Abstract
Reactive oxygen species (ROS), generated as a result of various reactions, control an array of cellular processes. The role of ROS during megakaryocyte (MK) development has been a subject of interest and research. The bone marrow niche is the major site of MK differentiation and maturation. In this environment, a gradient of oxygen tension, from normoxia to hypoxia results in different levels of ROS, impacting cellular physiology. This article provides an overview of major sources of ROS, their implication in different signaling pathways, and their effect on cellular physiology, with a focus on megakaryopoiesis. The importance of ROS-generating oxidases in MK biology and pathology, including myelofibrosis, is also described.
Keywords: megakaryocytes, oxidases, reactive oxygen species, bone marrow
Introduction
Reactive oxygen species (ROS) may be beneficial for the organism, as in the classic example of ROS produced by nicotinamide adenine dinucleotide phosphate (NADPH) for defense against pathogens. They may also arise as harmful by-products of other oxidative reactions. ROS levels influence a number of basic physiological processes, ranging from cell differentiation and proliferation to cell death. The underlying mechanisms for these functions have been gradually elucidated.
There has been increasing interest in the role of ROS and oxygen stress in the regulation of hematopoiesis. A large body of studies has shown that control of intracellular levels of ROS is essential for maintenance of quiescence and self-renewal potential of hematopoietic stem cells (HSCs). The bone marrow (BM) niche seems to be an essential component of the regulation of ROS in HSCs. Like HSCs, megakaryocytes (MKs) differentiate and mature within the BM niche. These cells undergo a unique cell cycle termed endomitosis, during which the DNA content of the cell replicates without corresponding cell divisions, before platelets are released into the circulation. The present article seeks to provide an overview of the recent literature concerning the effects of ROS on different stages of MK development (Figure 1). Although not a focus of this review, important aspects of HSC biology with parallels in the MK lineage will be noted for reference and analysis. More detailed information on the role of ROS in HSCs and other lineages is available elsewhere (Eliasson and Jonsson, 2010; Ghaffari, 2008; Sardina et al., 2011; Suda et al., 2011).
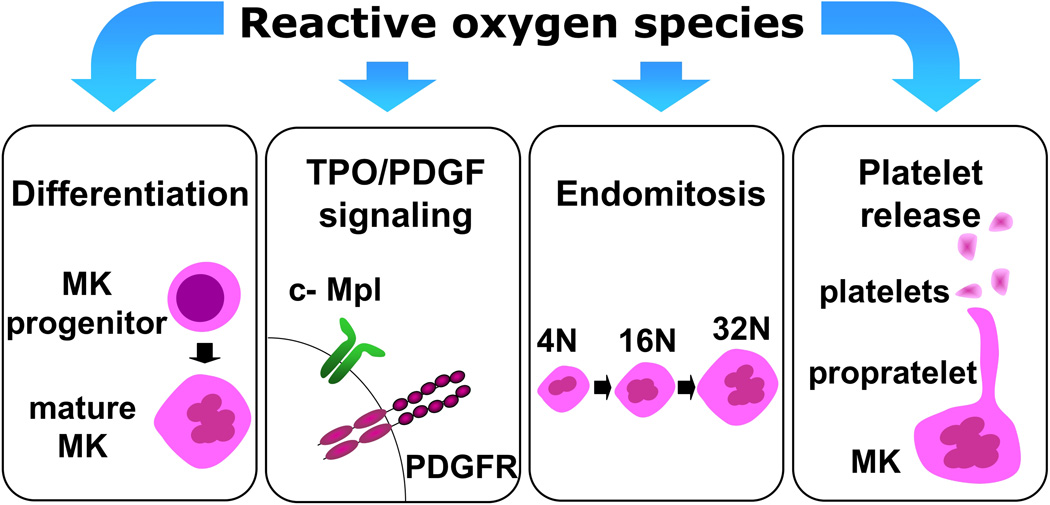
Figure 1. Effects of ROS on MK biology.
Schematic illustration of the major reported effects of ROS on MK biology, covered in this review. MK: megakaryocyte; TPO: thrombopoietin; c-Mpl: thrombopoietin receptor; PDGF: platelet-derived growth factor; PDGFR: PDGF receptor.
ROS and oxidases
Physiological processes in organisms involve oxidizing/reducing reactions (donation/gaining of an electron). ROS can be generated as a result of those reactions. ROS are free radicals (highly reactive molecules with unpaired electrons in their highest atomic orbital) containing partially reduced forms of molecular oxygen. Examples of ROS include highly reactive oxygen radicals, such as superoxide anions (O2·−), hydroxyl (OH·), peroxyl (RO2·) and alkoxyl (RO·) radicals, and non-radicals that are oxidizing agents and/or easily converted into radicals, such as hypochlorous acid (HOCl), ozone (O3), singlet oxygen (1O2) and hydrogen peroxide (H2O2) (Bedard and Krause, 2007). Although H2O2 is not a free radical (having no unpaired electrons), it is treated as one because it can easily give rise to reactive hydroxyl radicals (Boonstra and Post, 2004).
Sources of ROS can be either extracellular (pollutants, tobacco smoke, ultraviolet radiation, ionizing radiation, iron salts) or intracellular (mitochondrial respiratory chain, NADPH oxidase complexes, xanthine oxidases (XOs), amine oxidases, nitric oxide synthases (NOSs), myeloperoxidase (MPO), peroxysomes, and metabolism of arachidonic acid by lipoxygenases and cyclooxygenases (Bedard and Krause, 2007; Stocker and Keaney, 2004; Turrens, 2003).
The mitochondrion is the major intracellular source of ROS. Energy production through the mitochondrial respiratory chain is mediated by five enzyme complexes that oversee the reduction of oxygen to water, one electron at a time. Complex IV (cytochrome oxidase) retains all partially reduced intermediates until full reduction is achieved. However, the Q-cytochrome c oxireductase (Complex III), as well as the nicotamide adenine dinucleotide quinone (NADH-Q) reductase (Complex I), are well-documented sources of ROS, as they may leak electrons to oxygen, partially reducing this molecule to superoxide anion (O2·−) (Kowaltowski et al., 2009; Turrens, 2003). Apart from the mitochondria, membrane-bound NADPH-oxidase (NOX) induces production of ROS in phagocytes, which is important for defense against pathogens (Rossi and Zatti, 1964). XOs are key enzymes in purine metabolism catalyzing oxidative reactions to produce uric acid, which is an antioxidant and free-radical scavenger (Stocker and Keaney, 2004). Nitric oxide synthases (NOS) produce the free radical NO from oxidation of L-arginine. Three isoenzymes of NOSs are known: nNOS, expressed in neuronal tissues; eNOS, expressed in endothelial cells; and iNOS, the inducible form expressed in response to cytokines or bacterial products. The inducible form of NO is involved in proinflammatory reactions, functioning as a cytostatic and cytotoxic molecule (Knowles and Moncada, 1994). MPO is produced in myeloid phagocytic cells and its primary function is the destruction of microorganisms. The oxidation of chloride by MPO and H2O2 results in HOCl, a highly reactive oxidizing agent (Klebanoff, 2005). Breakdown of fatty acid chains by β-oxidation in peroxisomes is another source of intracellular ROS (Boonstra and Post, 2004). Lipoxygenases catalyze the insertion of molecular oxygen into polyunsaturated fatty acids to give rise to active lipids, including prostaglandins, thromboxanes, and leukotrienes (Kuehl and Egan, 1980).
Antioxidants
Although ROS are important for normal physiology, accumulation of ROS at high levels can be harmful to the organism, causing damage to a variety of biomolecules, such as lipids, DNA, carbohydrates and proteins. Consequently, a number of defense systems have evolved to prevent such damage. However, under conditions of increased accumulation of ROS, these defense mechanisms are not always sufficient to regulate the intracellular ROS balance, leading to oxidative stress with detrimental effects on cellular homeostasis.
