Abstract
Objective
To describe the relationship of change in retinal vessel diameters to the subsequent 6-year incidence and progression of diabetic retinopathy (DR) and incidence of proliferative DR (PDR) and macular edema (ME) in persons with diabetes.
Design
1098 persons with diabetes participated in examinations in 1980-1982, 1984-1986, and 1990-1992, who had DR graded from fundus photos and had computer-assisted measurements of the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE).
Results
Over the first 4-year period, the mean change in CRAE and CRVE was −0.37 μm and 2.54 μm, respectively. The 6-year incidence and progression of DR and incidence of PDR and ME from 1984-1986 to 1990-1992 were 56%, 39%, 15%, and 11%, respectively. In multivariate analyses, while controlling for duration, diabetes type, and other factors, an increase of 10 μm in CRVE from 1980-1982 to 1984-1986 was associated (odds ratio; 95% confidence interval) with increases in the 6-year incidence of DR (1.26; 1.10-1.43), progression of DR (1.21; 1.12-1.30), incidence of PDR (1.19; 1.07-1.32) and incidence of ME (1.16; 1.03-1.31). There were no interactions of these associations by diabetes type (data not shown). Change in CRAE was unrelated to the incidence or progression of DR (data not shown).
Conclusions
Independent of DR severity level, glycemic control, and other factors, widening of the retinal venular but not arteriolar diameter was associated with subsequent incidence and progression of DR. CRVE may provide additional information regarding the risk of incidence and progression of DR beyond traditional risk factors.
INTRODUCTION
Persons with diabetes are at risk of developing diabetic retinopathy (DR) and having it progress to proliferative DR (PDR) and macular edema (ME) with visual loss.1 While traditional risk factors (e.g., glycosylated hemoglobin [HbA1c], blood pressure, duration of diabetes) have been shown to be statistically significantly associated with the incidence and progression of DR, they explain only a limited amount of the risk of developing these complications.2 Other indicators of increased risk have been suggested, including retinal vessel diameters.3-5
Wider retinal venules had been shown in some studies to provide additional information, independent of retinopathy severity, hyperglycemia, hypertension, and other factors, regarding the risk of progression but not incidence of DR.3,4,6,7 The relationship of retinal arteriolar diameters to the incidence and progression of DR has been less consistent.3,4,6-8 These observations were usually based on a single measurement of retinal vessel diameter at baseline. In this report, we examine the relation of change in retinal vessel diameter over a four-year interval to the incidence and progression of DR over the next 6 years in people with diabetes participating in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR).
METHODS
Study Population
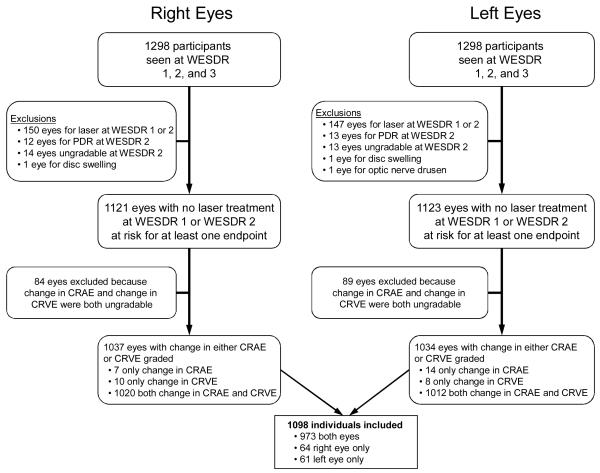
The population, which has been described in previous reports, consisted of a probability sample selected from 10,135 diabetic patients who received primary care in an 11-county area in southern Wisconsin from 1979 to 1980.9-16 This sample was composed of a “younger-onset” group with type 1 diabetes (all patients diagnosed with diabetes before 30 years of age who took insulin, n=1210) and an “older-onset” group with type 2 diabetes (a sample of persons diagnosed with diabetes at or after 30 years of age who were treated with diet, oral hypoglycemic agents and/or insulin, n=1780). Of those selected, 2366 individuals (996 with type 1 diabetes and 1370 with type 2 diabetes) participated in the baseline exam. Data used in the analyses included all participants from both groups (n=1098) who participated in the baseline (1980 to 1982),10,11 4-year follow-up (1984 to 1986),12,13 and 10-year follow-up (1990 to 1992),14 had gradable fundus photographs in at least one eye for DR and for measurement of retinal vessel diameter, and did not meet one of the criteria for exclusion involving both eyes (Figure 1). CRAE and CRVE were considered ungradable if one of the largest 6 arterioles or venules, respectively, were not gradable. Reasons for nonparticipation and comparisons between participants and nonparticipants at baseline and all the follow-up examinations have been presented elsewhere.10-14 The principal reason for nonparticipation was death.
