Abstract
The rodent acidic saline model of hyperalgesia uses repeat injections of acidic saline in the right lateral gastrocnemius muscle, spaced five days apart, to induce a persistent decrease in bilateral hindpaw withdrawal thresholds. The objective of this study was to determine if alternate injection sites would permit development of hyperalgesia. For this, the location of the first muscle injection was varied between 3 groups of rats to include the right lateral gastrocnemius, the right medial gastrocnemius or the left lateral gastrocnemius. All second injections were placed in the right lateral gastrocnemius. As reported by others, placing both injections in the right lateral gastrocnemius produced a significant reduction in paw withdrawal thresholds 24 hours after the second injection (p<0.05). Relocating the first injection to the right medial gastrocnemius or the left lateral gastrocnemius also produced significant reductions in paw withdrawal thresholds (p<0.05 for both). Hyperalgesia was also observed if the first muscle injection was replaced with a systemic injection of lipopolysaccharide. Further experiments tested whether glia cells may contribute to the priming process. Pretreatment with minocycline prior to the first injection completely blocked the development of hyperalgesia but was ineffective if injected before the second injection. These data indicate that anatomically diverse peripheral stimuli can converge within the central nervous system to produce hyperalgesia.
Keywords: hyperalgesia, muscle, priming, microglia, minocycline
1. Introduction
Chronic widespread musculoskeletal pain is a major cause of disability. One of the few experimental models of this type of pain is that developed by Sluka and colleagues in which two injections of acidic saline spaced 5 days apart into a lateral gastrocnemius muscle produce bilateral hyperalgesia which persists for 4-5 weeks (Sluka et al., 2001). This model is characterized by distinct phases including priming, the period between the 1st and 2nd injections when no hyperalgesia is present; induction, the 1st 24 hours after the 2nd injection when hyperalgesia is established; and maintenance, the period of chronic hyperalgesia that persists for 4-5 weeks.
Central factors which maintain the hyperalgesia include excitatory amino acid neurotransmission in the spinal cord and descending inputs from the rostral ventromedial medulla (RVM) (Skyba et al., 2002; Skyba et al., 2005; Tillu et al., 2008). Specifically, blockade of NMDA receptors within the rostral ventrolateral medulla (RVM) reversed the hyperalgesia and knockdown of NMDA-NR1 expression in the RVM prevented the development of hyperalgesia (Da Silva, 2010). In contrast, c-AMP-response element binding protein (CREB/phospho-CREB), protein kinase C and microglia do not appear to play a role (Hoeger-Bement and Sluka, 2003; Ledeboer et al., 2006). The maintenance phase also appears to be independent of peripheral input; there is neither ongoing peripheral inflammation in, nor primary afferent activity from, the injected muscle during the maintenance phase (Sluka et al. 2001; Sluka et al. 2003).
During the induction phase, release of glutamate and aspartate is increased at the L4 and L5 spinal segments (Skyba et al., 2002). An increase in aspartate and glutamate release was also observed in the RVM during the second acidic saline injection along with a decline in glycine release (Radhakrishnan and Sluka, 2009). The induction phase is also characterized by increased immunostaining for CREB and phospho-CREB, and enhancing or inhibiting adenylate cyclase/protein kinase A activity increased and decreased the hyperalgesia responses, respectively (Hoeger-Bement and Sluka, 2003).
Less is known regarding the priming stage of the acidic saline model. Extending this period between injections precluded the development of hyperalgesia (Sluka et al., 2001) suggesting the first intramuscular injection creates an altered state of cellular function somewhere within the nociceptive pathway that extends from the injection site to the spinal cord and the brain. The only change within the CNS reported to date is a decline in glycine release within the RVM (Radhakrishnan and Sluka, 2009). The purpose of the present study was to determine whether priming events may be generated from diverse anatomical locations. Additional studies were completed to explore a potential role of glia cells in the priming process.
