAPmm Hg | HRbpm | RBFml⋅min−1 | RVCµl(min⋅mmHg)−1 | ƒRSNAmV |
| Sham | 319±6 | 123±4 | 346±15 | 3.3±0.2 | 26.7±1.1 | 185±14.6 |
| A2 lesion | 323±5 | 123±2 | 326±15 | 3.1±0.5 | 25.0±4.1 | 183±28.1 |
Values are means ± S.E.M. b.wt., body weight; MAP, mean arterial pressure; HR, heart rate; RBF, renal blood flow; RVC, renal vascular conductance; ∫RSNA, integrate of renal sympathetic nerve activity.
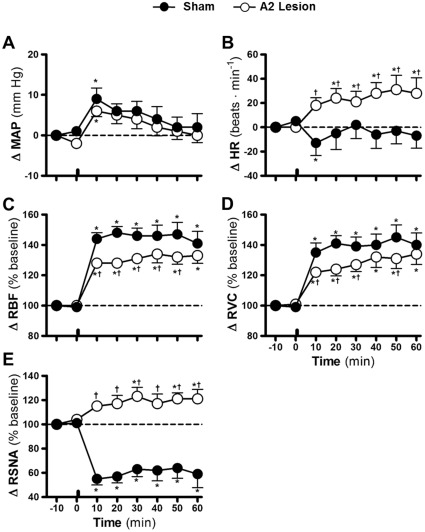
In sham animals, the infusion of HS increased MAP by 9±2.7 mmHg 10 min after infusion (Figures 3A and 4A). MAP returned to basal levels 20 min after the HS infusion (6±1.8 mmHg of baseline values; Figures 3A and 4A). In sham animals, a discrete reduction in the heart rate 10 min after the HS infusion (−13±10.3 beats•min−1 of baseline values; Figures 3A and 4B) was observed. Ten minutes after the HS infusion, the RBF and RVC increased to 144±4.0 and 135±6.3% of baseline values, respectively, and they remained augmented after 60 min (141±7.9% and 140±7.9% of baseline values, respectively; Figures 3A, 4C–D). The reduction in the RSNA reached a nadir approximately 10 min after the HS infusion (−45±5.0% of baseline values; Figures 3A and 4E) and remained lower for the remainder of the experimental period (−41±11.3% of baseline values at 60 min after HS infusion; Figures 3A and 4E).
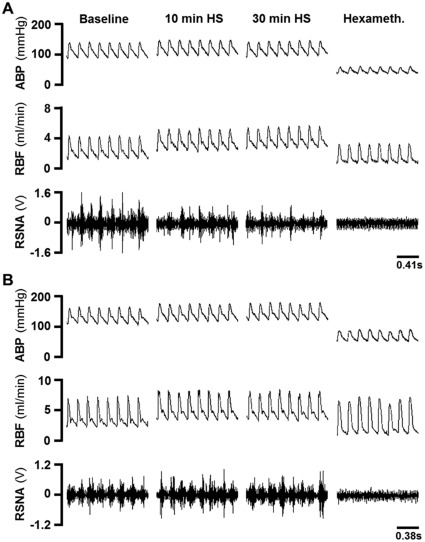
Figure 3. Typical examples.
Digitized record of cardiovascular and sympathetic responses induced by hypertonic saline infusion in sham (A) and A2-lesioned rats (B). ABP = arterial pressure, RBF = renal blood flow, RSNA = renal sympathetic nerve activity (RSNA), hexameth. = hexamethonium.
Figure 4. Effects of A2 noradrenergic neuron lesions on cardiovascular and autonomic responses induced by hypernatremia.
Effects of infusion of hypertonic saline (3 M NaCl, 1.8 ml·Kg−1 body weight) on mean arterial pressure (Δ MAP; A), heart rate (Δ HR; B), renal blood flow (Δ RBF; C), renal vascular conductance (Δ RVC; D) and renal sympathetic nerve activity (Δ RSNA; E) in sham and A2-lesioned rats. The bars indicate the moment of hypertonic saline infusion. Error bars indicate S.E.M. * p<0.05 compared with baseline; † p<0.05 compared with sham lesion.