Figure 1.
Participation and reasons for exclusion in the study. CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; PDR, proliferative diabetic retinopathy; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Procedures
The baseline and follow-up examinations were performed in a mobile examination van in or near the cities where the participants resided. All examinations followed a similar protocol that was approved by the institutional human subjects committee of the University of Wisconsin and that conformed to the tenets of the Declaration of Helsinki. The pertinent parts of the ocular and physical examinations included measuring blood pressure,17 measuring height and weight, measuring refractive error using Early Treatment Diabetic Retinopathy (ETDRS) protocol, dilating the pupil, determining the presence of cataract using standard lens photographs, taking 30° stereoscopic color fundus photographs of seven standard fields,18,19 performing a semi-quantitative determination of protein levels in the urine using Labstix (Ames, Elkhart, IN), and determining HbA1c levels from a capillary blood sample.20 A structured interview was conducted by the examiners including questions on smoking and specific medications for control of hyperglycemia and blood pressure. If there was any question about medication usage or medical history, it was verified by a physician’s report.
Grading Protocols
Grading protocols for diabetic retinopathy have been described in detail elsewhere14,21 and are modifications of the ETDRS adaptation of the modified Airlie House classification of diabetic retinopathy.22,23 Inter-observer and intra-observer variations and the validity of the systems have been evaluated, and the results have been presented elsewhere.14,21-23
Definitions
For each eye, the maximum grade in any of the seven standard photographic fields was determined for each of the lesions and used in defining the retinopathy levels, varying from level 10 (no retinopathy) to level 60 or greater (proliferative retinopathy); definitions have appeared elsewhere.22,23 The retinopathy level for a participant was derived by concatenating the levels for the two eyes, giving the eye with the higher level greater weight. This scheme provided a 15-step DR severity scale.
The incidence of any retinopathy was estimated from all eyes that had no retinopathy at the first follow-up examination (severity level 10) and were examined again at the following examination. Progression to PDR was estimated from all eyes free of this complication at the second examination. For eyes with no or only nonproliferative retinopathy, progression was defined as the instance of an increase in the severity of retinopathy by two steps or more along the 15-step scale from the second to the third examination.
Macular edema was defined as thickening of the retina with or without partial loss of transparency within 1 disc diameter from the center of the macula24 or the presence of focal photocoagulation scars in the macular area associated with a history of development of ME as documented by stereoscopic fundus photographs. The incidence of ME was estimated from data for all eyes that had no ME and had not been treated previously with photocoagulation at the second examination and were examined again at the third examination.
Diameters of retinal vessels were measured after converting the photographs of Field 1 to digital images. All retinal arterioles and venules were measured in the area between one-half and one disc diameter from the optic disc margin using a computer-assisted program. Computer-assisted measurements of individual arterioles and venules were each combined according to formulas developed by Parr and Spears25 and modified by Hubbard et al.26 and Knudtson et al.27 as average diameters of the six largest retinal arterioles (central retinal arteriolar equivalent [CRAE]) and venules (central retinal venular equivalent [CRVE]) in that eye. Intraclass correlation coefficients were extremely high (>0.90) for both inter- and intra-grader comparisons for both arteriolar and venular measurements (data not shown). Eyes treated with photocoagulation, or that had retinal vein or arterial occlusion or other nondiabetic ocular pathology affecting the retinal blood vessels were excluded (n=200).
Definition of Related Factors
Age was defined as the age at the time of the baseline examination in 1980–82. Age at diagnosis of diabetes was defined as the age at the time the diagnosis was first recorded by a physician on the patient’s chart or in a hospital record. The duration of diabetes was defined as the period between the age at diagnosis and the age at the baseline examination.
