Reduced rates of consultations for otitis media after introduction of pneumococcal conjugate vaccines (PCVs) have been overinterpreted. This systematic review suggests that PCV is only somewhat modestly effective against all-cause otitis media.
Abstract
Acute otitis media (AOM) is a leading cause of visits to physicians and of antibiotic prescriptions for young children. We systematically reviewed studies on all-cause AOM episodes and physician visits in which impact was attributed to pneumococcal conjugate vaccines, either as efficacy or effectiveness. Of 18 relevant publications found, most used the 7-valent pneumococcal conjugate vaccine (7vCRM). The efficacy of 7vCRM against all-cause AOM episodes or visits was 0%–9% in randomized trials and 17%–23% in nonrandomized trials. In observational database studies, physician visits for AOM were already declining in the 3–5 years before 7vCRM introduction (mean change, −15%; range, +14% to −24%) and continued to decline afterward (mean, −19%; range, +7% to −48%). This vaccine provides some protection against OM, but other factors have also contributed to the recent decline in OM incidence. Future effectiveness studies should thus use better-controlled methods to estimate the true impact of vaccination on AOM.
By the age of 3 years, more than two-thirds of children experience ≥1 episode of acute otitis media (AOM), and about half experience ≥3 episodes [1]. AOM is a leading cause of physician visits and antibiotic prescriptions. Pathogenic bacteria are isolated from middle ear fluid in up to 70% of cases [2], with Streptococcus pneumoniae and nontypeable Haemophilus influenzae together representing 60%–80% of bacterial cases [3–5]. Vaccines against these pathogens thus offer potential public health gains.
Use of the 7-valent (7vCRM; Pfizer) pneumococcal conjugate vaccine (PCV) in infants became widespread over the last decade [6]. Two PCVs with higher valency were recently licensed and are gradually replacing 7vCRM. The 10-valent PCV (PHiD-CV; GlaxoSmithKline Biologicals) includes 3 additional serotypes and uses an H. influenzae protein D carrier [7]. The 13-valent PCV (13vCRM; Pfizer) includes the same serotypes as PHiD-CV, plus another 3 [8].
7vCRM has dramatically reduced invasive pneumococcal disease (IPD), with >90% efficacy in clinical studies [9] and virtual elimination of vaccine-type IPD in immunized cohorts [10]. However, the impact on AOM, a polymicrobial mucosal disease, is less clear. A previous meta-analysis of efficacy trials [11] did not include observational database studies, and the 2 types of results need to be reconciled. Accumulation of effectiveness results for new vaccines takes some years, so OM policy decisions must still be based partly on 7vCRM effectiveness data.
METHODS
Search Strategy
PubMed was searched for articles in English, French, German, and Italian published between January 1998 and September 2010, using the terms “S. pneumoniae,” “pneumococcal conjugate vaccin*,” “vaccine,” “acute otitis media,” “otitis media,” “efficacy,” “effectiveness,” “effect(s),” “impact,” “visit(s),” “episode(s),” “claims,” “trends,” “retrospective,” and “observational” combined with “All child: 0–18 years.” Potentially relevant publications were screened for (1) original study, (2) assessment of PCV efficacy/effectiveness against all-cause AOM episodes or physician visits, and (3) a study population of children aged ≤12 years. Publication bibliographies and recent reviews were examined for further articles. Publications were noted but data not used in evidence tables if they focused specifically on hospitalizations/severe complications, recurrent AOM, and OM with effusion; used schedules other than 3 + 1 or 2 + 1; provided only data after administration of both PCV and the 23-valent pneumococcal polysaccharide vaccine; or calculated cost-effectiveness without providing new effectiveness data.
Calculations
Where necessary, rates were recalculated as the number of cases per 1000 person-years (PY). For observational database studies, pre-PCV rate changes were calculated as the difference between estimates reported for the first study year and the last year before PCV introduction, and post-PCV rate changes were calculated as the difference between estimates for the last year before PCV introduction and the last study year. Average rates for the periods before and after 7vCRM introduction were not calculated because consistently decreasing trends were seen in most studies. However, if rates were only reported for certain years combined [12–15], these data were used. Unpublished estimates were obtained directly from study investigators [13, 16] or approximated from figures [17]. For Poehling et al, the only available estimates for post-PCV changes were based on ratios of rates for <2 versus 3–5-year-olds [18]. For De Wals et al, we used the published post-PCV change adjusted by time-series regression [19].