In A2-lesioned rats, the pressor response induced by the HS infusion was similar to that of the sham group (6±1.4 mmHg above baseline at 10 min after HS infusion; Figures 3B and 4A). A sustained increase in the HR was also observed 10 min after the HS infusion (18±6.3 beats min−1 of baseline values; Figures 3B and 4B). The RBF and RVC increased after the HS infusion (128±2.5% and 122±2.2% of baseline values 10 min after HS infusion; Figures 3B and 4C–D) and remained augmented after 60 min (133±5.2% and 134±6.9% of baseline values; Figures 3B and 4C–D). However, these increases were slightly lower than those observed in the sham animals. In A2-lesioned rats, the reduction in the RSNA was abolished and reversed to a slight increase after the HS infusion (15±3.1% of baseline levels 10 min after HS infusion; Figures 3B and 4E); this difference reached statistical significance beginning at 30 min after the HS infusion.
Effects of A2 noradrenergic neuron lesions on baroreceptor reflexes
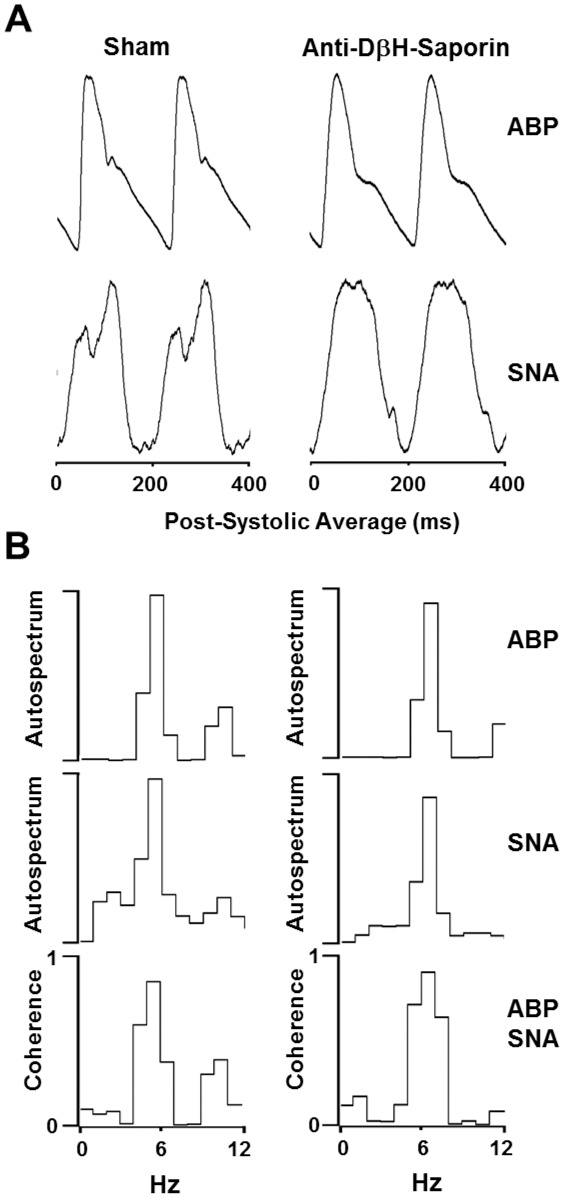
The functionality of the baroreceptor reflexes was evaluated by the analysis of the cardiac-related components of the RSNA and the coherence between the AP and RSNA. In A2-lesioned rats, RSNA bursts were synchronized with the cardiac cycle, as demonstrated by PAP-triggered averaging of the RSNA (Figure 5A). The RSNA power spectra displayed a peak that corresponded to the HR, indicating a prominent role of the baroreceptor reflexes in modulating spontaneous RSNA discharge. In these animals, a correlation between the RSNA bursts and the cardiac cycle led to high AP-RSNA coherence values (mean 0.83±0.04; Figure 5B). Similar results were observed in the A2-lesioned rats, as shown by the synchronization of the RSNA to the cardiac cycle (Figure 5A), a prominent peak in the power spectra corresponding to the HR, and high AP-SNA coherence (0.85±0.04; Figure 5B).
Figure 5. Effects of A2 noradrenergic neuron lesions on baroreceptor reflexes.
Post-systolic averages of arterial pressure (AP) and renal sympathetic nerve activity (SNA) waves (A) and coherence values between AP and SNA power spectrum (B) before hypertonic saline infusion in sham and A2-lesioned rats.