The means of both systolic and diastolic blood pressures were the averages of the last two of three measurements obtained according to the protocol of the Hypertension Detection and Follow-Up Program.17 Hypertension was defined as a mean systolic blood pressure ≥140 mmHg and/or a mean diastolic blood pressure ≥90 mmHg and/or a history of taking antihypertensive medication at the time of examination. Mean arterial blood pressure (MABP) was defined as (systolic blood pressure + [2 × diastolic blood pressure]) ÷ 3. Body mass index was defined as weight in kg divided by the square of height in m. Proteinuria was defined as urine protein concentration of 0.30 g/L or greater.
Smokers were identified as persons answering yes to having smoked ≥100 cigarettes in their lifetime, and further categorized as current smokers if they had not stopped smoking at the current examination. Cataract was classified as none/questionable, < photographic standard 1, ≥ photographic standard 1, or cataract surgery. Refraction was performed using a modification of the ETDRS protocol and modeled in categories as moderately to highly myopic (<−3 diopters), mildly myopic (−3 to <−1 diopters), emmetropic (−1 to 1 diopters), mildly hyperopic (>1 to 3 diopters) and hyperopic (>3 diopters). Nephropathy was defined as history of kidney transplant, proteinuria, or dialysis.
Statistical Methods
The change in the CRAE and CRVE were computed between the baseline 1980–82 examination and 1984–86 4-year follow-up examination while the 6-year incidence of DR, PDR, and ME, and progression of DR were computed between the 1984–86 and 1990–92 examinations. Generalized estimating equation models with independent correlation structure were used to analyze the relationship of changes in CRAE and CRVE to each diabetic outcome using eye-specific data.28 Univariate analyses examined a 10 μm change in CRAE and CRVE with the 6-year incidence of DR outcomes. Multivariable analyses additionally controlled for sex, diabetes type and duration, and changes in retinal photograph focus, cataract status, refraction, HbA1c, MABP, diabetic nephropathy status, smoking status, BMI, and retinopathy severity level (except in analyses for incident DR).
Change in area under the receiver operator characteristic curve (AUC) was used to measure improvement in prediction when each change in CRVE was added to the model based on traditional DR risk factors using the method described by DeLong et al. using right eyes only.29 Results were similar for left eyes (data not shown). Integrated discrimination improvement (IDI) was also computed following the method described by Pencina et al.30
RESULTS
Characteristics of the cohort at baseline and follow-up examinations are presented in Table 1. Persons were excluded if they did not participate in the 1984–86 and/or 1990–92 examination (n=1077), had photocoagulation for PDR or ME in both eyes at the 1980–82 or 1984–86 examinations (n=121), did not have gradable retinal vessels in at least one eye (n=58), were not at risk for any of the diabetic outcomes in either eye (n=20), or had nondiabetic retinal vascular conditions in both eyes, e.g., retinal vein or arterial occlusion (n=1). Persons included in the study were younger (40.9 vs. 59.5 years of age, p<0.001), had shorter duration of diabetes (10.7 vs. 15.1 years, p<0.001), lower systolic (128.6 vs. 145.8 mmHg, p<0.001) and diastolic blood pressure (78.5 vs. 79.4 mmHg, p=0.007), and were less likely to have hypertension (39% vs. 53%, p=0.01), gross proteinuria (9% vs. 34%, p<0.001), and PDR (3% vs. 69%, p<0.001) compared to nonparticipants.
Table 1.
Baseline Characteristics of Individuals Included and Excluded in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (1980-1982).