RESULTS
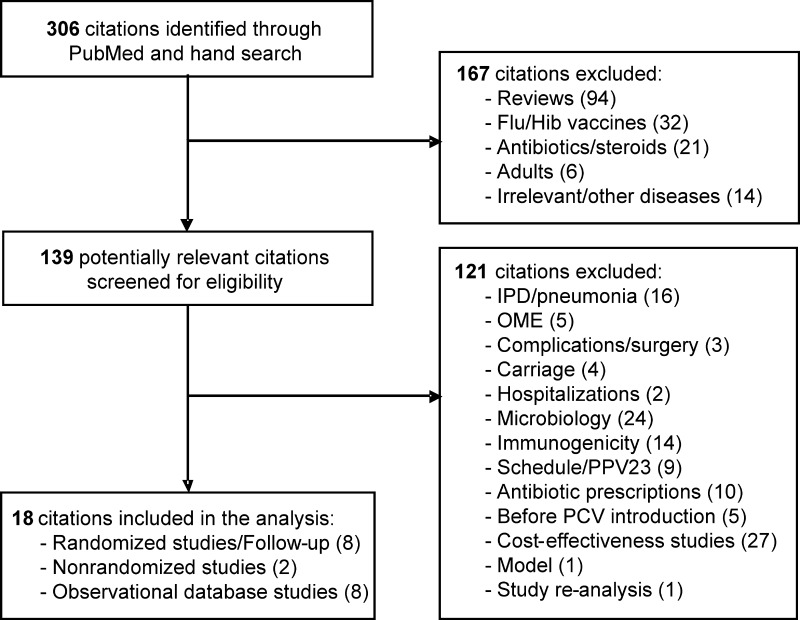
Of 306 candidate publications identified (Figure 1), 18 met inclusion criteria; 7 were clinical trials (Table 1), with some multiple publications; and 8 were observational database studies (Table 2). Five trials were randomized and double blinded: 3 were on 7vCRM [3, 9, 20], 1 was on the 7-valent vaccine candidate conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B (7vOMPC; Merck) [21], and 1 was on the 11-valent prototype version of PHiD-CV (11Pn-PD; GlaxoSmithKline Biologicals) [4]. Two 7vCRM trials were nonrandomized: 1 was observer blinded [22], and 1 was open label [23].
Figure 1.
Flow chart of the publications evaluated for inclusion in the analysis. Flu, influenza virus; Hib, Haemophilus influenzae type b; IPD, invasive pneumococcal disease; OME, otitis media with effusion; PCV, pneumococcal conjugate vaccine; PPV23, 23-valent pneumococcal polysaccharide vaccine.
Table 1.
Summary of the Clinical Trials Included in the Literature Analysis
| Reference | Country (State, Pop) | Data | PCV | Schedule (Months) | Age (Years) | No. of Subjects | Outcome | Case Definition | Case Ascertainment | Comparison | Baseline Rate (per 1000 Pop or PY) | PCV Efficacy (% [95% CI]) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Efficacy randomized clinical trials | ||||||||||||
| Black et al 2000 [9] Black et al 2002 [26] Fireman et al 2003 [25] (NCKP) |
United States (CA) | RCT | 7vCRM | 2, 4, 6, 12–15 | <3.5 | ∼38K | Episodes Visits | AOM | Computerized diagnoses, emergency physicians and pediatricians | Control vs 7vCRM arm | … Visits: ∼2000a | Episodes: PP: 5.8 (3.7–7.8) to 7.0 (4.1–9.7) ITT: 5.8 (3.7–7.9) to 6.4 (3.9–8.7) Visits: PP: 7.8 (5.4–10.2) to 8.9 (5.8–11.8) ITT: 7.0 (4.7–9.1) to 7.8 (5.2–10.5) |
| Eskola et al 2001 [3] (FinOM) |
Finland | RCT | 7vCRM | 2, 4, 6, 12 | <2 | 1662 | Episodes | AOM | Study physician according to case definition | Control vs 7vCRM arm | 1240 | PP: 6 (−4 to 16) |
| Palmu et al 2004 [24] |
FU | 2–5 | 756 | ITT: 9 (−35 to 38) | ||||||||
| O'Brien et al 2008 [20] |
United States (Native Americans) | RCT (community randomized) | 7vCRM | 2, 4, 6, 12–15 | <2 | 856 | Episodes | AOM | Treating physician | Control vs 7vCRM arm | 1500 | PP: −0.1 (−20.8 to 17.1)b |
| Kilpi et al 2003 [21] (FinOM) |
Finland | RCT | 7vOMPC | 2, 4, 6, 12 | <2 | 1666 | Episodes | AOM | Study physician according to case definition | Control vs 7vOMPC arm | 1240 | PP: −1 (−12 to 10)b |
| Prymula et al 2006 [4] (POET) |
Czech Republic/ Slovakia | RCT | 11Pn-PD | 3, 4, 5, 12–15 | <2.5 | 4968 | Episodes | AOM | Pediatrician, confirmed by ENT | Control vs 11Pn-PD arm | 125 | PP: 33.6 (20.8–44.3) |
| Nonrandomized clinical trials | ||||||||||||
| Esposito et al 2007 [22] |
Italy | Observer blinded | 7vCRM | 3, 5, 11–12 | <2.5 | 1555 | Episodes | AOM, excluding AOM with more severe concurrent illnesses | Reported by parents, confirmed by study pediatrician | Control vs 7vCRM arm | 469 | PP: 17 (−2 to 39) |
| Adam and Fehnle 2008 [23] |
Germany | Nonblinded | 7vCRM | 2, 3, 4, 12–15 | <2 | 7411 | Children with ≥1 episode | AOM | Treating physician | Control vs 7vCRM armc | 291 | ITT: 19.0 (10.7–26.4);MP: 23.2 (12.9–32.3) |
Abbreviations: 7vCRM, 7-valent pneumococcal vaccine conjugated to CRM197; 7vOMPC, 7-valent pneumococcal vaccine conjugated to OMPC; 11Pn-PD, 11-valent pneumococcal vaccine conjugated to protein D; AOM, acute otitis media; CA, California; ENT, ear, nose, and throat specialist; FU, follow-up; ITT, intention-to-treat; MP, matched pair; PCV, pneumococcal conjugate vaccine; Pop, population; PP, per protocol; PY, person-years; RCT, randomized controlled trial.