Discussion
Previous studies have indicated that A2 neurons are involved in the regulation of body fluid homeostasis [10]–[14], [22]–[25]. However, there was previously no evidence to support the contribution of these neurons to the autonomic control of the circulating volume composition. The present study provides a key new observation: lesions of the A2 noradrenergic cluster reduced HS-induced renal vasodilation. Moreover, in A2-lesioned rats, hypernatremia failed to decrease renal sympathetic activity during the observation period. Together, these results point out the involvement of A2 noradrenergic neurons in the autonomic responses that are induced by acute changes in the plasma sodium concentration, and the results specifically highlight their involvement in the regulation of renal sympathetic nerve activity.
Several studies have aimed to quantitate and validate the specificity of neuronal lesions induced by the central nanoinjection of anti-DβH-saporin, and the results obtained are compelling. Madden et al. [26] demonstrated that the nanoinjection of anti-DβH-saporin into the C1 adrenergic neuron rostral cluster, located in the rostroventrolateral medulla (RVLM) region, promoted lesion of these neurons as well as lesions of A1 and A5 catecholaminergic neurons. In addition to demonstrating that the lesion effect is caused by the uptake of synaptic terminals, these authors demonstrate that the anti-DβH-saporin could damage noradrenergic and adrenergic neurons located in the nanoinjection site itself. It is postulated that, in these neurons, the somatodendritic exocytosis of catecholamines resulted in the exposure of DβH and therefore the possible uptake of the anti-DβH-saporin complex via their soma and/or dendrites. In agreement with these results, we have recently demonstrated that the nanoinjection of anti-DβH-saporin into the caudoventrolateral medulla (CVLM) region promoted specific lesions to the A1 cluster located around the center of the nanoinjections [3], [27]. As noted earlier, the current study also shows that the nanoinjection of anti-DβH-saporin in the NTS promoted, on average, a reduction of approximately 70% in all of the catecholaminergic cells compared to the control cluster (nanoinjection of saporin) of A2 noradrenergic neurons.
An important aspect to be considered is whether nanoinjections of anti-DβH-saporin into the A2 area destroy the DβH-containing cells outside of the NTS and if the results observed may be attributed to these remote lesions. Our results indicate that nanoinjections of anti-DβH-saporin into the NTS/A2 area induced a discrete (16%) reduction in the total number of TH-positive cells in the CVLM/A1 cluster and an 11% reduction in the RVLM/C1 cluster. It is likely that these lesions result from the uptake of the conjugated toxin by efferent projections from the ventrolateral medulla to the A2 region of the NTS. Studies performed in our laboratory have demonstrated that lesions of less than 32% of the A1 noradrenergic cells produced by nanoinjections of anti-DβH-Saporin into the CVLM were ineffective in altering the renal sympathoinhibition induced by sodium overload (G.R.P. & S.L.C, unpublished observations). Consistent with these results, Madden & Sved [28] demonstrated that sympathoexcitatory responses induced by baroreceptor unloading or chemoreceptor activation were abolished only in animals with lesions in more than 80% of the C1 catecholaminergic group. These results suggest that extensive lesions of cell groups are necessary to induce significant and reproducible effects. Therefore, it is unlikely that the lack of renal sympathoinhibition in the A2-lesioned rats in our study may be due to the less than 20% lesions that were found in the A1/C1 areas.
Previous studies have attempted to establish the effects of hypernatremia on cardiovascular and renal parameters. Similar to the results obtained in the present study, they describe sustained increases of blood flow and renal vasculature conductance, combined with transient hypertension and bradycardia [2], [3], [8], [9], [27], [29], [30]. It is assumed that these adjustments are triggered by an increase in the sodium plasma concentration, part of an integrated set of responses to promote natriuresis and thus restore the regular volume conditions. Moreover, we have previously demonstrated that the vasodilation induced by sodium overload is specific to the renal bed, as there is no change in the aortic or mesenteric vascular conductance [9]. Evidence suggests the renal vasodilation induced by sodium overload seems to involve neural and humoral components, including a reduction of the renal sympathetic nerve activity and the release of vasoactive substances, such as ANP [2], [3], [5], [8], [31], [32].