2. Material and methods
2.1. Animals
Male Sprague-Dawley rats weighing 250-350 grams (Charles River, Quebec) were housed in pairs in standard rodent acrylic cages with ad libitum access to food and water within a controlled environment with automated light/dark cycle regulation. Animals were housed in these conditions for a minimum of two weeks prior to experimental manipulation. All experiments were subject to approval by the University of Manitoba Animal Protocol Management and Review process and were in accordance with guidelines established by the Canadian Council on Animal Care and the IASP Guidelines for the Use of Animals in Research.
2.2. Muscle Injections
Following the procedure of Sluka et al. (Sluka et al., 2001), animals were anesthetized with 2-4% isofluorane and injected in the medial or lateral gastrocnemius muscle with 100 ul of sterile saline at a pH of 7.2 or 4.0. The first (priming) injection occurred 5 days prior to the second (inducing) injection.
2.3. LPS Injections
Additional experiments were conducted to determine if a systemically-acting stimulus could be used as a priming signal. Animals were anesthetized with 2-4% isofluorane and injected intraperitoneally (ip) with 100 ug of bacterial endotoxin (lipopolysaccharide, LPS, from E. coli serotype 05:B55, Sigma). This dose and route of endotoxin administration is known to cause activation of distinct pathways within the CNS including those associated with the nociceptive system (Wan et al., 1992; Watkins et al., 1994). Due to the time course of the LPS effects, these animals received a single inducing injection of acidic saline in the right lateral gastrocnemius muscle 24 hours after LPS injection.
2.4. Mechanical Hyperalgesia Testing
Mechanical withdrawal thresholds were determined using Von Frey filaments (North Coast Touch Test, Morgan Hill, CA) applied to the plantar surface of the hindpaws. The reliability of this method has been previously documented (Gopalkrishnan and Sluka, 2000). Animals were placed in lucite testing cubicles over top of a mesh floor and allowed to acclimate for 30 minutes prior to testing. Filaments with bending forces of 39.2, 58.8, 78.4, 98.0, 147.0, 254.8, and 588.0 mN were used starting with the lowest force. Each filament was applied twice and the mechanical withdrawal threshold was taken as the filament which caused the limb to be abruptly withdrawn in response to both applications. This threshold was confirmed by re-testing the filaments above and below the withdrawal value. The testing was done immediately before and 24 hours after each injection.
2.5. Minocycline Injections
Further experiments were carried out to investigate the potential role of microglia cells in the priming and inducing phases of the acidic saline model. For this, animals were anesthetized with 2-4% isofluorane and injected intraperitoneally with 40 mg/kg minocycline (Sigma) (Raghavendra et al., 2003). Minocycline is an antibiotic that readily crosses the blood-brain barrier and has been shown to decrease central inflammatory responses and is commonly used as an inhibitor of microglial activation (Aronson, 1980; Raghavendra et al., 2003).
2.6. Experiment 1 – Anatomical aspects of priming in the acidic saline model
One group of animals (n=12/group) served as a common control group and were injected with 100 ul of sterile saline (pH 7.2) in the right lateral gastrocnemius on days 0 and 5. In three other groups, the first (priming) injection of 100 ul acidic saline (pH 4.0) was administered in different locations: right lateral gastrocnemius, right medial gastrocnemius or left lateral gastrocnemius. All animals in these groups subsequently received a second (inducing) injection of 100 ul acidic saline (pH 4.0) in the right lateral gastrocnemius muscle five days later. A fifth group of animals was used in which ip LPS served as the priming stimulus. These animals received the inducing injection (acidic saline, pH 4.0) in the right lateral gastrocnemius muscle 24 hours after the LPS injection rather than 5 days later. This time frame was chosen ensure that the inducing injection occurred during the period of time when some LPS effects including microglia activation are known to persist but after LPS-induced hyperalgesia is resolved (Johnston and Westbrook, 2003; Johnston and Westbrook, 2005). Behavioral testing to determine mechanical withdrawal thresholds for each hindlimb was performed as described above.
2.7. Experiment 2 - Role of glia in acidic saline model
Two groups of animals (n=12/group) received priming and inducing injections of acidic saline (pH 4.0) in the right lateral gastrocnemius muscle separated by 5 days. The first group was treated with minocycline one hour before receiving the priming injection and the second group was treated with minocycline one hour prior to the inducing injection. Behavioral testing to determine mechanical withdrawal thresholds for each hindlimb was performed as described above.