a Recalculated for the total population. Rate per 1000 PY was originally 2650 for children aged <1 years, 2010 for children 1–2 years, and 1180 for children >2–3.5 years.
b A negative efficacy indicates an increased risk in the vaccine group.
c Most vaccinated children had underlying medical conditions, in contrast to unvaccinated children.
Table 2.
Summary of the Observational Database Studies Included in the Literature Analysis
| Reference | Country (State, Prov, Pop) | Database | Age (Years) | No. of Subjects | Case Definition | Case Ascertainment | Comparison | Baseline Rate (per 1000 Pop or PY) | Pre-PCV Decrease (%) | Post-PCV Decrease (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Poehling et al 2004 [18] | United States (TN) | TN: Medicaid-managed care (government) | <2 | TN: 442K | OM, excluding concurrent IPD/pneumonia | Any listed ICD-9 code, outpatient only | 1998–2000 vs 2001– 2002 | 1775–2019 | … | 4a |
| United States (NY) | NY: Private- managed care | NY: 44K | 2125–2247 | … | 19a | |||||
| Poehling et al 2007 [15] | United States (TN) | TN: Medicaid-managed care (government) | <2 | TN: 150K | Frequent OM | Any listed ICD-9 code, outpatient only | 1998–1999 vs 1999– 2000 | … | TN: 16 NY: 16 | |
| United States (NY) | NY: Private-managed care | NY: 26K | 1999–2000 vs 2001–2002 | TN: −7b NY: 18 | ||||||
| Grijalva et al 2006 [12] | United States | NAMCS/NHAMCS | <2 | … | OM | Any listed ICD-9 code, ambulatory only | 1994–1995 vs 1998–1999 | 1415 | 24c | |
| 1998–1999 vs 2002–2003 | 12c | |||||||||
| Zhou et al 2008 [16] | United States | Employer insurance (private) | <2 | 20K–153Kd | OM | First listed ICD-9 code, ambulatory only | 1997 vs 1999 1999 vs 2004 | 2073e | −14e | 48e |
| Grijalva et al 2009 [13] | United States | NAMCS/NHAMCS | <5 | … | OM | Any listed ICD-9 code, ambulatory only | 1995–1996 vs 1999–2000 | 950 | 23 | |
| 1999–2000 vs 2005–2006 | 13 | |||||||||
| De Wals et al 2009 [19] | Canada (Quebec) | Physician claims | <5 | 25K–26Ke | OM | Any listed ICD-9 code, ambulatory only | 2000 vs 2007 | 587 | … | 13 |
| Singleton et al 2009 [14] (plus Curns et al 2002 [56]) | US (Native Americans) | Indian Health service database (government) | <5 | 775K | OM | Any listed ICD-9 code | 1994 vs 1996 1996 vs 2003–2005 | 1380 per 1000 children | 18f | 29f |
| Sox et al 2008 [17] | United States (MA) | Physician claims (private) | ≤12 | … | AOM | Any listed ICD-9 code, ambulatory only | 1996 vs 1999 1999 vs 2004 | 385 | 22g | 37g |
All studies analyzed the impact of 7vCRM on OM visits.
Abbreviations: 7vCRM, 7-valent pneumococcal vaccine conjugated to CRM197; AOM, acute otitis media; ENT, ear, nose, and throat specialist; ICD-9, International Classification of Diseases, Ninth Revision; IPD, invasive pneumococcal disease; MA, Massachusetts; NAMCS, National Ambulatory Medical Care Survey; NHAMCS, National Hospital Ambulatory Medical Care Survey; NY, New York; PCV, pneumococcal conjuage vaccine; OM, otitis media; Pop, population; Prov, province; PY, person-years; TN, Tennessee.
a Based on comparison of relative rates for <2-year-olds vs 3–5-year-olds during 1998–2000 vs 2001–2002.
b A negative effectiveness indicates an increased rate of AOM.
c Our recalculation from the rates of AOM visits in <2-year-olds.
d No. of children per year.
e Our recalculation from yearly estimates. Zhou et al originally presented a baseline of 2173 for 1997–1999 and a 43% decrease between 1997–1999 and 2004 [16].
f Our recalculation from [14] and [56]. Singleton et al originally presented a 35.5% decrease between 1994–1996 and 2003–2005 [14], whereas Curns et al presented the individual OM rates for 1994–1996 [56].
g Our recalculation estimated from Figure 1.
Baseline AOM Incidence
In clinical trials, baseline AOM episode rates among children aged <2 or <2.5 years differed 10-fold, from 125 to 1500 per 1000 PY or children [3, 4, 21, 24] (Table 1). The lowest rate was from the only study requiring otolaryngological confirmation upon pediatrician referral [4]. The nonrandomized trials observed baseline rates of <500 per 1000 PY or children [22, 23].