Studies have shown the importance of medullary noradrenergic neurons in the integration of the mechanisms involved in the autonomic, cardiovascular and respiratory systems [3], [10]–[15], [22], [24], [25], [27], [33]–[36]. With regard to the A1 noradrenergic cluster, several authors have shown that these neurons are important for the response induced by changes in the circulating volume [3], [12], [15], [22], [27], [33]–[37]. Studies have demonstrated that increases in the sodium plasma concentration induce the expression of c-Fos in these noradrenergic neurons, indicating their activation [15], [23]. Recently, we have shown that specific lesion of the A1 noradrenergic cluster abolishes the vasodilation and the renal sympathoinhibition that are induced by hypernatremia [3], [27]. These results indicate that A1 neurons are involved in both neural and humoral mechanisms in response to hypernatremia, once their specific lesion abolishes the renal sympathoinhibition and vasodilation that are induced by the sodium overload.
With regard to the A2 cluster of noradrenergic neurons, recent evidence has shown that the viral inactivation of these neurons decreased the daily water intake [10], showing their involvement in the behavioral regulation of the circulating volume. Other studies on the expression of immediate early genes show that these noradrenergic neurons located in the NTS are activated by increases in the osmolality or plasma volume [12], [23], [24]. Extracellular neural activity recordings have identified NTS neurons in which activity increases after sodium overload [24]. The present study shows that the specific lesion of A2 neurons abolishes the renal sympathoinhibition induced by the infusion of hypertonic saline. To the best of our knowledge, no other study has yet demonstrated the participation of these neurons in the autonomic component of the response to hypernatremia. In addition, we have also demonstrated that the renal vasodilation induced by sodium overload was not abolished but was reduced in animals with A2 lesions, suggesting that the humoral component of the response to hypernatremia is intact in these animals. Therefore, while A1 noradrenergic neurons appear to modulate both the endocrine and neural components that are observed in response to hypernatremia, the A2 noradrenergic neurons seem to be specifically related to the modulation of renal sympathetic activity.
Anatomical studies have shown that medullary noradrenergic neurons receive sensory information from baro- and cardiopulmonary receptors [16], [24], [34], [38], [39]. From medullary catecholaminergic and non-catecholaminergic neurons, the information about the circulating volume would be processed and transmitted to hypothalamic centers involved in the regulation of osmolality. In fact, both A1 and A2 catecholaminergic groups project densely to hypothalamic targets as the PVN and MnPO [18], [20], [21], [40]–[43]. The main difference is that, whereas A1 neurons project densely to both magno- and parvocellular regions of the PVN, A2 neurons project primarily to the dorsal and ventral parvocellular groups of this nucleus [41]. These anatomical data are compatible with the idea that A2 neurons may be selectively involved with the neural regulation of sympathetic activity in response to acute hypernatremia, whereas A1 neurons may be involved in the regulation of both neuronal and humoral components.
The noradrenergic neurotransmission in the hypothalamic regions appears to play a significant role in the responses induced by changes in the circulating volume. Data have shown that the nanoinjection of α-adrenergic-antagonists into the AV3V region reduces the hypertension response and secretion of ANP and oxytocin induced by sodium overload [44], [45]. Similarly, recent studies conducted by our laboratory have shown that the blockade of α1 or α2 adrenoceptors located in the MnPO abolishes the renal vasodilation induced by hypertonic saline infusion [4]. Thus, the lesion of medullary noradrenergic neurons and the α adrenoceptor blockade in the MnPO nucleus change the cardiovascular outcome in response to hypernatremia, supporting the idea that ascending projections from medullary noradrenergic neurons and the MnPO nucleus mediate the endocrine and autonomic adjustments involved in this response.
Together, the results obtained in this study are consistent with the hypothesis that the A2 noradrenergic neurons activated by peripheral afferents are part of the central pathways modulating the adjustments induced by acute changes in osmolality. Thus, as these noradrenergic neurons are part of the pathways involved in responses to changes in the plasma sodium concentration, a dysfunction in these cells would result in the inefficient operation of these adjustments, which could contribute to the development of diseases such as arterial hypertension.
Materials and Methods
Animals
All experiments were conducted on adult male Wistar rats (280–350 g). The animals came from the central animal house at the Universidade Federal de São Paulo. All protocols described here were approved by the Medical Ethics Committee at the Universidade Federal de São Paulo (protocol # 0739/06) and were performed in strict accordance with the Guidelines for Care and Use of Laboratory Animals of the National Health Institute.