2.8. Statistical Analysis
Consistent with other reports, hyperalgesia was not observed in all animals (Nielsen et al., 2004). For statistical purposes hyperalgesic responses were defined using two criteria. Animals that displayed a mechanical withdrawal threshold equal to or less than 98 mN after the second injection were categorized as hyperalgesic. This absolute threshold was chosen based on the data obtained from the saline control group (pH 7.2). The minimal withdrawal threshold observed in the control animals was 147 mN and the lower limit of the 95% confidence intervals excluded 98 mN at all time points. This criterion ensured that animals categorized as developing hyperalgesia behaved distinctly from saline controls. A second criterion required that mechanical withdrawal thresholds must undergo a decrease of at least two filament grades to ensure that animals with a baseline mechanical withdrawal level close to 98 mN were not categorized as hyperalgesic due to insignificant downward variation in withdrawal responses. For all groups treated with acidic saline, statistical analysis was limited to those animals demonstrating hyperalgesia.
Individual values for mechanical withdrawal thresholds (mN) underwent logarithmic transformation to approximate a linear graduation between filament bending forces and permit the use of parametric statistical analyses (Spataro et al., 2004; Zar 1984). The main effects of side and time were determined within each group using two-way ANOVA (Statistica 5.1, Statsoft, Inc, Tulsa, Oklahoma). Tukey’s tests were used for all posthoc analysis. A p value <0.05 was used to determine statistical significance.
3. Results
3.1. Experiment 1 - Anatomical aspects of acidic saline model
Similar to previous reports, animals receiving saline (pH 7.2) muscle injections did not develop hyperalgesia (Fig.1, Table 1). There were no statistically significant main effects for side (F1,5=3.544, p=0.118) or time (F3,15=2.806, p=0.075) nor was the side * time interaction significant (F3,15=0.243, p=0.865). Specifically, mechanical withdrawal thresholds did not change 24 hrs after the first (right: p=0.053; left: p=0.369) or second injection (right: p=0.999; left: p=0.999). Baseline withdrawal thresholds were similar between the first and second injections (right: p= 0.369; left: p= 0.639).
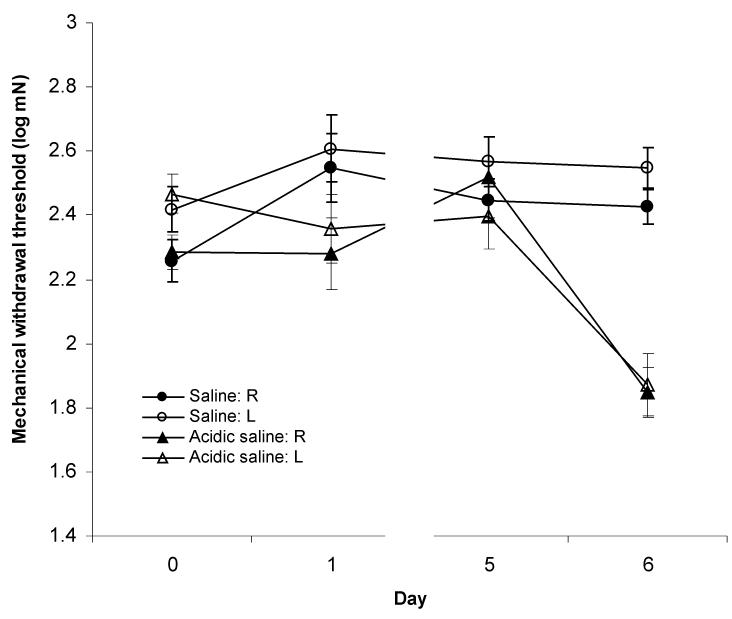
Figure 1.
Plantar mechanical withdrawal thresholds in rats receiving intramuscular injections in the right lateral gastrocnemius of either saline (pH 7.2) or acidic saline (pH 4.0). Injections were performed immediately following sensory testing on Day 0 and Day 5. Responses are shown for right (R) and left (L) hindlimbs which are ipsilateral and contralateral to the injected muscle, respectively. Withdrawal thresholds were significantly reduced (p<0.05) on Day 6 following the second injection in the acidic saline group indicating the development of bilateral hyperalgesia.