In observational database studies, baseline OM visit rates (per 1000 PY or children) were 1415–2247 for <2-year-olds [12, 18] and 610–1380 for <5-year-olds (Table 2) [14, 19]. The highest visit rates came from private insurance databases [16, 18], with lower rates in managed care (2032 per 1000 PY) than non–managed care (2429 per 1000 PY) [16].
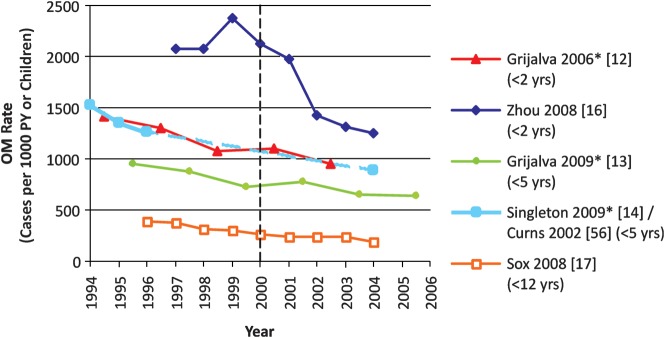
Six database studies presented trends in baseline OM visit rates over several years before 7vCRM introduction, and all [12–15, 17] but one [16] observed substantial declines (mean change, −15%; range, +14% to −24%) (Table 2, Figure 2). For example, OM rates declined by 23%–24% over 5 years before 7vCRM introduction, in 2 US population-based surveys [12, 13]. The exception was the analysis of nationwide employers’ insurance data by Zhou et al, which found a 14% increase in OM visit rates over the 2 years before 7vCRM introduction [16].
Figure 2.
Trends in otitis media rates among observational database studies presenting data for years before and after 7-valent pneumococcal conjugate vaccine introduction in 2000. Asterisks indicate studies for which the midpoint of the reported period was used to generate the graph. The age groups used for analysis are indicated in parentheses. Abbreviations: OM, otitis media; PY, person-years.
PCV Efficacy on AOM in Randomized and Nonrandomized Clinical Trials
In the 2 individually randomized trials on 7vCRM efficacy against AOM, the NCKP [9, 25, 26] and FinOM trials [3, 24], 7vCRM reduced all-cause episodes in 2-year-olds by 5.8%–7.0% and visits by 7.0%–8.9% (Table 1), achieving statistical significance in the NCKP trial. In a third, smaller, community-randomized trial among Native American infants, no effect was detected on clinically diagnosed AOM (−0.l%; 95% confidence interval [CI], −20.8% to 17.1%) [20]. In comparison, the nonrandomized trials in Italy (where parents chose whether their child received 7vCRM) and Germany (where most vaccinated children had comorbid conditions) observed 17%–23% reductions.
Only 2 publications reported efficacy of other PCVs. No efficacy of 7vOMPC was demonstrated against all-cause AOM (−1%; 95% CI, −12% to 10%) [21]. The POET trial showed 34% efficacy against all-cause AOM (95% CI, 21%–44%) for 11Pn-PD [4].
PCV Overall Impact on OM in Postimplementation Studies
The 8 observational database studies reported a 19% average reduction in OM visit rates (range, +7% to −48%) after 7vCRM introduction (Table 2). The 2 lowest estimates were in children aged <2 years receiving US governmental insurance: there was a 7% increase between 1999–2000 and 2001–2002 [15] and 4% (statistically nonsignificant) reduction between 1998–2000 and 2001–2002 [18]. The highest decrease was 48% and occurred during 1999–2004 (the decrease was 43% if the average from 1997–1999 was used) and was reported in the only study observing an increase (14%), rather than decrease, in OM rates before vaccine introduction [16].
DISCUSSION
In summary, 7vCRM efficacy against all-cause AOM episodes was an estimated 0%–9% in randomized trials and 17%–23% in nonrandomized clinical trials. Observational database studies showed that OM visit rates decreased 19% on average following 7vCRM introduction, with estimates ranging widely (+7% to −48%). Before 7vCRM introduction, OM visit rates were already declining in all but one study. These findings raise several issues to be considered when appraising policy options and designing studies, as discussed below.
Variability in Efficacy Trial Results
Efficacy against all-cause AOM assessed for 3 vaccine formulations (7vCRM, 7vOMPC, and 11Pn-PD) tested in 5 randomized trials yielded point estimates ranging from −1% to 34%. Whereas some of this variability is likely due to differing vaccine composition, it is difficult to distinguish this from confounding by local variability in viral and bacterial etiology, case ascertainment, diagnosis, and care-seeking behavior. Re-analyses of POET and FinOM trials, adjusting for severity of case definition and pathogen distributions, somewhat narrowed differences in PCV efficacy estimates [27, 28], but such reconciliation is not always feasible, and conclusions have to be based on central tendency across studies.