Nanoinjections of anti-DβH-saporin or saporin into the NTS
The animals were anaesthetized with halothane (2–3% in 100% O2) and mounted prone on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) with the incisor bar 11 mm below the interaural line. After partial removal of the occipital bone, the meninges covering the dorsal surface of the brainstem were cut and retracted, and the calamus scriptorius was visualized. Nanoinjections of anti-DβH-saporin (6.3 ng in 60 nl; Advanced Targeting Systems, San Diego, CA, USA) or an equimolar dose of saporin (1.3 ng in 60 nl; Advanced Targeting Systems, San Diego, CA, USA) were made bilaterally at two levels of the NTS. For all nanoinjections into the NTS, a glass micropipette was positioned as follows: −0.5 and −0.0 mm caudal to the calamusscriptorius, 0.0 mm lateral to the midline, and 0.3 mm ventral to the dorsal surface. These coordinates were based on the region of the NTS comprising the A2 noradrenergic group [46]. After nanoinjection, the micropipette was left in place for 3–5 min. The incision was then closed, and the animals were placed on a heated pad to maintain their body temperature during recovery. Penicillin (60 000 IU kg−1 b.wt., i.m.; Sigma-Aldrich, St. Louis, MO, USA) was injected for prophylaxis after the surgery. The animals were studied for 15–25 days after the nanoinjections into the NTS.
Surgical procedures
On the day of experiments, the rats were anesthetized with halothane (2–3% halothane in 100% O2), and the catheters were inserted into the right femoral vein and artery. The right jugular was also dissected, and a catheter was placed closer to the right atrium for the HS infusion (see below). After the catheter placement, the rats were removed from the halothane, and the anesthesia was maintained with urethane (1.2 g⋅kg−1 b.wt., i.v.; Sigma-Aldrich, St. Louis, MO, USA). The trachea was cannulated to reduce airway resistance, and the rats were mounted prone on a stereotaxic apparatus (David Kopf Instruments) with the bite bar set at 3.4 mm below the interaural line. The postganglionic renal sympathetic nerve activity (RSNA) was recorded from the left renal nerve with bipolar platinum electrodes in a monopolar configuration. In experiments measuring the renal blood flow (RBF), miniature ultrasonic transit-time flow probes (Transonic Systems Inc., Ithaca, NY, USA) were placed around the left renal artery. The body temperature was kept at 37±0.5°C with a thermostatically controlled heated table.
Recording of arterial pressure, heart rate, and renal blood flow
To register the blood pressure, the arterial catheter was connected to a pressure transducer attached to a bridge amplifier. The pulsatile pressure was recorded continuously with an MP150 analog-to-digital converter (Biopac Systems, Inc., Goleta, CA, USA). The mean arterial pressure (MAP) and heart rate (HR) were determined through the pulsatile signal with AcqKnowledge software (version 3.7.1.; Biopac Systems, Inc., Goleta, CA, USA). To measure the RBF, a flow probe was connected to an ultrasonic transit-time flowmeter (Transonic Systems, Inc., Ithaca, NY, USA).
Renal sympathetic nerve activity recording
The RSNA was recorded through the left renal nerve with bipolar platinum electrodes. After exposure and isolation, the renal nerve was placed on the recording electrodes and was covered with warm mineral oil. The RSNA signals were obtained using a high-impedance probe connected to an AC amplifier (ERS 100c; Biopac Systems, Inc., Goleta, CA, USA). The signal was amplified (20,000), passed through a band-pass filter (50–1,000 Hz) and digitized at 3 kHz using an MP150 analog-to-digital converter. The filtered nerve signal was rectified and integrated (1 s time constant) using AcqKnowledge software. At the end of each experiment, the background noise was determined as the average value of the integrated voltage after a bolus injection of hexamethonium (30 mg⋅kg−1 b.wt., i.v.).
Acute increase in plasma sodium concentration
Hypertonic saline (3 M NaCl, 1.8 ml•kg−1 b.wt., i.v.; Sigma-Aldrich, St. Louis, MO, USA) was infused through the jugular vein cannula for 1 min.
Blood sampling and analysis
Blood samples (200 µl each) were withdrawn from the femoral vein cannula 5 min before and 10, 30 and 60 min after HS infusion. After the sample was collected, an equal volume of sterile 0.15 M NaCl was injected to reduce changes in the extracellular fluid volume brought about by sampling. The blood hemoglobin concentration was measured immediately with a kit from Sigma (Drabkin's reagent, kit 525). The rest of the sample was centrifuged for 5 min at 6000 g. The plasma was removed and stored at −20°C. The plasma sodium concentration was measured with a flame photometer (model 943, Instrumentation Laboratory, Lexington, MA).