The antioxidant systems in mammalians include enzymes, such as superoxide dismutases (SOD), glutathione (GSH), glutathione peroxidase (GPX), glutashione S-transferases (GST), catalase, and peroxiredoxins (Prx). SOD2 has been reported to affect the erythrocyte lineage; no other effects of SODs have been reported in hematopoiesis. GSH is the hallmark redox buffer in living cellular systems. GSH is a tripeptide of glutamic acid, cysteine and glycine and is the predominant non-protein thiol in biological systems. The balance between reduced glutathione (GSH) and oxidized glutathione (GSSG) is critical in maintaining redox homeostasis (Grek et al., 2011). GPX cooperates with catalase and uses GSH for the conversion of H2O2 into water and GSSG. GPX requires selenium for catalytic activity. GST is another member of the glutathione-dependent antioxidant defenses, catalyzing the thioester conjugation to glutathione. TLK 199 or Telintra is a peptidometic inhibitor of GST, which recently showed promising results for treatment of myelodysplastic syndrome (MDS) (Grek et al., 2011). Prxs are a family of small antixodant proteins characterized by an amino-terminal catalytic cysteine residue that is converted to sulfenic acid via reaction with H2O2. Deficiency of Prx I and II in mice affects erythroid homeostasis; loss of Peroxiredoxin I further increases susceptibility to several malignancies (Ghaffari, 2008). Non-enzymatic molecules, including vitamin E, vitamin C and flavonoids act as antioxidants capable of neutralizing ROS (Halliwell, 1999).
Overview of megakaryopoiesis
MKs are highly specialized blood cells residing primarily in the BM but also in the spleen and lung capillaries (Kaushansky, 2008; Slater et al., 1983). These cells are responsible for the production of platelets, which are renewed on a daily basis (Kaushansky and Drachman, 2002). In adults, MKs derive from BM-residing HSCs. In response to physiological demand, the HSC gives rise to early progenitor cells including the early common myeloid progenitor (CMP); further lineage specification leads to development of the bipotential megakaryocyte/erythrocyte progenitor (MEP) (Akashi et al., 2000). Under certain cytokine stimulations, the bipotential MEP can develop into a highly proliferative early MK-burst-forming unit (MK-BFU) followed by maturation to a colony-forming unit-MK (CFU-MK) (Deutsch and Tomer, 2006). These MK progenitors eventually lose their proliferative ability and commit to an endomitotic cycle. This process leads to mature polyploid MKs that grow several-fold in size. Mature MKs can obtain a DNA content of up to 128N, with the average being 16N (Kaushansky, 1999; Tomer, 2004). During this process, MKs increase the production of proteins necessary for platelet biogenesis and function (Paulus, 1970). Mature MKs form proplatelet extensions that fragment and give rise to platelets (Italiano et al., 2007). Figure 1 illustrates the above described major steps in MK development.
Thrombopoietin (TPO), also known as c-Mpl ligand or MK growth and development factor (MGDF), is the key regulator of megakaryopoiesis. In pathological conditions, such as thrombocytopenia, where there is high demand for platelets, TPO release to the MK microenvironment is increased (Sungaran et al., 2000; Sungaran et al., 1997). Mutations in the regulatory regions of the TPO gene that result in overexpression of TPO, as well as activating mutations of the c-Mpl receptor, are associated with familial essential thrombocythaemia, characterized by enhanced megakaryocyte progenitor proliferation and platelet production (Deutsch and Tomer, 2006).
TPO is not the sole regulator of MK development. A number of cytokines including platelet-derived growth factor (PDGF) are involved is this process as well. Earlier studies in primary murine and human BM cultures, as well as in human umbilical cord blood CD34+ cells, have shown that PDGF-BB is a positive regulator of megakaryopoiesis (Su et al., 2001; Su et al., 2005; Yang et al., 1995). Interestingly, PDGF-BB upregulates the expression of MK-associated transcription factors, such as c-Fos, GATA-1 and NFE2 in megakaryocytic cell lines (Chui et al., 2003). Targeted deletion of the PDGF-B polypeptide chain is embryonic lethal. Hematological analysis of these embryos reveals erythroblastosis, macrocytic anemia, and thrombocytopenia (Kaminski et al., 2001; Leveen et al., 1994).
MK differentiation: significance of hypoxia in the BM niche
HSCs predominantly remain in a quiescent state in the low-oxygen environment of the osteoblastic niche (Adams and Scadden, 2006; Parmar et al., 2007). In contrast, HSCs and other progenitor cells actively undergo cell division for proliferation and further differentiation in the more oxygenic vascular niche (Kopp et al., 2005; Li and Li, 2006). Thus, an oxygen gradient from below 1% in the most hypoxic environment to 6% in the sinusoidal cavity provides conditions that allow different regulatory processes to take place, including self-renewal and differentiation (Eliasson and Jonsson, 2010). A study by Jang et al. indicates that HSCs that produce lower levels of ROS are more primitive, as shown by higher self-renewal capacity. On the other hand, ROShigh HSCs exhibit significant exhaustion in serial transplantation assays, and have increased levels of p38 MAPK and mTOR. Importantly, treatment with a p38 inhibitor or rapamycin (mTOR inhibitor) was able to restore HSC function in the ROShigh population (Jang and Sharkis, 2007). Pallota et al., (2009) developed an in vitro system combining human osteoblasts (hOST) and hypoxia to better model the BM osteoblastic niche. Differentiation of human CD34+ cells to MKs in this system revealed that in hypoxic (5% O2) but not in normoxic (20% O2) conditions, there is a progressive increase in type I collagen in the culture, demonstrating the requirement of hematopoietic progenitors for matrix deposition and modulation of the niche environment in hypoxic conditions. Moreover, hOST had an inhibitory effect on MK maturation and proplatelet formation, which was further exacerbated by hypoxia (Pallotta et al., 2009).
MKs grow and mature in proximity to the BM sinusoid space where there is increased oxygen tension (Junt et al., 2007; Li and Li, 2006). There, MKs shed platelets into the sinuses, the microvascular structures within the BM space (Junt et al., 2007). MKs are also present in another compartment of high oxygen concentration, the lung capillaries (Zucker-Franklin and Philipp, 2000). Since MKs mature in sites of high oxygen level relative to the average 5% pO2 in the BM hematopoietic niche (8% pO2 in the BM sinusoids and 16% pO2 in the lung capillaries), it is reasonable to hypothesize that oxygen levels contribute to MK maturation (Kietzmann et al., 1999; Pennathur-Das and Levitt, 1987). One of the earliest studies on oxygen tension during MK maturation showed that CD34+ peripheral blood (PB) cells gave higher numbers of CD41+ MKs under 20% pO2 than under 5% pO2 conditions. The TPO-induced increase in MK size was also greater under 20% pO2 culturing conditions (LaIuppa et al., 1998). MK differentiation under 20% pO2 also yielded higher ploidy MKs as well as platelet forming MKs. In contrast, 5% pO2 conditions gave greater numbers of CFU-MK (Mostafa et al., 2000). This increase in MK maturation, ploidy and terminal differentiation under 20% O2 was also associated with elevated expression of MK maturation-specific transcription factors GATA-1 and NFE2. In addition, the level of E2F-1, a ubiquitously expressed cell cycle transcription factor, was increased by day 6 under 20% pO2 culturing conditions (Mostafa et al., 2001).