Observed Versus Theoretical Effectiveness
Theoretical maximum effectiveness in real-life settings can be calculated by assuming no replacement with nonvaccine types, a stationary commensal profile, and 100% vaccine uptake. On the assumption that 70% of AOM episodes are bacterial [2], of which 50% are due to S. pneumoniae [5], of which 7vCRM serotypes represent 75% [29], and for which efficacy is 57% [3], then 7vCRM should prevent approximately 15% of the episodes of all-cause AOM (70% × 50% × 75% × 57%).
Vaccination rates of >80% would be expected to induce herd protection via decrease in nasopharyngeal carriage of vaccination serotypes [30, 31]. Although there is strong evidence for herd protection with IPD, herd protection against vaccine-type AOM in nonvaccinated age groups has not yet been directly demonstrated because tympanocentesis is not routinely performed. Dilution of vaccine-type herd protection within all-cause OM makes it hard to show, and one study failed to detect it in overall AOM visits in older children 2 years after 7vCRM implementation [19]. Near elimination of vaccine-type carriage some years after PCV use, on the assumption of maximum herd protection (with vaccine types eradicated), is a reasonable approximation. Effectiveness against vaccine types would then be 100% instead of 57%, yielding a theoretical effectiveness of approximately 26%.
The above calculations do not reflect any replacement with nonvaccine serotypes and bacteria, although some replacement is suggested in clinical studies and postintroduction surveillance [3, 32, 33]. Indeed, a recent model that used actual nasopharyngeal carriage rates in US children for both vaccine and nonvaccine serotypes, taking into account their specific abilities to cause AOM, projected a maximum theoretical effectiveness of 7vCRM against overall AOM of only 12% [34]. This suggests that estimates well beyond these theoretical limits may be substantially confounded and biased.
Variability in Baseline Incidence
Baseline AOM episode rates in clinical trials varied 10-fold. The high baseline rate in FinOM [3] is similar to US rates (900–1500 AOM episodes per 1000 children) [20, 35, 36], whereas the low rate in the POET trial is closer to those reported in other European studies (154–400 AOM episodes per 1000 PY) [37]. In general, stronger vaccine effects would be expected on samples that use tighter diagnostic definitions and, hence, lower baseline case incidence, but they face sample size challenges. This, plus possible intrinsic differences in populations or differences in healthcare uptake beyond those of diagnostic definition, suggest that one should be cautious in considering between-study comparisons of vaccines. Among the database analyses, baseline rates also varied across studies, even after taking into account age differences [12, 15, 16, 18]. Strong evidence for demographic, immunological, or microbiological differences between such populations is lacking, so such baseline rate differences are more appropriately attributed to differences in case severity or diagnostic code for case definition.
Changes Before Versus After Vaccine Introduction
In observational database studies, OM visit rates decreased by 19% on average after 7vCRM introduction. However, among studies also presenting data before 2000, all [12–15, 17] but one [16] observed OM visits declining by 15% on average before 7vCRM introduction. This suggests that long-term decreases in consultations before 7vCRM introduction, which are unlikely to have halted, have added to apparent postintroduction decreases. Poehling et al and Grijalva et al controlled for annual trend via differential effect by age, arriving at 4%–19% decreases due to 7vCRM [12, 18]. However, this minimizes any herd protection affecting the nonimmunized portion of the younger cohort. In addition, non–vaccine-related factors, such as age stratification of <2/≥2 years in antibiotic prescription guidelines, could affect OM visit rates over time differentially by age. De Wals et al moved in the appropriate direction by estimating a post-PCV rate with time-series regression to adjust for annual trend [19]; the raw decrease in OM claims in 2000–2007 was 25%, but the adjusted decrease attributable to 7vCRM was only approximately 13%.
To determine whether the decrease in consultations is due to 7vCRM introduction, analysis must be made over a few years and according to when and to what extent the vaccine was introduced. For example, a recent study in an Athens hospital found that, beginning in 2005, emergency department visits by children aged <15 years decreased by 38% and 48% for all-cause and pneumococcal otorrhea, respectively [38]. However, this drop occurred 1–2 years before mass pneumococcal vaccination in Greece, at the time of (presumably low) private market 7vCRM use, and, even after the decrease, vaccine serotypes still represented the majority of pneumococcal otorrhea. Upon implementation of mass vaccination in 2006, no further drop was seen, indicating that the reduction in 2005 was largely due to nonvaccine factors.
Potential Nonvaccine Factors
Several other factors might explain why OM rates decreased before PCV introduction and continued decreasing after. First, changes in AOM perception, consultation rates, and frequency and type of antibiotic use date from the early 1990s. The increasing acceptance by parents and physicians of observation without antibiotic use (“watchful waiting”), which is officially recommended for some AOM patients [39], could reduce the apparent AOM incidence if parents do not consult physicians for mild AOM if they expect little benefit for their child. Stricter diagnostic criteria [39] may have reduced not only inappropriate antibiotic use [13] but also apparent AOM consultation rates. Second, a shift to higher antibiotic dosage or the doubling of long-acting macrolide use in US children around the same time as 7vCRM introduction [40] could have reduced relapses and, therefore, reduced the total number of AOM visits per episode, thus reducing the healthcare burden [17].