| Included (n=1098) | Excluded (n=1268) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Risk Factor | N | Mean or % |
SD | N | Mean or % |
SD | P-valuea |
|
|
|||||||
| Age, years | 1098 | 40.91 | 20.25 | 1268 | 59.51 | 19.92 | <0.001 |
| Sex | |||||||
| Women | 589 | 53.64 | 629 | 49.61 | <0.001 | ||
| Men | 509 | 46.36 | 639 | 50.39 | |||
| Body mass index, kg/m2 | 1098 | 25.98 | 5.89 | 1258 | 27.05 | 5.46 | <0.001 |
| Diabetes duration, years | 1098 | 10.71 | 8.21 | 1268 | 15.06 | 9.84 | <0.001 |
| Glycosylated hemoglobin A1c, % | 1036 | 9.43 | 1.95 | 1184 | 9.53 | 2.00 | <0.001 |
| Systolic blood pressure, mmHg | 1094 | 128.62 | 20.20 | 1259 | 145.84 | 26.67 | <0.001 |
| Diastolic blood pressure, mmHg | 1092 | 78.47 | 10.70 | 1253 | 79.37 | 12.80 | 0.007 |
| Hypertensionb | |||||||
| No | 667 | 61.02 | 385 | 30.56 | <0.001 | ||
| Yes | 426 | 38.98 | 875 | 69.44 | |||
| Smoking status | |||||||
| Never | 669 | 60.93 | 691 | 54.50 | 0.07 | ||
| Past | 227 | 20.67 | 339 | 26.74 | |||
| Current | 202 | 18.40 | 238 | 18.77 | |||
| Cataract status (right eye) | |||||||
| No/questionable | 661 | 62.59 | 304 | 26.34 | <0.001 | ||
| <Standard 1 | 368 | 34.85 | 647 | 56.07 | |||
| ≥Standard 1 | 27 | 2.56 | 203 | 17.59 | |||
| Refraction, diopters (right eye) | 1060 | −0.49 | 2.20 | 1026 | 0.18 | 2.26 | 0.10 |
| Diabetic retinopathy level | |||||||
| None | 495 | 45.08 | 372 | 29.45 | <0.001 | ||
| Mild | 469 | 42.71 | 435 | 34.44 | |||
| Moderate | 99 | 9.02 | 149 | 11.80 | |||
| Proliferative | 35 | 3.19 | 307 | 24.31 | |||
| Macular edema present (right eye) | |||||||
| No | 1038 | 97.46 | 882 | 86.81 | <0.001 | ||
| Yes | 27 | 2.54 | 134 | 13.19 | |||
| Poor focus (right eye) | |||||||
| No | 1034 | 94.69 | 973 | 81.97 | <0.001 | ||
| Yes | 58 | 5.31 | 214 | 18.03 | |||
| Nephropathy present | |||||||
| No | 980 | 91.25 | 864 | 73.47 | <0.001 | ||
| Yes | 94 | 8.75 | 312 | 26.53 | |||
| CRAE, μm (right eye) | 1098 | 162.98 | 15.13 | 1113 | 154.08 | 17.92 | <0.001 |
| CRVE, μm (right eye) | 1097 | 243.94 | 23.81 | 1122 | 237.37 | 29.23 | 0.47 |
CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent.
Adjusted for age.
Defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or use of antihypertensive medication.
Between 1984–86 and 1990–92, the 6-year incidence of DR was 56%, progression of DR was 39%, incidence of PDR was 15%, and the 6-year incidence of ME was 11%. The mean change in CRAE between 1980–82 and 1990–94 was −0.4 μm (standard deviation [SD] 11.3 μm, range −73.0 to 81.4 μm) and the mean change in CRVE was 2.5 μm (SD 17.3 μm, range −108.0 to 78.5 μm). Change in CRAE and CRVE between the right and left eyes was correlated (Figure 2).
Figure 2.
Correlation of change between the right and left eyes of participants from baseline to the 4-year follow-up in the Wisconsin Epidemiologic Study of Diabetic Retinopathy in A. central retinal venular equivalent (CRVE) and B. central retinal arteriolar equivalent (CRAE).
The univariate relationships of 4-year change in CRAE and CRVE to the 6-year cumulative incidence of retinal outcomes are presented in Table 2. Increasing CRAE was not associated with any of the DR outcomes, while increasing CRVE was associated with increases in all DR outcomes. While controlling for sex, diabetes type and duration, and change in cataract status, refraction, image focus, HbA1c, MABP, smoking status, diabetic nephropathy, BMI, and DR level (only in the models of progression of DR and incidence of PDR and ME outcomes), the associations of CRVE but not CRAE remained statistically significant (Table 3). These relationships remained when CRAE was added to the CRVE model (data not shown) but an inverse relation of change in CRAE was found (odds ratio per 10 μm 0.77; 95% confidence interval 0.65–0.91, p=0.003) for the 6-year incidence of PDR when CRVE was added to the CRAE model. There were no other significant interactions of type or duration of diabetes or MABP with change in CRAE or CRVE. When analyses were rerun by type of diabetes, there were no statistically significant differences in any of the relationships of CRAE or CRVE to any of the DR endpoints (data not shown).
Table 2.