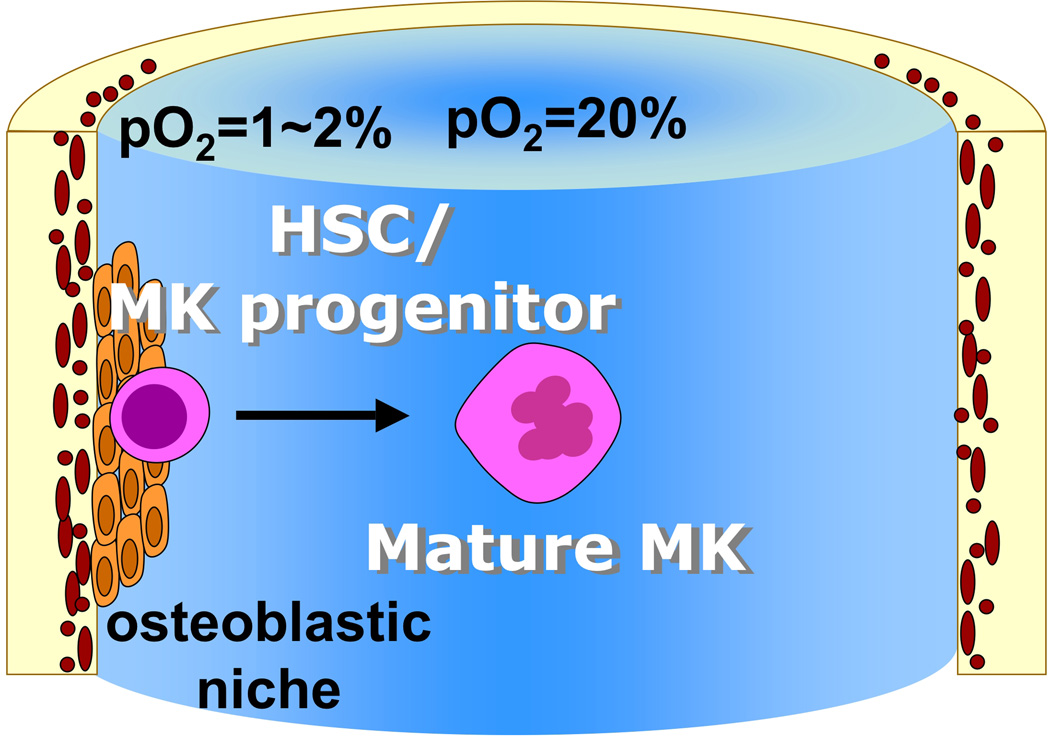
Collectively, these studies indicate that the hypoxic environment in the BM niche is favorable for maintenance of more immature MK progenitors while higher oxygen tension is essential for complete MK maturation and platelet production (Figure 2).
Figure 2. Spatial organization of the BM, with respect to distribution of oxygen tension and its effect on MK differentiation.
pO2: oxygen tension; HSC: hematopoietic stem cell; MK: megakaryocyte.
TPO and PDGF signaling and MK expansion: the role of ROS as signaling moieties
Fluctuating low levels of ROS are shown to be important in a number of regulatory processes. More specifically, H2O2 can serve as a second messenger promoting cell proliferation (Martindale and Holbrook, 2002). Although controversial, the notion of ROS as activators of signaling processes is slowly gaining ground (D'Autreaux and Toledano, 2007).
With respect to the mechanisms involved in the effect of ROS on proliferation, one study points to the possibility of H2O2 and O2·− being generated at sub-micromolar levels by cells in response to cytokine stimuli. These may act as signaling mediators, thus promoting growth responses. Indeed, hematopoietic growth factors that stimulate proliferation and differentiation of HSCs and progenitor cells signal through ROS. Studies in MO7e human megakaryoblastic leukemia cells showed that growth factors, including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3(IL-3), stem cell factor (SCF) and TPO induce a rapid increase in ROS levels (Prata et al., 2004). This peaking of ROS, in turn, may be essential for the activation of cytokine receptors.
The majority of signaling responses occur through the phosphorylation of tyrosine residues. Seminal works demonstrated that treatment of cells with H2O2 induces a global increase in proteins with phosphorylated tyrosine residues (pTyr), thus establishing a correlation between ROS and signaling activation (Heffetz et al., 1990). In vascular smooth muscle cells (VSMC) treated with PDGF, the time-course of H2O2 production was similar to that of PDGF-induced tyrosine phosphorylation.
Further studies aimed at discovering the proteins phosphorylated in response to ROS. In MO7e cells, the β common chain receptor for both GM-CSF and IL-3 is phosphorylated upon H2O2 stimulation, as upon stimulation with GM-CSF. H2O2 stimulation also induced tyrosine phosphorylation of PDGF α- and β-receptors (Gonzalez-Rubio et al., 1996). Addition of the antioxidant pyrrolidine dithiocarbamate (PDTC) to the cultures decreases the ROS levels and inhibits tyrosine phosphorylation induced by GM-SCF (Sattler et al., 1999). Mediators of signaling responses are also phosphorylated in response to ROS production. STAT5, which is a mediator of TPO responses, is phosphorylated in response to H2O2 stimulation (Sattler et al., 1999). Inhibition of ROS production hampered the activation of serine/threonine protein kinase (AKT), signal-transducer and activator of transcription 3 (STAT3) and STAT5 in an HEL cell line stimulated with TPO (Sardina et al., 2010). Other studies have focused on the activation of mitogen-activated protein kinases (MAPKs). This can be achieved by direct extracellular-regulated MAP kinase (ERK) phosphorylation and activation, where cells are exposed to H2O2, or indirectly by activating upstream effectors such as MAP kinase/ERK kinase (MEK) (stimulated by ONOO− or H2O2), Raf-1, or protein kinase C (PKC) (stimulated by H2O2) (Abe et al., 1998; Zhang et al., 2000). As a result, ROS-mediated MAPK activation can induce upregulation of transcription factors, such as c-fos and c-jun (Buscher et al., 1988; Rao, 1997). ERK activation in K562 and HEL cells occurs during phorbol myristate acetate (PMA)-induced MK differentiation, and this activation is hampered by inhibitors of ROS production (Sardina et al., 2010).
Signaling initiated by protein phosphorylation induced by tyrosine kinases is followed by de-phosphorylation by protein tyrosine phosphatases (PTPs) for adequate termination of the signaling. Recent studies indicate that perhaps the major role of ROS in cell signaling is the modulation of PTP function. The signature motif of the PTP superfamily contains an invariant Cys residue, which is important to catalytic activity and is particularly susceptible to oxidation (Tonks, 2005). The classical example is the MAP kinase phosphatase-3 (MKP-3), a specific regulator of Erk, oxidized in vitro by H2O2 (Kamata et al., 2005). The low molecular weight protein tyrosine phosphatase (LMW-PTP) negatively regulates PDGF signaling through binding and dephosphorylation of the receptor. LMW-PTP is oxidized and inactivated by exposure to H2O2 or to NO; this oxidization can also be effected by PDGF signaling, likely through H2O2 production. Moreover, glutathione, an antioxidant, most likely regulates the reversibility of LMW-PTP inactivation (Chiarugi, 2001). Exposure of cells to H2O2 or PDGF induces Akt phosphorylation and oxidation of phosphatase and tensin homolog (PTEN) (Kim et al., 2011). The antioxidant PrxII, has been demonstrated to locally regulate PDGF signaling in VSMC by decreasing the oxidation of PTPs, thereby functioning as a negative regulator of PDGF signaling (Choi et al., 2005).
IL-3 and erythropoietin (Epo) induce a transient increase in ROS levels when added to cultures of the hematopoietic progenitor cell line 32Dcl3. Furthermore, treatment of these cells with the antioxidant N-acetyl-L-cysteine (NAC) inhibits the IL-3-induced phosphorylation of JAK2, AKT and ERK. Moreover, upon treatment with the antioxidant, there is a downregulation of cyclin D2 and cyclin E concomitantly with an increase in expression of the cell cycle inhibitor p27, thus inhibiting G1 to S phase progression (Iiyama et al., 2006).
Studies in HeLa cells and primary fibroblasts demonstrate that exposure of cells to H2O2 or peroxynitrite induces Akt activation respectively mediated by epidermal growth factor (EGF) and PDGF receptors (Klotz et al., 2000; Wang et al., 2000). Other pathways activated by ROS are the p38 and c-Jun N-terminal kinases (JNK). These pathways are responsive to oxidative stress and are associated with apoptosis and mitotic arrest (Martindale and Holbrook, 2002).
The above ROS-affected signaling processes are clearly involved in expansion of primary BM MKs, as STAT and MAPK activation are important for MK proliferation (Miyakawa et al., 1996; Severin et al., 2010), and so are G1 phase cyclins (Eliades et al., 2010; Zimmet et al., 1997). Although the accumulated evidence is not sufficient to establish a definitive role of ROS in signaling affecting MK biology, the data indicates a strong effect of ROS on the activation of tyrosine kinase signaling and MK expansion. For PDGF signaling, there is a need to validate current findings in the megakaryocyte lineage. Further studies elucidating the precise mechanisms of this effect are awaited.