Perfusion, fixation, and tissue collection
At the end of the experiments, the animals' heart were perfused with saline (0.15 M NaCl), followed by a solution of 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M sodium phosphate buffer (500 ml at pH 7.4). The brain was removed and post fixed in a 4% paraformaldehyde solution for 1–2 h and was then kept in 30% sucrose solution. The serial coronal 40-µm brainstem sections were collected in four series and were stored in 0.02 M potassium phosphate-buffered saline (KPBS, Sigma; pH 7.4) at 4°C until immunohistochemical staining.
Immunohistochemistry
Each fourth brainstem section was processed for immunohistochemical detection of tyrosine hydroxylase (TH). The sections were incubated for 15 min in 0.5% hydrogen peroxide in 0.02 M KPBS (Sigma-Aldrich, St. Louis, MO, USA), followed by a 30-min incubation in 3.5% normal horse serum (Vector Laboratories Inc., Burlingame, CA, USA) in 0.02 M KPBS. The sections were incubated overnight at 4°C with mouse monoclonal antibody (1∶2000 dilution, Immuno Star Inc., Hudson, WI, USA) with 1.5% normal horse serum and 0.2% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), followed by a 1-h incubation with biotinylated horse anti-mouse IgG (1∶200 dilution with 0.1% Triton X-100; Vector Laboratories Inc., Burlingame, CA, USA). After these incubations, the sections were processed with the avidin-biotin procedure using Elite Vectastain reagents (Vector Laboratories Inc., Burlingame, CA, USA). Diaminobenzidine (DAB) was used to produce a brown cytoplasmic TH reaction product. The sections were set on slides, dehydrated in a series of alcohols, cleared in xylene and coverslipped.
Cell counting and imaging
The counting of labeled neurons was performed on every fourth medulla oblongata section (40 of each 160 µm). All of the immune-labeled neuronal perikarya in the ventrolateral medulla (VLM; A1/C1) and nucleus tractus solitarius (NTS; A2/C2) were counted bilaterally to quantify the extent of the anti-DβH-saporin-induced lesion. The neurons were counted at 200× magnification with a Nikon light microscope.
Data analysis
The changes in the RBF were expressed as a percentage of control values (means ± S.E.M). The renal vascular conductance (RVC) was calculated as the ratio of the RBF by MAP and was also expressed as a percentage of the baseline values. The changes in the RSNA were quantified by integration (1 s) after wave rectification and were expressed as percentages of the baseline after subtraction of the noise level. Trigger pulses matching the systole were used for the construction of normalized averages of the arterial pulse and the RSNA wave. For each average, a representative 100-s segment of the arterial pressure and the RSNA wave was used.
The effects of treatment with anti-DβH-saporin or saporin on the number of catecholaminergic medullary neurons are presented as means ± S.E.M. The number of cells counted for every section was compared by a one-way ANOVA. Additionally, the total cell counts for the A1, C1, A2 and C2 regions were calculated and compared between the groups by unpaired Student's t-tests. Cardiovascular, RSNA and plasma sodium and blood hemoglobin concentrations were analyzed by a two-way analysis of variance followed by the Fisher LSD test. The differences in the baseline levels (Table 1) between the groups were analyzed by unpaired Student's t-tests. A value of P<0.05 was considered to denote a significant difference.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Coordenadoria de Aperfeiçoamento em Pesquisa (CAPES; #BEX1380/07-9; www.capes.gov.br), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; #477832/2010-5; www.cnpq.br) and Fundação de Amparo a Pesquisa do Estado de Goiás (FAPEG; #200910267000352; www.fapeg.go.gov.br). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 2.May CN, McAllen RM, McKinley MJ. Renal nerve inhibition by central NaCl and ANG II is abolished by lesions of the lamina terminalis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1827–R1833. doi: 10.1152/ajpregu.2000.279.5.R1827. [DOI] [PubMed] [Google Scholar]
