Abstract
Existing clinical case definitions of pertussis are decades old and based largely on clinical presentation in infants and children, yet an increasing burden is borne by adolescents and adults who may manifest distinct signs/symptoms. Therefore, a “one-size-fits-all” clinical case definition is no longer appropriate. Seeking to improve pertussis diagnosis, the Global Pertussis Initiative (GPI) developed an algorithm that delineates the signs/symptoms of pertussis most common to 3 age groups: 0–3 months, 4 months to 9 years, and ≥10 years. These case definitions are based on clinical presentation alone, but do include recommendations on laboratory diagnostics. Until pertussis can be accurately diagnosed, its burden will remain underestimated, making the introduction of epidemiologically appropriate preventive strategies difficult. The proposed definitions are intended to be widely applicable and to encourage the expanded use of laboratory diagnostics. Determination of their utility and their sensitivity and/or specificity versus existing case definitions is required.
INTRODUCTION
In a previous report, Global Pertussis Initiative (GPI) participants described the difficulties in defining pertussis from a clinical perspective [1]. Today, many different case definitions are used throughout the world [1–8]. Most case definitions are supplemented with laboratory and epidemiologic data so that reports may be categorized as confirmed, probable, or suspect. Case definitions also vary depending on the situation in which they are used. For example, in vaccine efficacy trials, specificity is expected to be close to 100%. Yet, in outbreak situations in states or countries, specificity is sacrificed to achieve high sensitivity, which is important for disease prevention and control.
In the prevaccine era, pertussis was considered a disease of children, and all the present clinical case definitions reflect this bias. With the current awareness that pertussis is common in adolescents and adults and that disease manifestations may be different in older persons, it is apparent that the “one-size-fits-all” clinical pertussis case definition is no longer optimal. In addition, there is an increasing awareness that pertussis in early infancy has many unique characteristics that should be recognized in a separate case definition in order to improve recognition of disease in this population. In this communication, we provide background data relating to current case definitions and then propose age-stratified case definitions that we believe will increase diagnostic specificity without decreasing sensitivity.
SELECTED CURRENTLY USED CASE DEFINITIONS
Selected, currently used, clinical case definitions and additional laboratory and epidemiologic requirements are presented in Table 1. Most primary clinical case definitions, such as those by the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), Massachusetts Department of Health, European Union (EU), Pan American Health Organization (PAHO), and Australian Department of Health and Ageing, have in common a requirement for 2 weeks of cough. To increase specificity, most definitions require at least 1 additional symptom, such as paroxysms, inspiratory whoop, or posttussive vomiting.
Table 1.
Selected Presently Used Pertussis Case Definitions
| Organization/Country, Year | Clinical Criteria | Laboratory and Epidemiologic Criteria | Comment |
|---|---|---|---|
| WHO, 2000 | A case diagnosed as pertussis by a physician, or a person with a cough lasting ≥2 weeks with ≥1 of the following symptoms:
|
Isolation of B. pertussis, or detection of genomic sequences by PCR, or positive paired serology |
Case classification: clinical case: a case that meets the clinical case definition, but is not laboratory confirmed. laboratory-confirmed case: a case that meets the clinical case definition and is laboratory confirmed. |
| CSTE/CDC, 2010 | A cough illness lasting ≥2 weeks with 1 of the following: paroxysms of coughing, inspiratory “whoop,” or posttussive vomiting, without other apparent cause (as reported by a health professional) | Isolation of B. pertussis from clinical specimen PCR positive for pertussis |
Case classification: probable: in the absence of a more likely diagnosis, a cough illness lasting ≥2 weeks, with ≥1 of the following symptoms:
|
| France, 2009 | Patient coughing ≥14 days with 1 or more of the following:
|
Patient coughing ≥14 days with:
|
Epidemiologically confirmed: patient coughing ≥7 days and in contact in the past 20 days with a biologically confirmed case |
| Canada, 2009 |
Suspect case:
one or more of the following, with no other known cause:
Probable case: cough lasting 2 weeks or longer in the absence of appropriate laboratory tests and not epidemiologically linked to a laboratory – confirmed case AND ≥1 of the following, with no other known cause:
|
Confirmed case:
laboratory confirmation of infection:
|
|
| Massachusetts, 2009 | 1989–1992: ≥1 week with paroxysms or posttussive vomiting From 1993: cough ≥2 weeks with 1 of the following: paroxysms, whoop, or posttussive vomiting (CDC definition) |