Univariate Relationship of Change in Central Retinal Arteriolar and Venular Equivalents to Incidence and Progression of Diabetic Retinopathy and Incidence of Macular Edema and Proliferative Diabetic Retinopathy.
| Vessel Measurement | N at Risk | N of Events | Odds Ratio | 95% CI | P value |
|---|---|---|---|---|---|
|
|
|||||
| Change in CRAE (per 10 μm) | |||||
| Incidence of DR | 663 | 370 | 1.13 | 0.97, 1.32 | 0.13 |
| Progression of DR | 1968 | 761 | 1.07 | 0.97, 1.18 | 0.15 |
| Incidence of ME | 1885 | 199 | 1.10 | 0.96, 1.26 | 0.16 |
| Incidence of PDR | 1968 | 297 | 0.89 | 0.78, 1.03 | 0.11 |
| Change in CRVE (per 10 μm) | |||||
| Incidence of DR | 665 | 373 | 1.14 | 1.01, 1.28 | 0.03 |
| Progression of DR | 1963 | 761 | 1.13 | 1.05, 1.22 | <0.001 |
| Incidence of ME | 1884 | 197 | 1.20 | 1.09, 1.32 | <0.001 |
| Incidence of PDR | 1963 | 296 | 1.17 | 1.05, 1.31 | 0.004 |
CI, confidence interval; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DR, diabetic retinopathy; ME, macular edema; PDR, proliferative diabetic retinopathy.
Table 3.
Multivariatea Relationships of Change in Central Retinal Arteriolar and Venular Equivalents to Incidence and Progression of Diabetic Retinopathy and Incidence of Macular Edema and Proliferative Diabetic Retinopathy.
| Vessel Measurement | Odds Ratio | 95% CI | P value |
|---|---|---|---|
|
|
|||
| Change in CRAE (per 10 μm) | |||
| Incidence of DR | 1.17 | 0.97, 1.40 | 0.10 |
| Progression of DR | 1.11 | 0.99, 1.24 | 0.08 |
| Incidence of ME | 1.05 | 0.89, 1.23 | 0.56 |
| Incidence of PDR | 0.90 | 0.78, 1.03 | 0.13 |
| Change in CRVE (per 10 μm) | |||
| Incidence of DR | 1.26 | 1.10, 1.43 | <0.001 |
| Progression of DR | 1.21 | 1.12, 1.30 | <0.001 |
| Incidence of ME | 1.16 | 1.03, 1.31 | 0.004 |
| Incidence of PDR | 1.19 | 1.07, 1.32 | <0.001 |
CI, confidence interval; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DR, diabetic retinopathy; ME, macular edema; PDR, proliferative diabetic retinopathy.
Controlling for sex, diabetes type and duration and change in cataract status, refraction, focus, glycosylated hemoglobin, mean arterial blood pressure, smoking status, diabetic nephropathy, body mass index, and DR level in the models of progression of DR and incidence of PDR and ME outcomes
The highest increase and highest incremental gain in the AUC was for the inclusion of change in CRVE in the model including risk factors for incidence of DR (Table 4), which was not statistically significant (p=0.25). However, analysis using the IDI method showed that adding CRVE significantly increased the predictive ability of the model.
Table 4.
Change in Area Under the Curve for Traditional Risk Factor Model Including and Excluding Change in the Central Retinal Venular Equivalent.
| Outcome | AUC (95% CI) Traditional Risk Factor Model Only |
AUC (95% CI) Traditional Model + Change in CRVE |
% Change in AUC |
P valuea | IDI | P valueb |
|---|---|---|---|---|---|---|
|
|
||||||
| Incidence of DR | 0.6879 (0.6241, 0.7516) | 0.7067 (0.6450, 0.7685) | 2.70 | 0.25 | 0.0272 | 0.007 |
| Progression of DR | 0.6547 (0.6167, 0.6926) | 0.6698 (0.6323, 0.7072) | 2.30 | 0.12 | 0.0173 | <0.001 |
| Incidence of ME | 0.6993 (0.6382, 0.7604) | 0.7032 (0.6429, 0.7635) | 0.50 | 0.60 | 0.0060 | 0.04 |
| Incidence of PDR | 0.7029 (0.6506, 0.7552) | 0.7123 (0.6608, 0.7638) | 1.30 | 0.24 | 0.0097 | 0.03 |
AUC, area under the receiving operating characteristic curve; CI, confidence interval; CRVE, central retinal venular equivalent; DR, diabetic retinopathy; IDI, integrated discrimination improvement; ME, macular edema; PDR, proliferative diabetic retinopathy.