Table 1.
Frequency of hyperalgesia responses observed in animals receiving intramuscular injections of acidic saline. Group 1 received normal saline. Groups 2-4 received acidic saline injections as indicated. Group 5 received ip LPS followed by acidic saline.
| Group | Frequency of Responses |
Response Rate (%) |
|---|---|---|
|
| ||
| 1. Saline | 0/12 | 0 |
| 2. Right Lat Gastroc-Right Lat Gastroc | 6/12 | 50 |
| 3. Right Med Gastroc-Right Lat Gastroc | 8/12 | 67 |
| 4. Left Lat Gastroc-Right Lat Gastroc | 6/12 | 50 |
| 5. LPS-Right Lat Gastroc | 2/12 | 17 |
In contrast, when both of the acidic saline (pH 4.0) injections were delivered into the right lateral gastrocnemius muscle, 6 of the 12 animals (50%) developed mechanical hyperalgesia (Fig. 1, Table 1). Repeated measures ANOVA indicated a highly significant main effect of time (F3,15=11.291, p<0.001). Post-hoc analysis revealed that withdrawal thresholds did not change following the first injection (right: p=1.000; left: p= 0.795), however, a significant decrease was seen 24 hrs after the second injection (right: p<0.001; left: p<0.001). Neither the main effect of side (F1,5=2.851, p=0.152) nor the side * time interaction (F3,15=2.999, p=0.064) were statistically significant. Similar to saline treated animals, baseline withdrawal thresholds immediately prior to each injection were not different (right: p= 0.085; left: p= 0.974). Together, these data show that two injections of acidic saline targeted at the same location in the right lateral gastrocnemius muscle produces bilateral hyperalgesia as reported by others (Nielsen et al., 2004; Sluka et al., 2001).
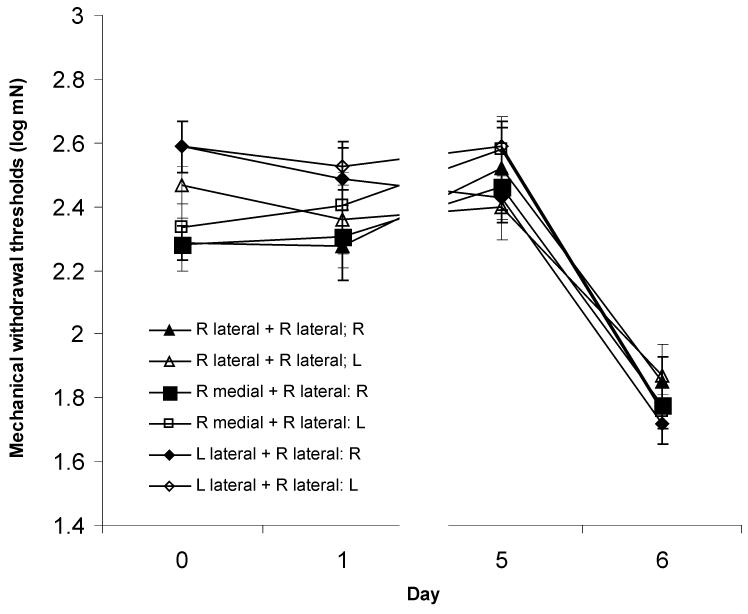
Relocation of the 1st (priming) acidic saline injection to the right medial gastrocnemius also produced mechanical hyperalgesia (Fig. 2, Table 1; main effect of time, F3,21=29.239, p<0.001). Mechanical withdrawal thresholds did not change after the 1st injection (right: p=0.999; left: p=0.809) whereas a significant decrease in thresholds occurred 24 hours after the second injection (right: p< 0.001; left: p< 0.001). A significant effect of side (F1,7=7.264, p=0.031) was observed in this group of animals which was present prior to the first injection and remained throughout the testing period (side × time interaction effect: F3,21=1.716, p=0.194). Baseline withdrawal thresholds were also higher prior to the 2nd injection (right: p= 0.021; left: p< 0.001).