Third, awareness of vaccination status could affect care-seeking behavior. In a recent observer-blinded randomized trial in Sweden of children at risk for recurrent AOM conducted before universal PCV, receipt of 7vCRM reduced overall reported AOM episodes by 26% and AOM hospital visits by 36% [41]. Because these apparent effects are larger than the above theoretical effectiveness estimate, there may have been some differential contribution from parents seeking medical assistance depending on vaccination status, with less care-seeking for vaccinated children because of the belief that vaccine would probably prevent the more serious forms or complications of disease.
Fourth, the decline in OM rates has paralleled the decreasing exposure of children to secondhand tobacco smoke, a strong AOM risk factor [42]. Fifth, influenza vaccination can reduce AOM incidence during the influenza season by reducing viral coinfection [43]. However, influenza routine vaccination in the US began in 2004, with the sharpest increase around 2007–2008 [44], after the attributable post-7vCRM decrease in OM.
Study Population
Possible differences among populations, chiefly their relative risks, cannot be overlooked in explaining the heterogeneity of results. However, convincing demonstrations are lacking. The failure of O'Brien et al to detect a statistically significant 7vCRM impact on AOM in high-risk American Indians may be due to the lack of statistical power [20]. Likewise, a favorable, although nonsignificant, vaccine effect (adjusted relative risk, 0.88 [95% CI, .69–1.13]) was found in successive cohorts of 51 nonvaccinated and 97 vaccinated (7vCRM plus a 23-valent polysaccharide booster dose) high-risk Australian aboriginal children [45]. Finally, the authors of the nonrandomized, nonblinded 7vCRM German trial [23] suggested that the achievable efficacy was possibly biased against the vaccine because more children in the 7vCRM group than the control group had a medical risk factor (66% vs 18%) or were born prematurely (40% vs 6%). The assumption behind all these studies is that high-risk, otitis-prone children generate a weaker immune response, for which there is some evidence [46]. Limited statistical power currently prevents clear conclusions, but possible differences in vaccine effectiveness between populations deserve consideration.
Diagnostic Codes Included as OM
Observational database studies identify OM cases according to broad diagnostic codes that are based often on a single clinician's judgment rather than on precise protocols and measurements. In the International Classification of Diseases, Ninth Revision coding system, codes 381.x refer mainly to nonsuppurative AOM, codes 382.x to suppurative AOM, and codes 383.x to mastoiditis. Code choice could greatly affect absolute OM visit count, and study-specific differences in case definition or even OM type distribution could influence 7vCRM effectiveness estimates [28, 47]. Unfortunately, no studies reported the proportions of the different codes used. Grijalva et al defined OM diagnosis as 381.x–382.x in one study [12] and as 381.x–383.x in the other [13], whereas Poehling et al used 381.0–381.4 and 382.x [15]. Zhou et al used 381.00–381.6, 382.00–382.02, 382.3, and 382.9 [16] but, unlike the other studies, only considered first-listed codes, possibly explaining why they reported the largest decrease (43%) [16]. Indeed, where AOM antibiotic use is strictly controlled, some physicians may use AOM less as a primary code, preferring a symptom-based equivalent code.
Design Considerations for Future Studies
Vaccine impact will always be assessed by large observational studies. However, one key requirement is adjustment for non–vaccine-related confounders. Adjustment for secular trends [48–50], preferably via time-series modeling [19], should always be performed. Modeling would also allow distinction between year-to-year variation (random and viral) and longer-term trends. At a minimum, projections from prevaccine trends should provide the expected null value from which an observed deviation may be taken as evidence for vaccine effect [48–50]. In addition, measurement of time trends of other diseases could provide additional control, with the caveat that some nonvaccine trends could affect unrelated diseases differently.
The central public health questions are whether vaccination causes an overall decrease in AOM and associated healthcare burden. Tympanocentesis-based efficacy studies, even at the population level, would at least help specify how much of an overall decrease is limited to target pathogens/serotypes, but it remains unusual and ethically problematic to perform routinely, and determining vaccine effectiveness against individual serotypes necessitates large sample sizes.
PCV effects on AOM can be measured economically and with good control in case-control studies, as for IPD [51–53]. However, finding appropriate controls in a well-immunized population is difficult. The presumably present herd protection is seen as a depressed incidence in controls and is not directly measurable with this design, meaning the effect is nearer to an efficacy than to an effectiveness estimate.
Finally, the problem of quality of case definition has long been remarked in AOM studies. Some hope of reducing variability from this source is given by 2 recent high-quality randomized studies on AOM treatment that used stringent and reproducible criteria applicable to all designs except routine practice databases [54, 55].
In conclusion, observed OM visit rates have decreased by approximately 19% following 7vCRM introduction, but long-term reductions in OM visits preceding 7vCRM introduction of approximately 15% suggest that continuing influences other than PCV vaccination have caused some of the subsequent reduction. Caution is therefore needed in the report and interpretation of these data, and no single study should be quoted as representing the “true” effect of 7vCRM on AOM. Study methods need to be improved to more accurately estimate true PCV effectiveness.