For difference in AUCs.
For IDI.
COMMENT
The data reported herein provide unique long-term population-based information regarding the relation of the change in CRAE and CRVE measurements to the subsequent incidence and progression of DR over 6 years of follow-up in people with diabetes. We found that change in CRVE but not CRAE was statistically significantly associated with the subsequent incidence and progression of DR, independent of other risk factors.
Our finding that increasing CRVE is associated with subsequent higher risk of incident DR may be due to the strong association of wider CRVE with endothelial dysfunction, inflammatory changes, and hyperglycemia, all of which are factors involved in the pathogenesis of DR.31-34 Our findings are consistent with the results from a small study that examined change in retinal vessel diameter with incidence of DR.35 In that study involving 45 children with type 1 diabetes, eyes with >10 μm retinal venular widening during the follow-up period were more likely to develop other signs of retinopathy more often than patients with less or no change in the venular diameter. All the other epidemiological studies that have examined these relationships were based on one measurement of retinal vessel diameter prior to the incidence or progression of DR.3-8 Roy et al. showed a direct association of CRVE measured once at baseline with the 6-year incidence of DR in persons with type 1 diabetes, while in the WESDR cohort we did not find an association of a single measurement of CRVE at baseline with the 4-year incidence of DR.3,4,7 Others have suggested that widening of retinal arteriolar diameters prior to the onset of retinopathy would increase the risk of incident retinopathy due to a breakdown in auto-regulation.36 While Cheung et al. found a direct relation of CRAE with incidence of DR in persons with type 2 diabetes,8 we found no association of change (either widening or narrowing) in the arteriolar diameter with the subsequent incidence of DR.
Our findings suggest that change in retinal venular diameter measurements in those without retinopathy may have prognostic value prior to the development of DR, independent of type or duration of diabetes, glycemic control, blood pressure level, and other traditional risk factors. Although the increase in AUC of 2.7% for inclusion of change in CRVE in the model was small and did not reach statistical significance, it compares favorably with other potential predictive factors used for other endpoints, e.g., C-reactive protein and serum HDL cholesterol, when added to the Framingham risk score for coronary heart disease in the ARIC study.30,37,38 It was a significant predictor of incidence and progression using an alternative approach, the IDI method, which suggested that adding information from change in CRVE to traditional risk variables, e.g., HbA1c, does substantially improve the discrimination between those who do and do not develop DR during a 6-year period. There is a need to further study whether measuring change in retinal venular diameter is a cost-effective approach to improving prediction of risk resulting in change in management (e.g., how often a patient without retinopathy should be seen) and whether such changes ultimately result in further reduction of loss of vision due to these changes.5,39
The association in the WESDR of an increase in the venular diameter with progression of DR and incidence of PDR and ME, independent of retinopathy severity, was not unexpected and is consistent with data from earlier studies.3,4,35,40-46 Increase in venular diameter in eyes with retinopathy is thought to result from retinal hypoxia47 and from lactate accumulation resulting from hyperglycemia.48 Retinal venous caliber abnormalities were important predictors of visual loss due to progression of retinopathy in the Diabetic Retinopathy Study.49 Developers of the classification system at the Airlie House meeting in 1969 were aware of retinal venular widening as a prognostic sign but chose to ignore it in scale “because it was considered too nonspecific and difficult to evaluate”. Retinal venous beading, a more qualitative measure and a sign of irregular venular dilation, was used instead to define severe nonproliferative retinopathy severity levels 47 and 53 in the ETDRS severity scale.24,50 It was significantly associated with progression of proliferative disease in the Diabetic Retinopathy Study and the ETDRS.23,24
In the WESDR, the association of change in CRVE to the 6-year progression of DR remained independent of previous change in DR, MABP, diabetic nephropathy and glycemic control, suggesting that larger venous caliber provides information regarding risk independent of the current ETDRS system used to classify retinopathy severity level. While the increases in AUC of 2.3% for progression of DR and 1.3% for incidence of PDR were small, the IDI values suggested some value in including these measures for better prognostic assessment beyond measuring only DR severity and other traditional risk factors.