Bacteriologic cases: positive culture (or + DFA until 1992), PCR added 2004 Serologic case: positive single-serum anti-pertussis toxin antibody (persons ≥11 years only) + clinical case definition Epidemiologically linked case: contact with a laboratory confirmed case + clinical case definition |
|
| EU, 2008 | Cough ≥2 weeks with ≥1 of the following:
or apnea episodes in infants |
Isolation of B. pertussis
Nucleic acids of B. pertussis B. pertussis–specific antibody response Epidemiologic link by human-to-human transmission |
Possible case: any person with clinical criteria Probable case: person with clinical criteria and epidemiologic link Confirmed case: person meeting the clinical and laboratory criteria |
| Australia, 2004 | Coughing ≥2 weeks or paroxysms of coughing or inspiratory whoop or posttussive vomiting |
Culture of B. pertussis
PCR for B. pertussis Seroconversion or significant increase of antibodies (without recent vaccination) Single IgA titer to whole cells Detection of B. pertussis by DFA |
Probable case: Any person with clinical criteria Confirmed case: Person meeting the clinical and laboratory criteria or epidemiologic link |
Abbreviations: CDC, Centers for Disease Control and Prevention; CSTE, Council of State and Territorial Epidemiologists; DFA, direct fluorescent antibody; EU, European Union; IgA, immunoglobin A; IU, international units; PCR, polymerase chain reaction; PT, pertussis toxin; WHO, World Health Organization.
France requires that cough be present for more than 7 days, whereas Australia accepts cough of any duration if it is accompanied by paroxysms, whooping, or vomiting. The EU also accepts any physician's diagnosis of pertussis and apnea as a clinically defining symptom in infants.
Almost all case definitions require laboratory or epidemiologic linkage data, and such data may affect whether the case is categorized as confirmed, probable, or possible. Laboratory confirmation tests include culture of Bordetella pertussis and polymerase chain reaction (PCR) assays that are specific for B. pertussis. Some countries, such as Australia, also accept direct fluorescent antibody (DFA) testing, whereas the PAHO definition specifically discourages DFA. Differences are also found concerning confirmation by serology: the CDC definition does not include serology, the WHO definition requires paired serology, and the EU definition elegantly compromises by requiring a “B. pertussis–specific antibody response.” France and Massachusetts also accept single serum serology with an elevated anti–pertussis toxin (anti-PT) titer, and Australia accepts an immunoglobin A (IgA) response to whole B. pertussis.
THE SENSITIVITY AND SPECIFICITY OF CLINICAL CASE DEFINITIONS
Recently, Ghanaie and associates [9] studied the sensitivity and specificity of the WHO pertussis clinical case definition in 328 children aged 6–14 years with a persistent cough for ≥2 weeks. Pertussis was diagnosed by culture and an IS481 PCR for B. pertussis or IS1001 PCR for B. parapertussis in nasopharyngeal swabs. All but 1 of these children had received 3 or more doses of whole-cell DTP vaccine. The sensitivity was 95.2% and the specificity was 15.0% with cough ≥2 weeks plus ≥1 of the WHO clinical criteria. With an increasing number of clinical findings, sensitivity decreased and specificity increased. Posttussive emesis was the symptom that had the most pronounced effect in increasing specificity. As the entry criterion for this study was cough of >2 weeks’ duration, the mean duration of cough in the study population was 20 days, and because diagnosis was made by PCR without serologic study, it is likely that cases were missed. This would lead to an artificially low specificity.
Between 2001 and 2005, Harnden et al. [10] performed a prospective cohort study involving 172 children aged 5–16 years who had a cough lasting ≥14 days. Bordetella pertussis infection was diagnosed by the demonstration of a 4-fold change in immunoglobin G (IgG) antibody to pertussis toxin (PT) in paired samples or a single IgG titer to PT that was greater than 100 enzyme-linked immunosorbent assay units/mL. In a subsequent analysis, Wang and Harnden [11] used the clinical data from the 2001–2005 study to examine the sensitivity and specificity of defined clinical features. In children with a persistent cough that was characterized as paroxysmal, the sensitivity was 86% and the specificity was 23%; persistent cough with posttussive vomiting had a sensitivity of 70% and a specificity of 61%; persistent cough with whooping gave a sensitivity of 50% and a specificity of 74%. To obtain clinical case definition data in adolescents and adults, Wang and Harnden also used the data in the prospective pertussis surveillance study of Strebel et al. [12] performed in Minnesota during 1995–1996. In this study, persons 10–49 years old who presented with an acute paroxysmal cough or a persistent cough illness of 7–34 days’ duration were enrolled. B. pertussis infection was diagnosed by culture, PCR, or serologic evidence of a titer rise or high single-serum specimen titer to PT. For paroxysmal cough, the sensitivity was 100% and the specificity was 12%. With posttussive vomiting, the sensitivity was 56% and the specificity was 68%; for whooping, the sensitivity was 28% and the specificity was 85%.