- 3.Pedrino GR, Rosa DA, Korim WS, Cravo SL. Renal sympathoinhibition induced by hypernatremia: involvement of A1 noradrenergic neurons. Auton Neurosci. 2008;142:55–63. doi: 10.1016/j.autneu.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Pedrino GR, Monaco LR, Cravo SL. Renal Vasodilation Induced By Hypernatremia: Role Of Alpha-Adrenoceptors In The Median Preoptic Nucleus. Clin Exp Pharmacol Physiol. 2009;36(12):e83–9. doi: 10.1111/j.1440-1681.2009.05280.x. [DOI] [PubMed] [Google Scholar]
- 5.Rauch AL, Callahan MF, Buckalew VM, Jr, Morris M. Regulation of plasma atrial natriuretic peptide by the central nervous system. Am J Physiol Regul Integr Comp Physiol. 1990;258:R531–R535. doi: 10.1152/ajpregu.1990.258.2.R531. [DOI] [PubMed] [Google Scholar]
- 6.Schoorlemmer GH, Johnson AK, Thunhorst RL. Effect of hyperosmotic solutions on salt excretion and thirst in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R917–R923. doi: 10.1152/ajpregu.2000.278.4.R917. [DOI] [PubMed] [Google Scholar]
- 7.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiss ML, Claassen DE, Hirai T, Kenney MJ. Nonuniform sympathetic nerve responses to intravenous hypertonic saline infusion. J Auton Nerv Syst. 1996;57:109–115. doi: 10.1016/0165-1838(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 9.Pedrino GR, Nakagawa Sera CT, Cravo SL, Colombari DS. Anteroventral third ventricle lesions impair cardiovascular responses to intravenous hypertonic saline infusion. Auton Neurosci. 2005;117:9–16. doi: 10.1016/j.autneu.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, et al. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res. 2007;76:184–193. doi: 10.1016/j.cardiores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol. 2007;504:379–403. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- 12.Howe BM, Bruno SB, Higgs KA, Stigers RL, Cunningham JT. FosB expression in the central nervous system following isotonic volume expansion in unanesthetized rats. Exp Neurol. 2004;187:190–198. doi: 10.1016/j.expneurol.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Kasparov S, Teschemacher AG. Altered central catecholaminergic transmission and cardiovascular disease. Exp Physiol. 2008;93:725–740. doi: 10.1113/expphysiol.2007.041814. [DOI] [PubMed] [Google Scholar]
- 14.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochstenbach SL, Ciriello J. Plasma Hypernatremia Induces C-Fos Activity in Medullary Catecholaminergic Neurons. Brain Res. 1995;674:46–54. doi: 10.1016/0006-8993(94)01434-j. [DOI] [PubMed] [Google Scholar]
- 16.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, et al. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 18.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 19.Ciriello J, Rosas-Arellano MP, Solano-Flores LP. Direct projections to subfornical organ from catecholaminergic neurons in the caudal nucleus of the solitary tract. Brain Res. 1996;726:227–232. [PubMed] [Google Scholar]
- 20.Fernandez-Galaz C, Dyer RG, Herbison AE. Analysis of brainstem A1 and A2 noradrenergic inputs to the preoptic area using microdialysis in the rat. Brain Res. 1994;636:227–232. doi: 10.1016/0006-8993(94)91021-9. [DOI] [PubMed] [Google Scholar]
- 21.Saphier D. Electrophysiology and neuropharmacology of noradrenergic projections to rat PVN magnocellular neurons. Am J Physiol. 1993;264:R891–R902. doi: 10.1152/ajpregu.1993.264.5.R891. [DOI] [PubMed] [Google Scholar]
- 22.Cravo SL, Lopes OU, Pedrino GR. Involvement of catecholaminergic medullary pathways in cardiovascular responses to acute changes in circulating volume. Braz J Med Biol Res. 2011;44:877–882. doi: 10.1590/s0100-879x2011007500092. [DOI] [PubMed] [Google Scholar]
- 23.Godino A, Giusti-Paiva A, Antunes-Rodrigues J, Vivas L. Neurochemical brain groups activated after an isotonic blood volume expansion in rats. Neuroscience. 2005;133:493–505. doi: 10.1016/j.neuroscience.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Hochstenbach SL, Ciriello J. Effects of plasma hypernatremia on nucleus tractus solitarius neurons. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1916–R1921. doi: 10.1152/ajpregu.1994.266.6.R1916. [DOI] [PubMed] [Google Scholar]
- 25.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 26.Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbetaH-saporin. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- 27.Pedrino GR, Maurino I, Almeida Colombari DS, Cravo SL. Role of catecholaminergic neurones of the caudal ventrolateral medulla in cardiovascular responses induced by acute changes in circulating volume in rats. Exp Physiol. 2006;91:995–1005. doi: 10.1113/expphysiol.2006.034611. [DOI] [PubMed] [Google Scholar]