Figure 2.
Plantar mechanical withdrawal thresholds in rats receiving intramuscular injections of acidic saline (pH 4.0) in the indicated gastrocnemius muscles on Day 0 and Day 5. Significant decreases in right (R) and left (L) withdrawal thresholds were observed in all groups following the second injection of acidic saline (p<0.05); relocation of the first injection site did not alter the development of hyperalgesia.
Moving the priming injection to the contralateral side (left lateral gastrocnemius) still permitted the development of mechanical hyperalgesia which was indistinguishable from that seen in other groups (Fig. 2, Table 1; main effect of time, F3,15=55.157, p<0.001). Baseline mechanical withdrawal thresholds prior to injections were similar (right: p=0.143; left: p=1.000) and withdrawal thresholds did not change after the first injection (right: p=0.626; left: p=0.950). However, a significant decrease in withdrawal thresholds was seen bilaterally after the second injection (right: p< 0.001; left: p< 0.001). A significant effect of side (F1,5=16.331, p= 0.001) was present with no side * time interaction (F3,15=1.539, p=0.245).
The remaining group within this experimental block received an ip injection of LPS as the priming signal. The standard inducing stimulus of acidic saline (pH 4.0) was then administered to the right lateral gastrocnemius muscle the following day. Although the response rate was low (Table 1), mechanical withdrawal thresholds were reduced 24 hours after the inducing injection (p=0.048) indicating the development of hyperalgesia. Importantly, withdrawal thresholds were not reduced 24 hours after the LPS injection (prior to the inducing injection; p=0.126) indicating that LPS-induced hyperalgesia was not present.
3.2. Experiment 2 – Role of Glia in Acidic Saline Model
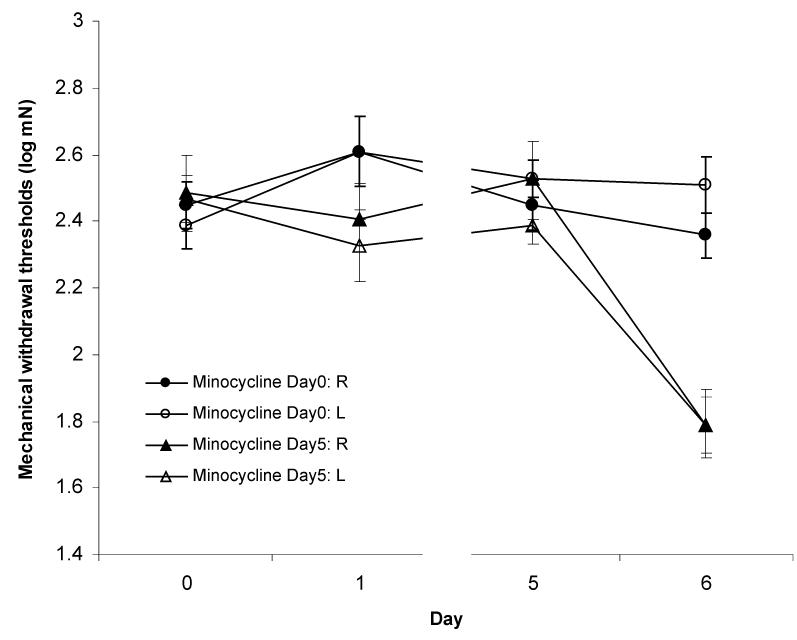
As a follow-up to the results from the LPS trial, an inhibitor of microglia function, minocycline, was injected 60 minutes prior to the priming or inducing acidic saline injections. Hyperalgesia was not observed in animals treated with minocycline before the priming injection (Fig. 3, Table 2). No significant effects were present for side (F1,5=0.476, p=0.521), time (F3,15=2.498, p=0.099 ) or the side * time interaction (F3,15=1.116, p=0.374). There was no change in withdrawal thresholds after the first injection (right: p= 0.603; left: p= 0.251). Most importantly, no reduction in withdrawal thresholds occurred after the second injection (right: p=0.962; left: p=0.999) indicating hyperalgesia did not develop in any of these animals. There were no significant differences between the baselines before the first or second injections (right: p=1.000; left: p=0.740).