In 1998, Patriarca et al. [13] evaluated 15 clinical case definitions for pertussis during community outbreaks and concluded that a definition of ≥14 days of cough was both sensitive (77%–91%) and specific (54%–71%) for monitoring culture-positive cases. However, in nonoutbreak situations, the use of their case definitions had low sensitivity [14].
CHALLENGES TO CURRENT CRITERIA AND THE DEVELOPMENT OF NEW CLINICAL DEFINITIONS OF PERTUSSIS
The present clinical case definitions of pertussis are inconsistent and are not used everywhere. In addition, they are not universally applicable. Different age groups must be evaluated by different clinical criteria. Pertussis case definitions may also differ between endemic and outbreak situations. Furthermore, resource-rich and resource-limited countries have unique problems related to the control of pertussis and its diagnosis. However, in all situations, the true burden of pertussis is unknown and is significantly underestimated.
In resource-rich countries, a major priority relates to the education, awareness, and recognition of pertussis in adolescents and adults and its transmission to infants [15–17]. In order to improve the awareness and recognition of the disease in these populations, precise criteria for clinical manifestations of pertussis are needed (Table 2). Awareness of proper sampling techniques for obtaining nasopharyngeal specimens (nasopharyngeal swabs, nasopharyngeal aspiration) for culture and PCR, as well as the usefulness of single-serum serology in diagnosis should also be fostered. Finally, awareness of appropriate treatment and chemoprophylactic regimens for pertussis should be promulgated.
Table 2.
Considerations Related to Adolescent and Adult Pertussis
| • How is a paroxysmal cough defined? How can it be distinguished from staccato coughing? |
| • Is a pertussis-related cough dry? Wet? Hacking? Productive? |
| • How is an inspiratory “whoop” defined? |
| • How is a pertussis-related apnea defined? When is it most likely to occur? |
| • How can a pertussis-related cough be differentiated from the cough seen with sinusitis? Asthma? Bronchitis? And that due to other infectious agents? |
| • Is the cough worse at night? Are we able to quantify worse? |
| • Does the cough significantly disturb ability to sleep? How are we defining sleep disturbance? |
In resource-limited countries, pertussis burden is especially underestimated because of a number of factors, including misdiagnosis, lack of recognition, and absent requirements for notification. As a result, the burden of pertussis is not recognized as clinically significant. Adding to the problem is that surveillance systems are often not established or data are collected only sparsely. Nevertheless, pertussis continues to be a serious health problem, especially among infants, in terms of both morbidity and mortality. In addition, because adolescent and adult pertussis is largely unrecognized, these age groups are not targeted for prevention and infected individuals are not treated, thereby facilitating spread of the disease in the community, including to vulnerable young infants. Finally, laboratory access for confirmatory diagnosis is very limited.
Until healthcare professionals in both resource-rich and research-poor countries diagnose their adolescent and adult pertussis patients correctly, the burden of disease will continue to be significantly underestimated. Without knowing that the disease predominantly occurs in this population, attempts to increase vaccine use in adolescents and adults are unlikely to be made.
HOW DO ADVANCES IN DIAGNOSTICS IMPACT CLINICAL PRACTICE?
In persons with pertussis, the median time from cough onset to seeking medical care differs by age group. For example, in 1 study setting, children aged 7–12 years were seen after 7.8 days of coughing, whereas adolescents aged 13–18 years were seen after 12.5 days and adults 17.3 days after symptoms began [18 and Riffelmann M, et al. unpublished data]. The interval from the onset of cough to when the patient seeks medical care has a major effect on the laboratory diagnosis of B. pertussis infection [19–22]. Culture obtained during the first 3 weeks of cough has 100% specificity, but low sensitivity, ranging from 20% to 80%, when compared with PCR and/or serology. In general, culture and PCR sensitivity is inversely related to age [23]. Other factors that may influence the sensitivity of culture are the type and quality of specimen, the type of transport media, and the duration of transport (optimally within 48 hours).