MK endomitosis and polyploidization: role of ROS and NAPDH oxidases
As described above, a number of studies have investigated the role of ROS in MK maturation. Therefore, it is of interest to determine the source of ROS in this lineage. An important insight came from the observation that diphenylene iodonium chloride (DPI), an inhibitor of flavoprotein-dependent enzymes, inhibited platelet aggregation (Salvemini et al., 1991). NADPH-oxidase is such an enzyme, and also a known enzymatic source of ROS (Rossi and Zatti, 1964). The family of NADPH-oxidase or NOX proteins consists of oxidases responsible for the transfer of electrons across biological membranes (Bedard and Krause, 2007). Members of the family include the phagocyte NOX2 (gp91phox), NOX1, NOX3, NOX4, NOX5 and the dual oxidases DUOX1 and DUOX2 (Bedard and Krause, 2007). Organizer subunits p47phox and NOXO1 as well as activator subunits p67phox and NOXA1 and modulator subunit p40 associate with NOXs (Bedard and Krause, 2007; Geiszt, 2006). All members of the NOX family contain six transmembrane domains, two binding sites for heme and conserved binding sites for NADPH and flavin (Geiszt, 2006).
Recent studies have focused on the source of ROS in HSCs. In PB-derived CD34+ cells, a low mitochondrial oxygen consumption rate was detected, thus qualifying HSCs as a poor oxidative phosphorylating cell type. In addition to low mitochondrial oxygen consumption, the authors depicted the contribution of NOX2 and NOX4 in extra-mitochondrial oxygen consumption (Piccoli et al., 2005). Moreover, both catalase and the NOX pharmacological inhibitors, Apocynin and DPI, inhibited ROS production by human BM-derived HSCs. These cells were also shown to express at least three different NOX isoforms - NOX1, NOX2 and NOX4 - at both the mRNA and protein level along with a set of their regulatory subunits (Piccoli et al., 2007b). Interestingly, CD34+ and CD133+ HSCs express hypoxia-inducible factor 1α (HIF-1α) under normoxic conditions and its levels are positively regulated by NOX-dependent production of ROS (Piccoli et al., 2007a). Seno et al. (2001), using a pharmacological approach to target NOXs, –demonstrated that they are possible sources of ROS in human platelets and in the megakaryocytic cell line MEG01. The authors also verified the expression of the NOX regulatory components p22phox and p67phox in both platelets and MEG01cells (Seno et al., 2001).
More recently, it was reported that Nox1 is the major Nox expressed in primary mouse megakaryocytes and contributes to the production of ROS in CD41+ MKs. Inhibition of Nox1 by Apocynin or DPI reduced polyploidization in wild-type MKs. This defect was due to reduced levels of G1 cyclins D3 and E, which have been shown to be important for MK polyploidy (Eliades et al., 2010; Wang et al., 1995). Thus, the effect of ROS on expression of G1 phase cyclins and G1-S cycle progression, along with the reported effect of NOX4 in VSMC polyploidy (McCrann et al., 2009b), point to NOX as an important player in polyploidization. The influence of ROS on G1 phase cyclins has been validated not only in MKs. NOX1-mediated increase in ROS in mouse lung epithelial cells promotes phosphorylation of Erk1/2 and expression of cyclin D1. Antioxidant treatment with catalase or diphylene iodonium, a potent NOX inhibitor, downregulates the levels of cyclin D1 (Ranjan et al., 2006). ROS level is known to increase as cells progress from G1 to S phase and this accumulation is required for entry into S phase (Havens et al., 2006).
MK maturation and platelet formation: the role of ROS
Endogenous ROS are detected in MKs from mouse BM (McCrann et al., 2009a). TPO induces a rapid increase in ROS that is necessary for the megakaryocytic differentiation of human HSCs (Sardina et al., 2010). ROS can control gene expression through the activation of redox-sensitive transcription factors, which in turn coordinate the expression of a number of downstream target genes with antioxidant roles. The classical example is the NF-κB transcription factor, activated by H2O2 and intermediates of oxygen radicals (Schmidt et al., 1996; Schreck et al., 1991). p45NF-E2 is a member of the cap ‘n’ collar-basic leucine zipper family of transcriptional activators, with a restricted expression in hematopoietic cells. This family is known to bind antioxidant response elements (ARE) in DNA, which are responsible for the expression of genes related to the defense against oxidative stress. Mice deficient for p45NF-E2 died in neonatal period due to complications of hemorrhage. Absolute thrombocytopenia was detected in those animals. MK ploidization was not affected, indicating that the lack of platelets was due not to differentiation defects in MKs, but rather to a defect in formation of platelet territories in the cytoplasm of MKs (Shivdasani et al., 1995). Recently, work by Motohashi et al., (2010) revealed that NF-E2 p45–promotes ROS accumulation that results in enhanced MK maturation. This is achieved by competition with Nrf2, a key activator of stress-responsive genes (Motohashi et al., 2010).
MK senescence and autophagy: the role of ROS
In conditions of hyperoxia and elevated ROS, cells resort to a decrease in proliferation, cell cycle arrest and senescence (Boonstra and Post, 2004; Shao et al., 2011). Hyperoxia increases the levels of p21, a cell cycle inhibitor that belongs to the family of Cip/Kip proteins (Cazzalini et al., 2010). This increase is mediated by p53 (Helt et al., 2001). In HSCs, senescence can be induced by activation of p38 MAPK (Ito et al., 2006). Recently, studies on UT-7/TPO cells treated with TPO have suggested that cell cycle arrest and senescence participate in the process of MK maturation. This process was accompanied by up-regulation of the senescence marker p21, and mediated by phosphorylation of ERK (Besancenot et al., 2010).
Autophagy is a catabolic pathway in which cells sequester organelles such as mitochondria into lysosomes for degradation. The process of autophagy is an important cellular survival mechanism, which can be activated in response to multiple physiological conditions, including starvation, hormonal imbalance and oxidative stress (Watson et al., 2011). The mammalian target of rapamycin (mTOR) is the central molecular component of autophagy. The Atg1-13 protein complex initiates autophagy, activated by the absence of signaling from mTOR. Mice deficient for Atg7 in the hematopoietic system develop myeloproliferation/MDS and exhibit high mitochondrial content and ROS in HSCs (Mortensen et al., 2011). Interestingly, autophagy has been observed in the megakaryocytic differentiation of the K562 cell line (Colosetti et al., 2009), and mTOR is a reported as a regulator of MK differentiation (Drayer et al., 2006; Raslova et al., 2006); these findings collectively suggest the involvement of ROS-dependent autophagic processes in MK.
Other effects of ROS in MKs
15d-PGJ2
One of the functions of 15d-PGJ2, a J-type prostaglandin, is generation of ROS. 15d-PGJ2 treatment of primary human MKs induced formation of ROS and increased platelet production. Treatment with antioxidants attenuated the platelet-producing effect of 15d-PGJ2 (O'Brien et al., 2008).
TLR2
Stimulation of the Toll-like receptor 2 (TLR2) using the agonist Pam3CSK4 increased production of ROS in the Meg-01 cell line and affected MK-related signaling and gene expression, as well as ploidy of mouse MKs in vitro and in vivo (Beaulieu et al., 2011).
NO
The addition of NO donors sodium nitroprusside (SNP) or (Z)-1-(2-(aminoethyl)-N-(2-ammonioethyl)amino)diazen-1-ium-1,2-diolate (DETA/NO) to cultures of human CD34+ cells has toxic effects on both total cell number and TPO-induced MK differentiation (as measured by total number of MK and percentage of CD41 expression) (Schattner et al., 2000). The most likely effect of NO on MKs is induction of apoptosis, as demonstrated in detail in megakaryocytic cell lines (Battinelli and Loscalzo, 2000) and MKs derived from human CD34+ cells (Schattner et al., 2000). Stimulation of CD34+ cells with tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) increased endogenous NO levels and suppressed MK growth (Schattner et al., 2000), whereas treatment with TPO suppressed the induction of apoptosis by NO (Battinelli and Loscalzo, 2000). These findings indicate that NO is a mediator of apoptosis in the MK lineage.
ROS in MK pathology
Although there are no reports associating ROS with specific megakaryocytic disorders, oxidative stress has been implicated in a variety of BM failure conditions, such as MDS and myelofibrosis, in which platelet deficiency requires therapeutic intervention.