Figure 3.
Plantar mechanical withdrawal thresholds in rats receiving intramuscular injections of acidic saline (pH 4.0) in the right gastrocnemius muscle on Day 0 and Day 5. Rats also received intraperitoneal injections of minocycline (40mg/kg) on Day 0 or Day 5. Minocycline injections where administered 60 minutes prior to acidic saline injections. Hyperalgesia responses were observed in animals treated with minocycline on Day 5 (p <0.05) but not in animals treated with minocycline on Day 0.
Table 2.
Frequency of hyperalgesia responses observed in animals pretreated with minocycline prior to the first or second injection of acidic saline.
| Group | Frequency of Responses |
Response Rate (%) |
|---|---|---|
| 1. Minocycline Day 0 | 0/12 | 0 |
| 2. Minocycline Day 5 | 6/12 | 50 |
In contrast, injecting minocycline before the inducing injection did not prevent the development of hyperalgesia (Fig. 3, Table 2). Significant effects were found for time (F3,6=72.585, p<0.001 ) but not side (F1,2=11.682, p=0.076) or side * time interaction (F3,6=2.350, p=0.172). Withdrawal thresholds did not change after the first injection (right: p=0.753; left: p= 0.950) but were reduced after the second injection (right: p< 0.001; left: p< 0.001) indicating the development of bilateral hyperalgesia. As was the case in all other groups, baseline withdrawal thresholds did not differ between the first and second muscle injections (right: p=1.000; left: p=0.512).
4. Discussion
The acidic saline model of chronic muscle pain induces bilateral hyperalgesia through repeated injections of acidic saline into the same muscle location (Sluka et al., 2001). Although primary hyperalgesia is present in this model (Tillu et al., 2008), it is most commonly used, including within the present study, to examine secondary hyperalgesia by measuring hindpaw withdrawal thresholds. It is unknown whether the induction of secondary hyperalgesia requires restimulation of cells that respond to the first injection such as somatic cells at the injection site or neural afferents that innervate the injection site. We tested this requirement by relocating the initial injection to different muscle sites, both ipsilateral and contralateral. Our data clearly show the necessary priming stimulus for the development of hyperalgesia can be generated from alternate locations. Further, systemically administered LPS was also able to serve as a priming stimulus, albeit with much lower efficacy. Lastly, systemic minocycline administration prior to the first (priming) injection prevented the development of hyperalgesia; minocycline was ineffective when given before the second (inducing) second injection. Together, these data indicate that priming events can arise from diverse anatomical locations and may create the primed state through activation of microglia.
The initial study describing the acidic saline model (Sluka et al., 2001) reported that extending the interval between injections from 5 to 10 days precluded the development of hyperalgesia. These data indicate that a distinct priming event is initiated by the first injection. While the exact mechanism of priming is unknown, it may involve cellular events at several anatomic locations such as the injection site within the muscle, the sensory nerve fibers that innervate the site along with the associated dorsal root ganglion (DRG) cells, and cells within the dorsal horn of the spinal cord. In the current study, the induction of bilateral mechanical hyperalgesia was unaffected by relocating the first injection from the lateral to the medial head of the right gastrocnemius muscle. The intent of displacing the first injection was to separate the injection sites such that the second injection was administered to a site that had not received any direct priming stimuli. The presence of hyperalgesia in these animals confirms that induction can occur independently of direct restimulation of the tissues that received a priming injection.
It was next determined whether priming could be achieved if the first injection was relocated to the contralateral gastrocnemius muscle. This would result in separate populations of dorsal horn neurons (and primary afferents/DRG cells) receiving the priming and inducing stimuli. Despite this further anatomical separation, animals receiving the priming and inducing injections in separate limbs developed bilateral hyperalgesia that was indistinguishable from that seen in animals given ipsilateral injections. Specifically, both the magnitude of the hyperalgesic response as well as the proportion of animals developing hyperalgesia were the same. Although the possibility cannot be excluded that injections in the left calf muscle provide some input to the right dorsal horn, there seems little support for this explanation since afferent projections that terminate in the contralateral dorsal horn are relatively rare (Novikov, 2001; Smith, 1983; Sugiura et al., 1989). Further, no responses were recorded in wide dynamic range neurons when the contralateral limb was subjected to brush or pinch mechanical stimuli (Sluka et al., 2003). These data provide evidence that the priming and induction processes converge at a common site, presumably in the central nervous system.