- 28.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol. 2003;285:H2734–H2748. doi: 10.1152/ajpheart.00155.2003. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffari MS, Roysommuti S, Shao ZH, Wyss JM. Renal responses to hypertonic saline infusion in salt-sensitive spontaneously hypertensive rats. American Journal of the Medical Sciences. 1997;314:370–376. doi: 10.1097/00000441-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–R2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes-Rodrigues J, Ramalho MJ, Reis LC, Menani JV, Turrin MQA, et al. Lesions of the Hypothalamus and Pituitary Inhibit Volume-Expansion-Induced Release of Atrial-Natriuretic-Peptide. Proc Natl Acad Sci U S A. 1991;88:2956–2960. doi: 10.1073/pnas.88.7.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida Y, Sugimoto I, Morita H, Murakami H, Hosomi H, et al. Suppression of renal sympathetic nerve activity during portal vein infusion of hypertonic saline. Am J Physiol Regul Integr Comp Physiol. 1998;274:R97–103. doi: 10.1152/ajpregu.1998.274.1.R97. [DOI] [PubMed] [Google Scholar]
- 33.Buller KM, Smith DW, Day TA. Differential recruitment of hypothalamic neuroendocrine and ventrolateral medulla catecholamine cells by non-hypotensive and hypotensive hemorrhages. Brain Res. 1999;834:42–54. doi: 10.1016/s0006-8993(99)01539-5. [DOI] [PubMed] [Google Scholar]
- 34.Day TA, Sibbald JR, Smith DW. A1 neurons and excitatory amino acid receptors in rat caudal medulla mediate vagal excitation of supraoptic vasopressin cells. Brain Res. 1992;594:244–252. doi: 10.1016/0006-8993(92)91131-w. [DOI] [PubMed] [Google Scholar]
- 35.Smith DW, Sibbald JR, Khanna S, Day TA. Rat vasopressin cell responses to simulated hemorrhage: stimulus-dependent role for A1 noradrenergic neurons. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1336–R1342. doi: 10.1152/ajpregu.1995.268.5.R1336. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka J, Hatakenaka S, Miyakubo H, Nomura M. Noradrenaline release in the median preoptic nucleus area caused by hemorrhage in the rat. Brain Res Bull. 2003;60:233–240. doi: 10.1016/s0361-9230(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 37.Colombari DS, Pedrino GR, Freiria-Oliveira AH, Korim WS, Maurino IC, et al. Lesions of medullary catecholaminergic neurons increase salt intake in rats. Brain Res Bull. 2008;76:572–578. doi: 10.1016/j.brainresbull.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Day TA, Sibbald JR. Involvement of the A1 cell group in baroreceptor inhibition of neurosecretory vasopressin cells. Neurosci Lett. 1990;113:156–162. doi: 10.1016/0304-3940(90)90296-l. [DOI] [PubMed] [Google Scholar]
- 39.Spyer KM. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol. 1981;88:24–124. [PubMed] [Google Scholar]
- 40.Ciriello J, Caverson MM. Ventrolateral medullary neurons relay cardiovascular inputs to the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 1984;246:R968–R978. doi: 10.1152/ajpregu.1984.246.6.R968. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka J, Hayashi Y, Watai T, Fukami Y, Johkoh R, et al. A1 noradrenergic modulation of AV3V inputs to PVN neurosecretory cells. Neuroreport. 1997;8:3147–3150. doi: 10.1097/00001756-199709290-00028. [DOI] [PubMed] [Google Scholar]
- 43.Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J Comp Neurol. 1987;259:591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- 44.Antunes-Rodrigues J, Marubayashi U, Favaretto AL, Gutkowska J, McCann SM. Essential role of hypothalamic muscarinic and alpha-adrenergic receptors in atrial natriuretic peptide release induced by blood volume expansion. Proc Natl Acad Sci U S A. 1993;90:10240–10244. doi: 10.1073/pnas.90.21.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bealer SL. Preoptic recess alpha-adrenoceptors control cardiovascular responses to hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1283–R1289. doi: 10.1152/ajpregu.1997.272.4.R1283. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong DM, Ross CA, Pickel VM, Joh TH, Reis DJ. Distribution of dopamine-, noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol. 1982;212:173–187. doi: 10.1002/cne.902120207. [DOI] [PubMed] [Google Scholar]