Notes
Acknowledgments. We are grateful to Drs Carlos Grijalva and Fangjun Zhou for kindly providing us with additional data on request. We also thank Drs Bernard Hoet and Carlos Grijalva for critical reading of a preliminary version of the manuscript. Editorial assistance and manuscript coordination was provided by Drs Véronique Mouton and Barbara Pelgrims (GlaxoSmithKline Biologicals, Belgium).
Financial support. This work was supported by GlaxoSmithKline Biologicals, which paid for all costs associated with the development and writing of the manuscript.
Manuscript preparation. S. T. and W. H. conceived this systematic review, identified the eligible studies, and analyzed the data. S. T. prepared the tables. J. H. did the literature search and wrote the first draft of the manuscript. S. T., W. H., P. M., A. V., and M. H. interpreted the results. All authors made critical revisions to the manuscript and approved the final version. All authors had full access to the data, and the corresponding author had final responsibility for submission of the manuscript.
Potential conflicts of interest. M. H. declares that he received consulting fees and honoraria/travel expenses from GlaxoSmithKline Biologicals in the past 3 years. P. M. declares that she received honoraria/travel grants from GlaxoSmithKline Biologicals in the past 3 years. A. V. declares that she received advisory board honoraria from GlaxoSmithKline Biologicals, MSD, AstraZeneca, and Pfizer and speaker/travel grants from GlaxoSmithKline Biologicals and Pfizer in the last 3 years. J. H. is a medical writer and declares that she received funding from GlaxoSmithKline Biologicals and Pfizer. S. T. and W. P. H. declare that they are employed by GlaxoSmithKline Biologicals. W. P. H. owns stock in GlaxoSmithKline Biologicals, which has a licensed pneumococcal conjugate vaccine.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 2.Cripps AW, Otczyk DC. Prospects for a vaccine against otitis media. Expert Rev Vaccines. 2006;5:517–34. doi: 10.1586/14760584.5.4.517. [DOI] [PubMed] [Google Scholar]
- 3.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 4.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typeable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–8. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 5.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–52. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Progress in introduction of pneumococcal conjugate vaccine–worldwide, 2000–2008. MMWR Morb Mortal Wkly Rep. 2008;57:1148–51. [PubMed] [Google Scholar]
- 7.Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J. 2009;28:S66–76. doi: 10.1097/INF.0b013e318199f8ef. [DOI] [PubMed] [Google Scholar]
- 8.Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866–75. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 9.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 11.Jansen AG, Hak E, Veenhoven RH, Damoiseaux RA, Schilder AG, Sanders EA. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev. 2009:CD001480. doi: 10.1002/14651858.CD001480.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865–73. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singleton RJ, Holman RC, Plant R, et al. Trends in otitis media and myringotomy with tube placement among American Indian/Alaska native children and the US general population of children. Pediatr Infect Dis J. 2009;28:102–7. doi: 10.1097/INF.0b013e318188d079. [DOI] [PubMed] [Google Scholar]
- 15.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121:253–60. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 17.Sox CM, Finkelstein JA, Yin R, Kleinman K, Lieu TA. Trends in otitis media treatment failure and relapse. Pediatrics. 2008;121:674–9. doi: 10.1542/peds.2007-1565. [DOI] [PubMed] [Google Scholar]
- 18.Poehling KA, Lafleur BJ, Szilagyi PG, et al. Population-based impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755–61. doi: 10.1542/peds.2003-0592-F. [DOI] [PubMed] [Google Scholar]
- 19.De Wals PD, Carbon M, Sevin E, Deceuninck G, Ouakki M. Reduced physician claims for otitis media after implementation of pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2009;28:e271–5. doi: 10.1097/INF.0b013e3181bad212. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien KL, David AB, Chandran A, et al. Randomized, controlled trial efficacy of pneumococcal conjugate vaccine against otitis media among Navajo and White Mountain Apache infants. Pediatr Infect Dis J. 2008;27:71–3. doi: 10.1097/INF.0b013e318159228f. [DOI] [PubMed] [Google Scholar]
- 21.Kilpi T, Ahman H, Jokinen J, et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003;37:1155–64. doi: 10.1086/378744. [DOI] [PubMed] [Google Scholar]
- 22.Esposito S, Lizioli A, Lastrico A, et al. Impact on respiratory tract infections of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months of age. Respir Res. 2007;8:12. doi: 10.1186/1465-9921-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adam D, Fehnle K. Safety and effectiveness against respiratory tract infections for pneumococcal conjugate vaccine co-administered with routine vaccine combinations. Vaccine. 2008;26:5944–51. doi: 10.1016/j.vaccine.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 24.Palmu AA, Verho J, Jokinen J, Karma P, Kilpi TM. The seven-valent pneumococcal conjugate vaccine reduces tympanostomy tube placement in children. Pediatr Infect Dis J. 2004;23:732–8. doi: 10.1097/01.inf.0000133049.30299.5d. [DOI] [PubMed] [Google Scholar]
- 25.Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10–6. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Black S, Shinefield H. Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from Northern California. Eur J Pediatr. 2002;161(Suppl 2)):S127–31. doi: 10.1007/s00431-002-1064-z. [DOI] [PubMed] [Google Scholar]