One central priming mechanism that may be shared by these anatomically diverse stimuli is activation of microglia cells. Microglia have been shown to contribute to other pain models such as intraplantar injection of formalin (Fu et al., 1999; Sweitzer et al., 1999; Watkins et al., 1997), ligation of spinal nerves and nerve roots (Hashizume et al., 2000; Winkelstein et al., 2001) and adjuvant-induced arthritis (Raghavendra et al., 2004). To investigate whether microglia activation may contribute to the priming phase of the acidic saline model we incorporated a previous report that microglia cells remain in an activated state 24 hours after intraperitoneal injection of LPS (Johnston and Westbrook, 2005). We, therefore, injected rats with LPS and then administered a single acidic saline injection in the right gastrocnemius muscle 24 hours later. Although the number of responding animals was low, bilateral hyperalgesia could be induced in these animals. Importantly, no effects of LPS were seen on the mechanical withdrawal threshold when tested 24 hours after LPS injection (i.e., prior to the single acidic saline injection) which is consistent with other reports that the effects of LPS on nociception are resolved within 24 hours (Johnston and Westbrook, 2003; Watkins et al., 1994).
The role of microglia cells was further examined using minocycline which is commonly used as an inhibitor of microglia activation (Aronson, 1980; Raghavendra et al., 2003). Treatment with minocycline before the priming injection completely prevented the development of hyperalgesia but minocycline was without effect when given prior to the inducing injection. Although limited, these data suggest a role for microglia activation in the priming phase rather than the induction phase of the acidic saline model. Our results are consistent with other reports of early, transient activation of microglia (DeLeo et al., 2004; Svensson et al., 2003; Li et al. 2010b) and the known lack of a contribution from microglia during the maintenance phase of hyperalgesia in this model (Ledeboer et al., 2006).
Microglia priming has been reported recently by other investigators. A single intraperitoneal injection of LPS produced a long lasting mechanical allodynia only when preceded by an experimental laparotomy whereas LPS injection on its own did not result in altered behavioral responses (Hains et al., 2010). The effect of laparotomy was blocked by intrathecal administration of minocycline. A similar priming effect was described by Li et al., whereby prior formalin injection in the hindpaw resulted in exaggerated hyperalgesia responses to a second formalin injection (Li et al., 2010a). Again, treatment with minocycline reversed the effect. The data from the minocycline experiments in the present study are consistent with the priming actions of microglia reported by others.
While the actions of minocycline may not be restricted to microglia, it has been shown that minocycline is an effective inhibitor of microglial activation. Specifically, intrathecal injection of substance P or peripheral injection of formalin induce rapid phosphorylation of the p38 protein kinase within microglia cells in the dorsal horn of the spinal cord (Svensson et al., 2003; Li et al., 2010b). The expression of p-p38 and the accompanying hyperalgesia are blocked by intrathecal or systemic minocycline treatment (Hua et al., 2005; Li et al. 2010a). Despite readily crossing the blood-brain barrier (Aronson, 1980), a limitation of studies utilizing systemic injections of minocycline, including the present one, is the potential for minocycline to act on peripheral targets. Systemic minocycline administration has been reported to inhibit both paw edema and hyperalgesia responses following carageenan injection (Cho et al., 2006). Although minocycline had no effect on the function of dorsal root ganglion neurons (Cho et al., 2006), these findings raise the possibility that the reduced central responses seen after systemic minocycline treatment may be secondary to decreased inflammatory processes in the periphery. However, this would seem an unlikely possibility given the similar effects of minocycline whether given systemically or intrathecally. Further, intramuscular injections of acidic saline do not stimulate peripheral inflammation beyond that associated with the placement of the needle (Sluka et al., 2001). Lastly, this minor inflammation would be similar between the first and second injections but minocycline was only effective when given prior to the first muscle injection. Thus, if minocycline did inhibit peripheral processes in the present study, these were not required for the induction (vs priming) of hyperalgesia. Indeed, the relative independence of the acidic saline model from ongoing peripheral inflammation sets it apart from many other models of chronic pain and provides a means to distinguish central processes which are directly related to altered pain signaling rather than secondary to overt peripheral inflammation.