Real-time (RT)–PCR is more sensitive than culture and is the diagnostic method of choice in patients with cough illness of ≤3 weeks’ duration [19]. Selected issues with RT-PCR are presented in Table 3. In general, by the time most adults seek medical care, the time windows for both culture and RT-PCR have passed; therefore, serologic diagnosis should be the method of choice [18, 19].
Table 3.
Issues Relating to Real-Time Polymerase Chain Reaction and Pertussis Serology
| PCR: |
| • More expensive than culture |
| • May be difficult to perform (requires trained staff) and to implement outside the hospital setting (requires dedicated laboratory space) |
| • Sensitivity decreases with increasing cough duration |
| • Commercial kits are not widely available |
| • Subject to contamination, especially during outbreak situations |
| Serology: |
| • Testing is mostly done in immunologically nonnaive populations |
| • Testing is done with an antigen (pertussis toxin) that is contained in all acellular vaccines |
| • Immune response to vaccine antigens cannot be distinguished from response to infection |
| • Interpretation of serology depends on vaccination history |
| • Population-based cutoffs may need verification after change of vaccination calendar |
| • Problems in serodiagnosis of B. parapertussis infections |
Abbreviation: PCR, polymerase chain reaction.
Since all adults and most adolescents will have had a previous B. pertussis infection and/or pertussis immunization, they will have a rapid anamnestic antibody response to new B. pertussis infection; consequently, by the time they seek care for a persistent cough illness, they are likely to have developed high antibody levels to B. pertussis antigens [21]. PT is unique to B. pertussis and is highly immunogenic; therefore, it is the antigen that should be used for single serum diagnosis of B. pertussis cough illness. Single-serum IgG anti-PT testing has been used successfully in Massachusetts and in various countries in Europe for approximately 2 decades [24–26]. High levels of IgA and/or IgG antibodies to PT were described in many studies of prolonged cough illness as an accurate indicator of recent pertussis disease [21, 22, 24–29]. In Europe, single-serum serology for the diagnosis of pertussis has been intensively studied in the Netherlands [30], and commercial test kits with a variety of pertussis antigens and varying degrees of sensitivity and specificity are available [31]. EU reference laboratories have recently suggested recommendations for the serologic diagnosis of pertussis; these include mainly quantifying IgG antibodies to PT and reporting results in international units/mL [32, 33]. In the United States, tests done in commercial laboratories have varying degrees of sensitivity and specificity [18]. One widely used test has a specificity of ∼95% [34]. However, the tests with the greatest sensitivity and specificity are those that quantifiably measure IgG and IgA antibodies to PT (personal clinical observation of one of the authors [J. D. C.]). Selected issues with pertussis serology are presented in Table 3.
SUGGESTED CASE DEFINITIONS FOR PERTUSSIS
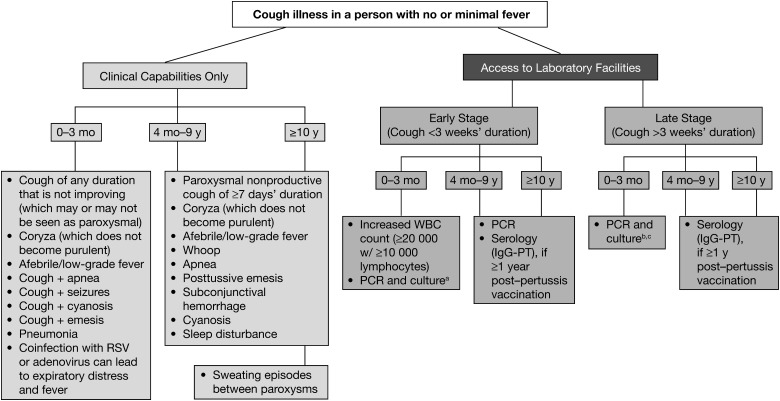
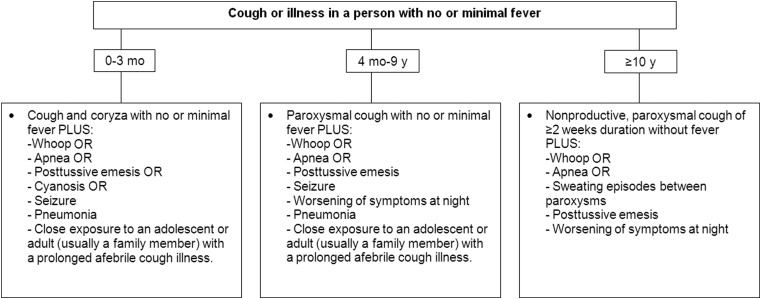
In recognition of the fact that the signs and symptoms of pertussis differ by age, we have tailored criteria for pertussis diagnosis in 3 different age cohorts (0–3 months, 4 months–9 years, and ≥10 years). These criteria are presented in Figure 1. In Figure 2, clinical case definitions of pertussis for surveillance purposes are presented.