ROS in MDS
MDS is a clonal stem cell disorder characterized by ineffective maturation of the erythroid, granulocytic, or megakaryocytic lineage. The natural history of the disease is progression from cytopenia to myeloid leukemia. MDS is a disease of the elderly, with a mean age of 70 years at diagnosis (Corey et al., 2007). Age itself is an established critical factor for accumulation of oxidative damage in HSCs (Finkel and Holbrook, 2000). Moreover, accumulating evidence has suggested a major causative role for ROS in the pathogenesis of MDS (Farquhar and Bowen, 2003), beginning with a seminal study that demonstrated the presence of oxidative DNA damage in CD34+ cells from MDS patients (Tauro et al., 2001). A high superoxide concentration has also been detected in supernatant from MDS stroma compared to normal stroma (Tauro et al., 2001). Exposure to cigarette smoke and benzene are reported to be associated with risk of MDS. The progression to disease is also likely to be associated with a deficiency in the detoxification system, such as the NAD(P)H:quinine oxidoreductase (NQO1) deficiency in the hematopoietic stem cells (Rothman et al., 1997). An experimental model of cigarette smoke exposure in guinea pigs demonstrated the correlation between NQO1 deficiency, cigarette smoke exposure, and progression to MDS (Das et al., 2011). Moreover, as noted in our discussion of senescence and autophagy, mice deficient for Atg7 progress to a myeloproliferative disorder/MDS (Mortensen et al., 2011). Mitochondria are the major source of ROS within the cell. Signs of defective mitochondrial autophagy have been observed in erythroid precursors of MDS patients (Houwerzijl et al., 2009), and mutations of mitochondrial DNA are commonly detected in MDS patients (Gattermann, 2004). However, the precise role of autophagy in MDS is still under discussion (Watson et al., 2011).
Lysyl oxidase (LOX) in myelofibrosis
LOX is a copper-dependent amine oxidase that catalyzes the oxidative deamination of lysine and hydroxylysine residues on collagen and elastin precursors. The resulting semialdehydes form covalent cross-linkages, thus stabilizing the extracellular matrix fiber deposits (Lucero and Kagan, 2006). LOX is synthesized as a 50 kDa glycosylated precursor (pro-LOX) which is then secreted and undergoes proteolytic cleavage by pro collagen C-proteinases, including bone morphogenetic protein 1 (BMP1), to release a catalytically active 30 kDa enzyme (LOX) and an 18 kDa propeptide (LOX-PP) (Kagan and Li, 2003; Trackman et al., 1992). The importance of this oxidase was demonstrated in LOX knockout mice, which die soon after birth due to aortic rupture, incomplete diaphragm development and cardiovascular dysfunction (Maki et al., 2002). Studies from our group and others have shown that LOX mobilizes monocytes, fibroblasts and VSMCs (Lazarus et al., 1995; Li et al., 2000; Nelson et al., 1988). LOX has been associated with various pathologies, including cardiovascular diseases (Rodriguez et al., 2008), neurodegenerative disorders (Gilad et al., 2001; Gilad et al., 2005), tumor progression and metastasis (Erler et al., 2006; Min et al., 2009; Payne et al., 2005). An interesting insight into the regulation of MKs by LOX came from a recent study from our laboratory showing that LOX can oxidize cell surface proteins, including PDGFR-α, in rat aortic smooth muscle cells (Lucero et al., 2008). This effect was blocked by α-aminopropionitrile (BAPN), an inhibitor of LOX enzyme activity (Lucero et al., 2008; Tang et al., 1983). The BAPN-mediated inhibition of PDGFR oxidation diminished the binding affinity for its correspondent ligand, PDGF-BB. This inhibition resulted in an accelerated rate of decay of phosphorylated downstream effectors of PDGFR signaling, such as Akt and ERK1/2 (Lucero et al., 2008). This effect of LOX was also identified in MKs, showing dependency of PDGF-BB on an active LOX to promote MK proliferation. Furthermore, LOX is primarily expressed in low-ploidy MKs (Eliades et al., 2011).
Our laboratory also uncovered an important role for LOX in controlling MK-induced bone marrow fibrosis. The term myelofibrosis indicates BM deposition of reticulin, collagen or both. However, regardless of the pathogenic background, MK-induced myelofibrotic conditions share a common denominator: defective MK development and a dense extracellular matrix (Kuter et al., 2007). BM fibrosis, in the context of acute megakaryocytic leukemia (AMKL) and myeloproliferative disorders usually involves deposition of reticulin and/or collagen fibers (Kuter et al., 2007; McCarthy, 1985). The evolving hypothesis is that MKs release growth factors such as Transforming Growth Factor β (TGF-β), PDGF and Fibroblast Growth Factor (FGF), which accentuate the production of collagen by fibrogenic cells (Le Bousse-Kerdiles and Martyre, 1999; Le Bousse-Kerdiles et al., 2008; Reilly et al., 1993; Terui et al., 1990; Vannucchi et al., 2002). LOX not only affects the proliferative effect of PDGF, but through its ability to cross-link the extracellular matrix, is a major regulator of the fibrotic phenotype in the above pathologies. Pharmacological inhibition of LOX significantly attenuated matrix deposition and myelofibrosis in GATA-1low mice (Eliades et al., 2011). In GATA-1low mice, the abrogation of the distal promoter of GATA-1 and the DNAse hypersensitive region leads to downregulated GATA-1. These mice display myelofibrosis with a significantly increased number of MKs arrested between the stage of megakaryoblast and immature MK and decrease in total marrow cellularity (Centurione et al., 2004; Vannucchi et al., 2002). The effects of LOX on MKs are summarized in Figure 3.
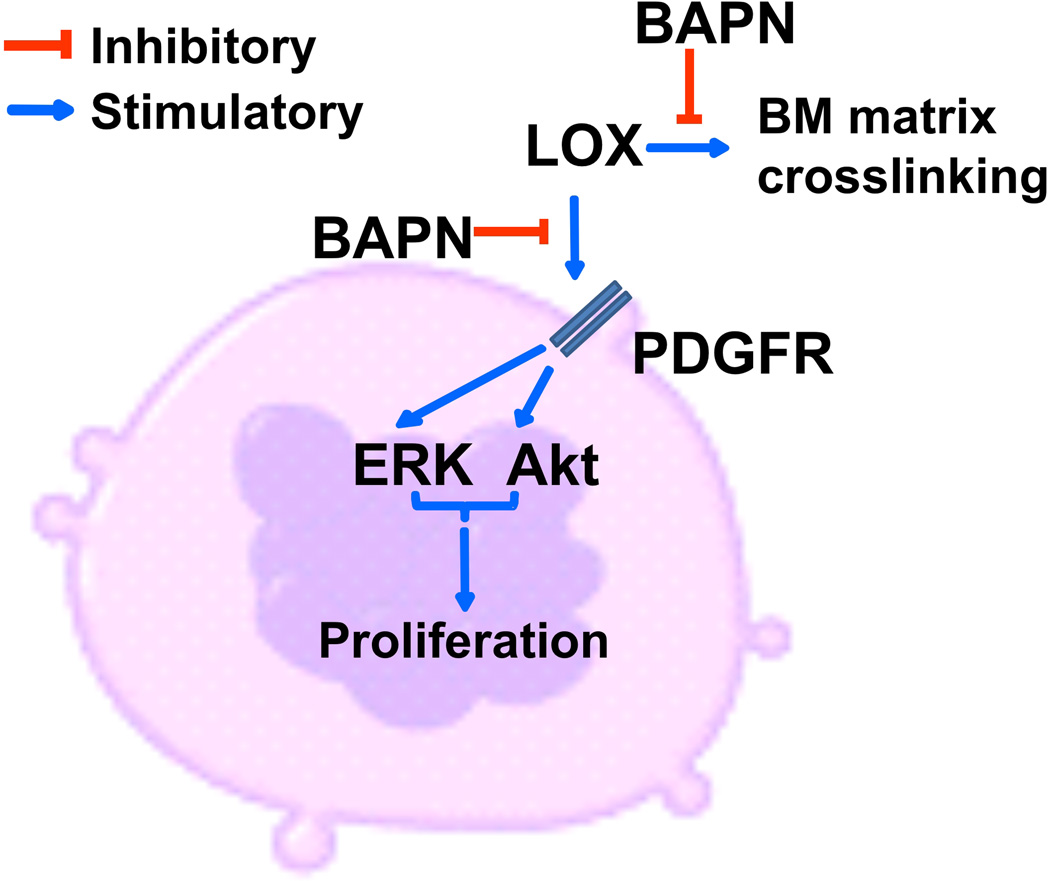
Figure 3. Effect of LOX on MK biology and progression of BM fibrosis.