In summary, our data demonstrate that anatomically diverse priming and inducing stimuli can converge within the central nervous system to generate persistent secondary hyperalgesia. Further, activation of microglia within the central nervous system represents one central target that may respond to priming stimuli although few details are known regarding how peripheral events create a primed state within the central nervous system. Priming may result from a relatively brief afferent input that creates a persistent change within the central nervous system. Alternatively, central changes may be maintained by ongoing input from a peripheral site. Despite the suggestion of relatively low afferent stimulation due to the absence of any behavioral signs of distress following acidic saline injections, additional experiments that assess and/or block peripheral processes during the period between the priming and inducing injections would be required to address this question. Further investigation of how acidic saline generates persistent hyperalgeisa is highly warranted given the similarities between this model and chronic muscle pain conditions in humans where no overt inflammatory condition exists to account for the symptoms.
Acknowledgments
We would like to acknowledge the generous assistance of Dr. K. Sluka in providing training regarding the acidic saline model. LJ supported by Manitoba Graduate Scholarship - University of Manitoba. This study was funded by NIMH (MH43778).
Footnotes
Authorship Responsibility LLJ and BJMN were both involved in the design, collection and analysis of data, interpretation of results, and the preparation of this manuscript.
There is no conflict of interest.
Reference List
- Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176(10):2061–8. [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Cho IH, Chung YM, Park CK, Park SH, Li HY, Kim D, Piao ZG, Choi SY, Lee SJ, Park K, Kim JS, Jung SJ, Oh SB. Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Res. 2006;1072(1):208–14. doi: 10.1016/j.brainres.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Da Silva LF, Desantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2009;11(4):378–87. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehab. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11(10):1004–14. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Westbrook RF. Acute and conditioned sickness reduces morphine analgesia. Behav Brain Res. 2003;142:89–97. doi: 10.1016/s0166-4328(02)00398-4. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Westbrook RF. Inhibition of morphine analgesia by LPS: role of opioid and NMDA receptors and spinal glia. Behav Brain Res. 2004;156:75–83. doi: 10.1016/j.bbr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Mahoney JH, Milligan ED, Martin D, Maier SF, Watkins LR. Spinal cord glia and interleukin-1 do not appear to mediate persistent allodynia induced by intramuscular acidic saline in rats. J Pain. 2006;7:757–767. doi: 10.1016/j.jpain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Li K, Fu KY, Light AR, Mao J. Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception. J Neuroimmunol. 2010a;221(1-2):25–31. doi: 10.1016/j.jneuroim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Lin T, Cao Y, Light AR, Fu KY. Peripheral formalin injury induces 2 stages of microglial activation in the spinal cord. J Pain. 2010b;11(11):1056–65. doi: 10.1016/j.jpain.2010.01.268. [DOI] [PubMed] [Google Scholar]
- Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol. 2004;487:93–103. doi: 10.1016/j.ejphar.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Novikov LN. Labeling of central projections of primary afferents in adult rats: a comparison between biotinylated dextran amine, neurobiotin and Phaseolus vulgaris-leucoagglutinin. J Neurosci Methods. 2001;112:145–154. doi: 10.1016/s0165-0270(01)00461-7. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett. 2009;457:141–145. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Smith CL. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983;220:29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989;62:834–840. doi: 10.1152/jn.1989.62.4.834. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Janz L, Vriend CY, Sorensen CM, Greenberg AH, Nance DM. Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res Bull. 1993;32:581–587. doi: 10.1016/0361-9230(93)90158-8. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice Hall; Engelwood Cliffs, NJ: 1984. [Google Scholar]