- 27.Jokinen J, Hausdorff WP, Palmu AA, et al. Re-analysis of clinical trial data for comparison of conjugate vaccine efficacy against acute otitis media. [poster G-598]. Presented at: 4th Europaediatrics; Moscow, Russia. 2009. [Google Scholar]
- 28.Palmu A, Jokinen J, Kilpi T. Impact of different case definitions for acute otitis media on the efficacy estimates of a pneumococcal conjugate vaccine. Vaccine. 2008;26:2466–70. doi: 10.1016/j.vaccine.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–21. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 30.Dagan R. The potential effect of widespread use of pneumococcal conjugate vaccines on the practice of pediatric otolaryngology: the case of acute otitis media. Curr Opin Otolaryngol Head Neck Surg. 2004;12:488–94. doi: 10.1097/01.moo.0000145958.12395.ec. [DOI] [PubMed] [Google Scholar]
- 31.Musher DM. Pneumococcal vaccine—direct and indirect (“herd”) effects. N Engl J Med. 2006;354:1522–4. doi: 10.1056/NEJMe068038. [DOI] [PubMed] [Google Scholar]
- 32.Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829–33. doi: 10.1097/01.inf.0000136868.91756.80. [DOI] [PubMed] [Google Scholar]
- 33.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shea KM, Weycker D, Stevenson AE, Strutton DR, Pelton SI. Modeling the decline in pneumococcal acute otitis media following the introduction of pneumococcal conjugate vaccines in the US. Vaccine. 2011;29:8042–8. doi: 10.1016/j.vaccine.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 35.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 37.Liese J, Silfverdal S-A, Giaquinto C, et al. Comparing incidence figures of acute otitis media in young children in five European countries with the published literature. 2010 Presented at: 28th Annual Meeting of the European society for Paediatric Infectious Diseases, Nice, France. [Google Scholar]
- 38.Stamboulidis K, Chatzaki D, Poulakou G, et al. The impact of the heptavalent pneumococcal conjugate vaccine on the epidemiology of acute otitis media complicated by otorrhea. Pediatr Infect Dis J. 2011;30:551–5. doi: 10.1097/INF.0b013e31821038d9. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Pediatrics and American Academy of Family Physicians. Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 40.Van Effelterre T, Moore MR, Fierens F, et al. A dynamic model of pneumococcal infection in the United States: implications for prevention through vaccination. Vaccine. 2010;28:3650–60. doi: 10.1016/j.vaccine.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Gisselsson-Solen M, Melhus A, Hermansson A. Pneumococcal vaccination in children at risk of developing recurrent acute otitis media—a randomized study. Acta Paediatr. 2011;100:1354–8. doi: 10.1111/j.1651-2227.2011.02332.x. [DOI] [PubMed] [Google Scholar]
- 42.Alpert HR, Behm I, Connolly GN, Kabir Z. Smoke-free households with children and decreasing rates of paediatric clinical encounters for otitis media in the United States. Tob Control. 2011;20:207–11. doi: 10.1136/tc.2010.038711. [DOI] [PubMed] [Google Scholar]
- 43.Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J. 2007;26:97–106. doi: 10.1097/01.inf.0000253053.01151.bd. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Influenza vaccination coverage among children aged 6–23 months—United States, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep. 2009;58:1063–6. [PubMed] [Google Scholar]
- 45.Mackenzie GA, Carapetis JR, Leach AJ, Morris PS. Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination. BMC Pediatr. 2009;9:14. doi: 10.1186/1471-2431-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veenhoven R, Rijkers G, Schilder A, et al. Immunoglobulins in otitis-prone children. Pediatr Res. 2004;55:159–62. doi: 10.1203/01.PDR.0000099776.66136.39. [DOI] [PubMed] [Google Scholar]
- 47.De Wals P, Erickson L, Poirier B, Pepin J, Pichichero ME. How to compare the efficacy of conjugate vaccines to prevent acute otitis media? Vaccine. 2009;27:2877–83. doi: 10.1016/j.vaccine.2009.02.102. [DOI] [PubMed] [Google Scholar]
- 48.Hanquet G, Lernout T, Vergison A, et al. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29:2856–64. doi: 10.1016/j.vaccine.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 50.Flasche S, Slack M, Miller E. Long term trends introduce a potential bias when evaluating the impact of the pneumococcal conjugate vaccination programme in England and Wales. Euro Surveill. 2011;16:19868. [PubMed] [Google Scholar]
- 51.Barricarte A, Castilla J, Gil-Setas A, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine: a population-based case-control study. Clin Infect Dis. 2007;44:1436–41. doi: 10.1086/516779. [DOI] [PubMed] [Google Scholar]
- 52.Dominguez A, Ciruela P, Garcia-Garcia JJ, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine in the prevention of invasive pneumococcal disease in children aged 7–59 months. A matched case-control study. Vaccine. 2011;29:9020–5. doi: 10.1016/j.vaccine.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 53.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 54.Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364:105–15. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364:116–26. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 56.Curns AT, Holman RC, Shay DK, et al. Outpatient and hospital visits associated with otitis media among American Indian and Alaska native children younger than 5 years. Pediatrics. 2002;109 doi: 10.1542/peds.109.3.e41. E41-1. [DOI] [PubMed] [Google Scholar]