Extracellular LOX stimulates PDGF-mediated Erk and Akt signaling, contributing to MK progenitor proliferation. Inhibition of LOX by BAPN eliminates this effect. BAPN also inhibits collagen cross-linking through inhibition of the catalytic activity of LOX, hampering progression of myelofibrosis.
Finally, a study analyzing the levels of oxidative stress in patients with primary myelofibrosis detected significantly raised ROS concentrations and significantly lowered total antioxidant capacity (Vener et al., 2010). Added to the finding that ROS were implicated in the expression of hypoxia-induced LOX in endothelial cells (Guadall et al., 2011), the data accumulated so far suggest a novel and central role of ROS and LOX in the progression of marrow fibrotic disorders.
Therapeutic targeting of ROS
ROS homeostasis deregulation has been reported in many hematological disorders. Together with evidence of ROS involvement in regulation of critical cellular events such as proliferation, differentiation and survival, targeting ROS for therapeutic purposes is a promising approach for further development. However, since ROS are also important for homeostasis of normal hematopoietic cells, specific targeting of malignant cells has proven trickier than initially hoped. Two basic approaches are possible: the pro-oxidant and anti-oxidant, which aim respectively to increase or decrease ROS in target cells (Grek et al., 2011; Hole et al., 2011; Sardina et al., 2011).
The rationale of the pro-oxidant approach is that since malignant cells already have high levels of ROS, increasing their ROS to toxic levels is more easily achievable than with normal cells. This effect may be achievable by inhibiting intracellular anti-oxidants. TLK 199, Telintra, is a peptido-mimetic inhibitor of an isoform of GST, GSTP (π). In preclinical mouse studies Telintra raised circulation of blood cells of all lineages, an effect associated with increase in BM progenitor cells. Telintra has shown positive results in an ongoing Phase II clinical trial for MDS; multilineage hematologic improvement has been observed, including decreased requirements for red blood cell, platelet, and growth factor support (Grek et al., 2011).
The anti-oxidant approach is based on the idea that malignant cells depend on high levels of ROS for survival. Amifostine has been used with traditional anti-cancer drugs as a cytoprotective agent. Amifostine is converted to its active metabolite by the membrane-bound enzyme alkaline phosphatase. The active metabolite prevents or repairs oxidative stress-induced DNA damage by scavenging free radicals, donating hydrogen ions to free radicals and direct binding and inactivation of cytotoxic drugs. Because normal tissues generally have higher levels of alkaline phosphatase, better vascularization and higher pH, the active metabolite preferentially locates there. Studies have also indicated that amifostine stimulates HSCs. These features have inspired ongoing clinical use of amifostine in MDS patients (Grek et al., 2011; Hole et al., 2011).
Summary
There is increasing evidence that oxygen tension in the microenvironment affects MK differentiation, maturation, polyploidy and proplatelet fragmentation. Further research is needed to better understand how different oxygen tensions translate into signals that control MK biology. As outlined above, an array of oxidases, including NOX or LOX, affect the bone marrow niche and its matrix, as well as cell cycle properties. Together, these influences have a major impact on lineage development and cell propagation. Developing selective inducers or inhibitors of specific oxidases to control ROS in the BM niche will aid investigations at both the basic and translational levels.
Acknowledgements
This work was supported by NHLBI grant HL80442 to KR. KR is an established Investigator with the American Heart Association. We apologize to all authors whose important work in the field could not be cited because of word count limitations.
Footnotes
Conflict of Interest
The authors of this article have no conflict of interest to declare.
References
- Abe MK, Kartha S, Karpova AY, Li J, Liu PT, Kuo WL, Hershenson MB. Hydrogen peroxide activates extracellular signal-regulated kinase via protein kinase C, Raf-1, and MEK1. Am J Respir Cell Mol Biol. 1998;18(4):562–569. doi: 10.1165/ajrcmb.18.4.2958. [DOI] [PubMed] [Google Scholar]
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7(4):333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Battinelli E, Loscalzo J. Nitric oxide induces apoptosis in megakaryocytic cell lines. Blood. 2000;95(11):3451–3459. [PubMed] [Google Scholar]
- Beaulieu LM, Lin E, Morin KM, Tanriverdi K, Freedman JE. Regulatory effects of TLR2 on megakaryocytic cell function. Blood. 2011;117(22):5963–5974. doi: 10.1182/blood-2010-09-304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Besancenot R, Chaligne R, Tonetti C, Pasquier F, Marty C, Lecluse Y, Vainchenker W, Constantinescu SN, Giraudier S. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010;8(9) doi: 10.1371/journal.pbio.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Buscher M, Rahmsdorf HJ, Litfin M, Karin M, Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988;3(3):301–311. [PubMed] [Google Scholar]
- Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704(1–3):12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Centurione L, Di Baldassarre A, Zingariello M, Bosco D, Gatta V, Rana RA, Langella V, Di Virgilio A, Vannucchi AM, Migliaccio AR. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1(low) mice. Blood. 2004;104(12):3573–3580. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- Chiarugi P. The redox regulation of LMW-PTP during cell proliferation or growth inhibition. IUBMB Life. 2001;52(1–2):55–59. doi: 10.1080/15216540252774775. [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435(7040):347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Chui CM, Li K, Yang M, Chuen CK, Fok TF, Li CK, Yuen PM. Platelet-derived growth factor up-regulates the expression of transcription factors NF-E2, GATA-1 and c-Fos in megakaryocytic cell lines. Cytokine. 2003;21(2):51–64. doi: 10.1016/s1043-4666(02)00499-4. [DOI] [PubMed] [Google Scholar]
- Colosetti P, Puissant A, Robert G, Luciano F, Jacquel A, Gounon P, Cassuto JP, Auberger P. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy. 2009;5(8):1092–1098. doi: 10.4161/auto.5.8.9889. [DOI] [PubMed] [Google Scholar]
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7(2):118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Das A, Dey N, Ghosh A, Das T, Chatterjee IB. NAD(P)H: quinone oxidoreductase 1 deficiency conjoint with marginal vitamin C deficiency causes cigarette smoke induced myelodysplastic syndromes. PLoS One. 2011;6(5):e20590. doi: 10.1371/journal.pone.0020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch VR, Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134(5):453–466. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- Drayer AL, Olthof SG, Vellenga E. Mammalian target of rapamycin is required for thrombopoietin-induced proliferation of megakaryocyte progenitors. Stem Cells. 2006;24(1):105–114. doi: 10.1634/stemcells.2005-0062. [DOI] [PubMed] [Google Scholar]
- Eliades A, Papadantonakis N, Bhupatiraju A, Burridge KA, Johnston-Cox HA, Migliaccio AR, Crispino JD, Lucero HA, Trackman PC, Ravid K. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286(31):27630–27638. doi: 10.1074/jbc.M111.243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliades A, Papadantonakis N, Ravid K. New roles for cyclin E in megakaryocytic polyploidization. J Biol Chem. 2010;285(24):18909–18917. doi: 10.1074/jbc.M110.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Farquhar MJ, Bowen DT. Oxidative stress and the myelodysplastic syndromes. Int J Hematol. 2003;77(4):342–350. doi: 10.1007/BF02982641. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gattermann N. Mitochondrial DNA mutations in the hematopoietic system. Leukemia. 2004;18(1):18–22. doi: 10.1038/sj.leu.2403209. [DOI] [PubMed] [Google Scholar]
- Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71(2):289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Kagan HM, Gilad VH. Lysyl oxidase, the extracellular matrix-forming enzyme, in rat brain injury sites. Neurosci Lett. 2001;310(1):45–48. doi: 10.1016/s0304-3940(01)02089-4. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Kagan HM, Gilad VH. Evidence for increased lysyl oxidase, the extracellular matrix-forming enzyme, in Alzheimer's disease brain. Neurosci Lett. 2005;376(3):210–214. doi: 10.1016/j.neulet.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rubio M, Voit S, Rodriguez-Puyol D, Weber M, Marx M. Oxidative stress induces tyrosine phosphorylation of PDGF alpha-and beta-receptors and pp60c-src in mesangial cells. Kidney Int. 1996;50(1):164–173. doi: 10.1038/ki.1996.299. [DOI] [PubMed] [Google Scholar]