Our study has many strengths. The population is large and involves both types of diabetes, the distribution of severity of retinopathy based on objective recording of DR and ME using stereoscopic fundus photographs of seven standard fields is broad, and there was a low refusal rate. In addition, standardized protocols of measurement, including computer-assisted measurement of retinal vessel caliber, were consistent over time. However, caution must be exercised in interpreting the findings in the present study. Relationships may have been attenuated by selective survival. Retinopathy severity and retinal arteriolar narrowing and venular widening have been shown to be related to mortality.51 This would reduce the strength of the associations between narrowing of the CRAE and progression of disease and incident PDR and ME.
In summary, measurement of change in CRVE may provide additional information regarding incidence and progression of DR and risk of development of PDR and ME than DR severity by itself in persons with type 1 and type 2 diabetes. The association of change in CRVE with incidence of DR raises the question of whether measurement of CRVE will provide an even earlier clinically meaningful stage of DR before the onset of microaneurysms and blot hemorrhages.
ACKNOWLEDGMENTS
Funding/Support: Funding was provided by research grant EY016379 (Drs R. Klein and B.E.K. Klein) from the National Institutes of Health, Bethesda, MD, for the entire study including collection and analyses of data. Additional funding for data analyses was provided by Senior Scientific Investigator Awards from Research to Prevent Blindness (Drs R. Klein and B.E.K. Klein), New York, NY. We are grateful to the participants and the 452 Wisconsin physicians and their staffs who have participated in and supported this study. Dr R. Klein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Financial Disclosure: None reported.
Publisher's Disclaimer: Disclaimer: The content of this report is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
REFERENCES
- 1.Klein R, Klein BEK. Vision disorders in diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. 2nd Edition. National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; Bethesda, MD: 1995. pp. 293–338. NIH Publication No. 95-1468. [Google Scholar]
- 2.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004;164(17):1917–1924. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122(1):76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114(10):1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY. Retinal vessel diameter as a clinical predictor of diabetic retinopathy progression: time to take out the measuring tape. Arch Ophthalmol. 2011;129(1):95–96. doi: 10.1001/archophthalmol.2010.347. [DOI] [PubMed] [Google Scholar]
- 6.Alibrahim E, Donaghue KC, Rogers S, et al. Retinal vascular caliber and risk of retinopathy in young patients with type 1 diabetes. Ophthalmology. 2006;113(9):1499–1503. doi: 10.1016/j.ophtha.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2011;129(1):8–15. doi: 10.1001/archophthalmol.2010.340. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31(9):1842–1846. doi: 10.2337/dc08-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, DeMets DL, Kaufman I, Voss PS. Prevalence of diabetes mellitus in southern Wisconsin. Am J Epidemiol. 1984;119(1):54–61. doi: 10.1093/oxfordjournals.aje.a113725. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244–249. doi: 10.1001/archopht.1989.01070010250031. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112(9):1217–1228. doi: 10.1001/archopht.1994.01090210105023. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–1868. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Hypertension Detection and Follow-up Program Cooperative Group The Hypertension Detection and Follow-up Program. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 18.ETDRS Research Group . Early Treatment Diabetic Retinopathy Study (ETDRS) Manual of Operations. National Technical Information Service; Springfield, VA: 1985. Chapter 12: Procedures for Completing Eye Examinations; pp. 1–74. NTIS Accession No. PB85-223006/AS. [Google Scholar]
- 19.ETDRS Research Group . Early Treatment Diabetic Retinopathy Study (ETDRS) Manual of Operations. National Technical Information Service; Springfield, VA: 1985. Chapter 18: Classification of diabetic retinopathy from stereo color fundus photographs; pp. 1–54. NTIS Accession No. PB85-223006/AS. [Google Scholar]
- 20.Moss SE, Klein R, Klein BE, Spennetta TL, Shrago ES. Methodologic considerations in measuring glycosylated hemoglobin in epidemiologic studies. J Clin Epidemiol. 1988;41(7):645–649. doi: 10.1016/0895-4356(88)90116-3. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93(9):1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 22.ETDRS Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 23.ETDRS Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5 Suppl):823–833. [PubMed] [Google Scholar]