- Grek CL, Townsend DM, Tew KD. The impact of redox and thiol status on the bone marrow: Pharmacological intervention strategies. Pharmacol Ther. 2011;129(2):172–184. doi: 10.1016/j.pharmthera.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadall A, Orriols M, Alcudia JF, Cachofeiro V, Martinez-Gonzalez J, Rodriguez C. Hypoxia-induced ROS signaling is required for LOX up-regulation in endothelial cells. Front Biosci (Elite Ed) 2011;3:955–967. doi: 10.2741/e301. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic Res. 1999;31(4):261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- Havens CG, Ho A, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol Cell Biol. 2006;26(12):4701–4711. doi: 10.1128/MCB.00303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D, Bushkin I, Dror R, Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990;265(5):2896–2902. [PubMed] [Google Scholar]
- Helt CE, Rancourt RC, Staversky RJ, O'Reilly MA. p53-dependent induction of p21(Cip1/WAF1/Sdi1) protects against oxygen-induced toxicity. Toxicol Sci. 2001;63(2):214–222. doi: 10.1093/toxsci/63.2.214. [DOI] [PubMed] [Google Scholar]
- Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117(22):5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- Houwerzijl EJ, Pol HW, Blom NR, van der Want JJ, de Wolf JT, Vellenga E. Erythroid precursors from patients with low-risk myelodysplasia demonstrate ultrastructural features of enhanced autophagy of mitochondria. Leukemia. 2009;23(5):886–891. doi: 10.1038/leu.2008.389. [DOI] [PubMed] [Google Scholar]
- Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal. 2006;18(2):174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Italiano JE, Jr, Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. J Thromb Haemost. 2007;5(Suppl 1):18–23. doi: 10.1111/j.1538-7836.2007.02487.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kaminski WE, Lindahl P, Lin NL, Broudy VC, Crosby JR, Hellstrom M, Swolin B, Bowen-Pope DF, Martin PJ, Ross R, Betsholtz C, Raines EW. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood. 2001;97(7):1990–1998. doi: 10.1182/blood.v97.7.1990. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. The enigmatic megakaryocyte gradually reveals its secrets. Bioessays. 1999;21(4):353–360. doi: 10.1002/(SICI)1521-1878(199904)21:4<353::AID-BIES12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111(3):981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K, Drachman JG. The molecular and cellular biology of thrombopoietin: the primary regulator of platelet production. Oncogene. 2002;21(21):3359–3367. doi: 10.1038/sj.onc.1205323. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Hirsch-Ernst KI, Kahl GF, Jungermann K. Mimicry in primary rat hepatocyte cultures of the in vivo perivenous induction by phenobarbital of cytochrome P-450 2B1 mRNA: role of epidermal growth factor and perivenous oxygen tension. Mol Pharmacol. 1999;56(1):46–53. doi: 10.1124/mol.56.1.46. [DOI] [PubMed] [Google Scholar]
- Kim I, Han SJ, Kim Y, Ahn Y, Chay KO, Lee SR. Tyr740 and Tyr751 residues of platelet-derived growth factor beta receptor are responsible for the redox regulation of phosphatase and tensin homolog in the cells stimulated with platelet-derived growth factor. Redox Rep. 2011;16(4):181–186. doi: 10.1179/1351000211Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Klotz LO, Schieke SM, Sies H, Holbrook NJ. Peroxynitrite activates the phosphoinositide 3-kinase/Akt pathway in human skin primary fibroblasts. Biochem J. 2000;352(Pt 1):219–225. [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kuehl FA, Jr, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210(4473):978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- LaIuppa JA, Papoutsakis ET, Miller WM. Oxygen tension alters the effects of cytokines on the megakaryocyte, erythrocyte, and granulocyte lineages. Exp Hematol. 1998;26(9):835–843. [PubMed] [Google Scholar]
- Lazarus HM, Cruikshank WW, Narasimhan N, Kagan HM, Center DM. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. 1995;14(9):727–731. doi: 10.1016/s0945-053x(05)80015-0. [DOI] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles MC, Martyre MC. Dual implication of fibrogenic cytokines in the pathogenesis of fibrosis and myeloproliferation in myeloid metaplasia with myelofibrosis. Ann Hematol. 1999;78(10):437–444. doi: 10.1007/s002770050595. [DOI] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles MC, Martyre MC, Samson M. Cellular and molecular mechanisms underlying bone marrow and liver fibrosis: a review. Eur Cytokine Netw. 2008;19(2):69–80. doi: 10.1684/ecn.2008.0127. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li W, Liu G, Chou IN, Kagan HM. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem. 2000;78(4):550–557. [PubMed] [Google Scholar]
- Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31(10):589–595. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Ravid K, Grimsby JL, Rich CB, DiCamillo SJ, Maki JM, Myllyharju J, Kagan HM. Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells. J Biol Chem. 2008;283(35):24103–24117. doi: 10.1074/jbc.M709897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192(1):1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- McCarthy DM. Annotation. Fibrosis of the bone marrow: content and causes. Br J Haematol. 1985;59(1):1–7. doi: 10.1111/j.1365-2141.1985.tb02956.x. [DOI] [PubMed] [Google Scholar]
- McCrann DJ, Eliades A, Makitalo M, Matsuno K, Ravid K. Differential expression of NADPH oxidases in megakaryocytes and their role in polyploidy. Blood. 2009a;114(6):1243–1249. doi: 10.1182/blood-2008-12-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009b;8(6):902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69(16):6685–6693. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, Ikeda Y. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood. 1996;87(2):439–446. [PubMed] [Google Scholar]
- Mortensen M, Watson AS, Simon AK. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy. 2011;7(9):1069–1070. doi: 10.4161/auto.7.9.15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa SS, Miller WM, Papoutsakis ET. Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. Br J Haematol. 2000;111(3):879–889. [PubMed] [Google Scholar]
- Mostafa SS, Papoutsakis ET, Miller WM. Oxygen tension modulates the expression of cytokine receptors, transcription factors, and lineage-specific markers in cultured human megakaryocytes. Exp Hematol. 2001;29(7):873–883. doi: 10.1016/s0301-472x(01)00658-0. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Kimura M, Fujita R, Inoue A, Pan X, Takayama M, Katsuoka F, Aburatani H, Bresnick EH, Yamamoto M. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood. 2010;115(3):677–686. doi: 10.1182/blood-2009-05-223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JM, Diegelmann RF, Cohen IK. Effect of beta-aminopropionitrile and ascorbate on fibroblast migration. Proc Soc Exp Biol Med. 1988;188(3):346–352. doi: 10.3181/00379727-188-42745. [DOI] [PubMed] [Google Scholar]
- O'Brien JJ, Spinelli SL, Tober J, Blumberg N, Francis CW, Taubman MB, Palis J, Seweryniak KE, Gertz JM, Phipps RP. 15-deoxy-delta12,14-PGJ2 enhances platelet production from megakaryocytes. Blood. 2008;112(10):4051–4060. doi: 10.1182/blood-2008-05-158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta I, Lovett M, Rice W, Kaplan DL, Balduini A. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS One. 2009;4(12):e8359. doi: 10.1371/journal.pone.0008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus JM. DNA metabolism and development of organelles in guinea-pig megakaryocytes: a combined ultrastructural, autoradiographic and cytophotometric study. Blood. 1970;35(3):298–311. [PubMed] [Google Scholar]
- Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SFT, Csiszar K, Hendrix MJC, Kirschmann DA. Lysyl Oxidase Regulates Breast Cancer Cell Migration and Adhesion through a Hydrogen Peroxide–Mediated Mechanism. Cancer Research. 2005;65(24):11429–11436. doi: 10.1158/0008-5472.CAN-05-1274. [DOI] [PubMed] [Google Scholar]