- 24.ETDRS Research Group Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 25.Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77(4):472–477. doi: 10.1016/0002-9394(74)90457-7. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 27.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 28.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 29.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 31.Tso MO, Abrams GW, Jampol LM. Hypertensive retinopathy, choroidopathy, and optic neuropathy: a clinical and pathophysiological approach to classification. In: Singerman LJ, Jampol LM, editors. Retinal and Choroidal Manifestations of Systemic Disease. Williams and Wilkins; Baltimore, MD: 1991. pp. 79–127. [Google Scholar]
- 32.Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 33.Garner A, Ashton N, Tripathi R, Kohner EM, Bulpitt CJ, Dollery CT. Pathogenesis of hypertensive retinopathy. An experimental study in the monkey. Br J Ophthalmol. 1975;59(1):3–44. doi: 10.1136/bjo.59.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150(3):263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 35.Falck A, Laatikainen L. Retinal vasodilation and hyperglycaemia in diabetic children and adolescents. Acta Ophthalmol Scand. 1995;73(2):119–124. doi: 10.1111/j.1600-0420.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 36.Brinchmann-Hansen O, Heier H, Myhre K. Fundus photography of width and intensity profiles of the blood column and the light reflex in retinal vessels. Acta Ophthalmol. 1986;64(S179):9–19. [Google Scholar]
- 37.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56(9):880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 38.Folsom AR, Chambless LE, Ballantyne CM, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med. 2006;166(13):1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 39.McGeechan K, Macaskill P, Irwig L, Liew G, Wong TY. Assessing new biomarkers and predictive models for use in clinical practice: a clinician’s guide. Arch Intern Med. 2008;168(21):2304–2310. doi: 10.1001/archinte.168.21.2304. [DOI] [PubMed] [Google Scholar]
- 40.Oakley NW, Joplin GF, Kohner EM, Fraser TR. Practical experience with a method for grading diabetic retinopathy. In: Goldberg MF, Fine SL, editors. Symposium on the Treatment of Diabetic Retinopathy; Washington, DC: US Department of Health, Education and Welfare; 1968. pp. 3–6. Public Health Service publication 1890. [Google Scholar]
- 41.Skovborg F, Nielsen AV, Lauritzen E, Hartkopp O. Diameters of the retinal vessels in diabetic and normal subjects. Diabetes. 1969;18(5):292–298. doi: 10.2337/diab.18.5.292. [DOI] [PubMed] [Google Scholar]
- 42.Wallace J. Vessel measurements in diabetic fundi. Proc R Soc Med. 1970;63(8):788–791. doi: 10.1177/003591577006300822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefansson E, Landers MB, III, Wolbarsht ML. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg. 1983;14(3):209–226. [PubMed] [Google Scholar]
- 44.Grunwald JE, Riva CE, Sinclair SH, Brucker AJ, Petrig BL. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol. 1986;104(7):991–996. doi: 10.1001/archopht.1986.01050190049038. [DOI] [PubMed] [Google Scholar]
- 45.Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. BMJ. 1992;305(6855):678–683. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen HW. Diabetic retinopathy. An ophthalmoscopic study with a discussion of the morphologic changes and the pathogenetic factors in this disease. Acta Ophthalmol Suppl. 1960;(Suppl 60):1–89. [PubMed] [Google Scholar]
- 47.Meehan RT, Taylor GR, Rock P, Mader TH, Hunter N, Cymerman A. An automated method of quantifying retinal vascular responses during exposure to novel environmental conditions. Ophthalmology. 1990;97(7):875–881. doi: 10.1016/s0161-6420(90)32500-9. [DOI] [PubMed] [Google Scholar]
- 48.Keen H, Chlouverakis C. Metabolic factors in diabetic retinopathy. In: Graymore CN, editor. Biochemistry of the Retina. Academic Press; New York, NY: 1965. pp. 123–131. [Google Scholar]
- 49.Rand LI, Prud’homme GJ, Ederer F, Canner PL. Factors influencing the development of visual loss in advanced diabetic retinopathy. Diabetic Retinopathy Study (DRS) Report No. 10. Invest Ophthalmol Vis Sci. 1985;26(7):983–991. [PubMed] [Google Scholar]
- 50.Davis MD, Norton EWD, Myers FL. The Airlie Classification of Diabetic Retinopathy. In: Goldberg MF, Fine SL, editors. Symposium on the Treatment of Diabetic Retinopathy; Washington, DC: US Department of Health, Education and Welfare; 1968. pp. 7–22. Public Health Service publication 1890. [Google Scholar]
- 51.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117(11):1487–1495. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]