- Pennathur-Das R, Levitt L. Augmentation of in vitro human marrow erythropoiesis under physiological oxygen tensions is mediated by monocytes and T lymphocytes. Blood. 1987;69(3):899–907. [PubMed] [Google Scholar]
- Piccoli C, D'Aprile A, Ripoli M, Scrima R, Boffoli D, Tabilio A, Capitanio N. The hypoxia-inducible factor is stabilized in circulating hematopoietic stem cells under normoxic conditions. FEBS Lett. 2007a;581(16):3111–3119. doi: 10.1016/j.febslet.2007.05.077. [DOI] [PubMed] [Google Scholar]
- Piccoli C, D'Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Commun. 2007b;353(4):965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- Piccoli C, Ria R, Scrima R, Cela O, D'Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280(28):26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- Prata C, Maraldi T, Zambonin L, Fiorentini D, Hakim G, Landi L. ROS production and Glut1 activity in two human megakaryocytic cell lines. Biofactors. 2004;20(4):223–233. doi: 10.1002/biof.5520200406. [DOI] [PubMed] [Google Scholar]
- Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH. Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal. 2006;8(9–10):1447–1459. doi: 10.1089/ars.2006.8.1447. [DOI] [PubMed] [Google Scholar]
- Rao GN. Protein tyrosine kinase activity is required for oxidant-induced extracellular signal-regulated protein kinase activation and c-fos and c-jun expression. Cell Signal. 1997;9(2):181–187. doi: 10.1016/s0898-6568(96)00139-8. [DOI] [PubMed] [Google Scholar]
- Raslova H, Baccini V, Loussaief L, Comba B, Larghero J, Debili N, Vainchenker W. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107(6):2303–2310. doi: 10.1182/blood-2005-07-3005. [DOI] [PubMed] [Google Scholar]
- Reilly JT, Barnett D, Dolan G, Forrest P, Eastham J, Smith A. Characterization of an acute micromegakaryocytic leukaemia: evidence for the pathogenesis of myelofibrosis. Br J Haematol. 1993;83(1):58–62. doi: 10.1111/j.1365-2141.1993.tb04631.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Martinez-Gonzalez J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79(1):7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- Rossi F, Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia. 1964;20(1):21–23. doi: 10.1007/BF02146019. [DOI] [PubMed] [Google Scholar]
- Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, Li GL, Dosemeci M, Linet M, Zhang L, Xi L, Wacholder S, Lu W, Meyer KB, Titenko-Holland N, Stewart JT, Yin S, Ross D. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57(14):2839–2842. [PubMed] [Google Scholar]
- Salvemini D, de Nucci G, Vane JR. Superoxide dismutase cooperates with prostacyclin to inhibit platelet aggregation: a comparative study in washed platelets and platelet rich plasma. Thromb Haemost. 1991;65(4):421–424. [PubMed] [Google Scholar]
- Sardina JL, Lopez-Ruano G, Sanchez-Abarca LI, Perez-Simon JA, Gaztelumendi A, Trigueros C, Llanillo M, Sanchez-Yague J, Hernandez-Hernandez A. p22phox-dependent NADPH oxidase activity is required for megakaryocytic differentiation. Cell Death Differ. 2010;17(12):1842–1854. doi: 10.1038/cdd.2010.67. [DOI] [PubMed] [Google Scholar]
- Sardina JL, Lopez-Ruano G, Sanchez-Sanchez B, Llanillo M, Hernandez-Hernandez A. Reactive oxygen species: Are they important for haematopoiesis? Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93(9):2928–2935. [PubMed] [Google Scholar]
- Schattner M, Pozner RG, Gorostizaga AB, Lazzari MA. Effect of thrombopoietin and granulocyte colony-stimulating factor on platelets and polymorphonuclear leukocytes. Thromb Res. 2000;99(2):147–154. doi: 10.1016/s0049-3848(00)00238-3. [DOI] [PubMed] [Google Scholar]
- Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. Identification of hydrogen peroxide as the relevant messenger in the activation pathway of transcription factor NF-kappaB. Adv Exp Med Biol. 1996;387:63–68. doi: 10.1007/978-1-4757-9480-9_9. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata KI, Kawashima S, Tawa R, Imajoh-Ohmi S, Sakurai H, Yokoyama M. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res. 2001;103(5):399–409. doi: 10.1016/s0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Severin S, Ghevaert C, Mazharian A. The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J Thromb Haemost. 2010;8(1):17–26. doi: 10.1111/j.1538-7836.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- Shao L, Li H, Pazhanisamy SK, Meng A, Wang Y, Zhou D. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol. 2011;94(1):24–32. doi: 10.1007/s12185-011-0872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81(5):695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- Slater DN, Trowbridge EA, Martin JF. The megakaryocyte in thrombocytopenia: a microscopic study which supports the theory that platelets are produced in the pulmonary circulation. Thromb Res. 1983;31(1):163–176. doi: 10.1016/0049-3848(83)90017-8. [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Su RJ, Li K, Yang M, Zhang XB, Tsang KS, Fok TF, Li CK, Yuen PM. Platelet-derived growth factor enhances ex vivo expansion of megakaryocytic progenitors from human cord blood. Bone Marrow Transplant. 2001;27(10):1075–1080. doi: 10.1038/sj.bmt.1703042. [DOI] [PubMed] [Google Scholar]
- Su RJ, Li K, Zhang XB, Pan Yuen PM, Li CK, James AE, Liu J, Fok TF. Platelet-derived growth factor enhances expansion of umbilical cord blood CD34+ cells in contact with hematopoietic stroma. Stem Cells Dev. 2005;14(2):223–230. doi: 10.1089/scd.2005.14.223. [DOI] [PubMed] [Google Scholar]
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sungaran R, Chisholm OT, Markovic B, Khachigian LM, Tanaka Y, Chong BH. The role of platelet alpha-granular proteins in the regulation of thrombopoietin messenger RNA expression in human bone marrow stromal cells. Blood. 2000;95(10):3094–3101. [PubMed] [Google Scholar]
- Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89(1):101–107. [PubMed] [Google Scholar]
- Tang SS, Trackman PC, Kagan HM. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. Journal of Biological Chemistry. 1983;258(7):4331–4338. [PubMed] [Google Scholar]
- Tauro S, Hepburn MD, Bowen DT, Pippard MJ. Assessment of stromal function, and its potential contribution to deregulation of hematopoiesis in the myelodysplastic syndromes. Haematologica. 2001;86(10):1038–1045. [PubMed] [Google Scholar]
- Terui T, Niitsu Y, Mahara K, Fujisaki Y, Urushizaki Y, Mogi Y, Kohgo Y, Watanabe N, Ogura M, Saito H. The production of transforming growth factor-beta in acute megakaryoblastic leukemia and its possible implications in myelofibrosis. Blood. 1990;75(7):1540–1548. [PubMed] [Google Scholar]
- Tomer A. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood. 2004;104(9):2722–2727. doi: 10.1182/blood-2004-02-0769. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121(5):667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Trackman PC, Bedell-Hogan D, Tang J, Kagan HM. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J Biol Chem. 1992;267(12):8666–8671. [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, Migliaccio G, Migliaccio AR. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice) Blood. 2002;100(4):1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- Vener C, Novembrino C, Catena FB, Fracchiolla NS, Gianelli U, Savi F, Radaelli F, Fermo E, Cortelezzi A, Lonati S, Menegatti M, Deliliers GL. Oxidative stress is increased in primary and post-polycythemia vera myelofibrosis. Exp Hematol. 2010;38(11):1058–1065. doi: 10.1016/j.exphem.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275(19):14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Kamen D, Lees E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86(10):3783–3788. [PubMed] [Google Scholar]
- Watson AS, Mortensen M, Simon AK. Autophagy in the pathogenesis of myelodysplastic syndrome and acute myeloid leukemia. Cell Cycle. 2011;10(11):1719–1725. doi: 10.4161/cc.10.11.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chesterman CN, Chong BH. Recombinant PDGF enhances megakaryocytopoiesis in vitro. Br J Haematol. 1995;91(2):285–289. doi: 10.1111/j.1365-2141.1995.tb05291.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang YZ, Kagan E, Bonner JC. Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J Biol Chem. 2000;275(29):22479–22486. doi: 10.1074/jbc.M910425199. [DOI] [PubMed] [Google Scholar]
- Zimmet JM, Ladd D, Jackson CW, Stenberg PE, Ravid K. A role for cyclin D3 in the endomitotic cell cycle. Mol Cell Biol. 1997;17(12):7248–7259. doi: 10.1128/mcb.17.12.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157(1):69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]