Figure 1.
Algorithm for the diagnosis of pertussis. Abbreviations: IgG, immunoglobin G; PCR, polymerase chain reaction; PT, pertussis toxin; RSV, respiratory syncytial virus; WBC, white blood cell. aIn resource-limited areas where PCR is not available, samples may be sent to a reference laboratory for culture confirmation. bFalse-negatives possible. cSerology not useful in this age cohort.
Figure 2.
Clinical case definition of pertussis for surveillance purposes.
These case definitions are intended to: (1) be more specific and/or more sensitive than existing case definitions of pertussis (which were developed more than 40 years ago and were primarily designed either for surveillance purposes or for vaccine efficacy studies), (2) be applicable to both resource-rich and resource-poor settings, (3) encourage the increased use of laboratory confirmation, and (4) increase the sensitivity and specificity of pertussis reporting.
If a patient meets 1 or more of the criteria for pertussis diagnosis, the physician should treat the patient and report the case to the appropriate health agencies. General comments on the clinical presentation of pertussis and its laboratory diagnosis are presented in Tables 4 and 5, respectively.
Table 4.
General Comments on Clinical Presentation of Pertussis
| • Pertussis should be increasingly suspected in patients who are afebrile with increasing cough duration and severity |
| • Coryza is associated with illness onset and, in contrast with most viral respiratory infections, does not become purulent |
| • The key to identifying a paroxysmal cough is that the patient does not inhale until he has run out of breath (possibly resulting in an inspiratory “whoop”) |
| • Paroxysmal cough episodes are more disturbing to the patient at night |
| • Among young infants, apnea and seizures may not be noted to occur with recognized paroxysms |
| • Most infants with pertussis will have had a close exposure to an adolescent or adult (usually a family member) with a prolonged afebrile cough illness |
| • The cough in pertussis is not truly productive |
| • Sweating episodes occur in adolescents and adults in time periods when coughing is not occurring |
Table 5.
General Comments on Laboratory Diagnostics of Pertussis
| • PCR and culture are most useful in the first 3 weeks after illness onset |
| • Serology should not be used to diagnose pertussis in patients <1 year after inoculation with an acellular or whole-cell vaccine formulation |
| • IgG anti-PT ELISA is preferred to IgA anti-PT testing because the IgA response following infection is less common, and thus a negative IgA anti-PT test should not be relied upon as diagnostic evidence of a pertussis infection |
| • The attendees strongly discouraged the use of DFA to detect B. pertussis. They also strongly discouraged the use of ELISA tests that employed whole B. pertussis as the antigen |
Abbreviations: DFA, direct fluorescent antibody; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobin; PCR, polymerase chain reaction; PT, pertussis toxin.
There are a number of strong indicators of pertussis that differ by age group. In young infants, the occurrence of coryza and cough in an afebrile child is usually not alarming. When these young infants are seen by physicians, they are thought to have a viral respiratory infection, and the parents are reassured. However, over the next day or two, the parents recognize the worsening of symptoms, but more often than not, the physicians do not (based on author experience in California in 2010 [J. D. C.]). The key indicators of pertussis in these young infant cases are the afebrile nature of the illness combined with a cough that is increasing in frequency and severity and a coryza that remains watery. Therefore, the presence of this triad would be expected to have high sensitivity and good specificity. The addition of apnea, seizures, cyanosis, emesis, or pneumonia would result in both high sensitivity and specificity. In these young infant cases, an elevated white blood cell count (≥20 000 cells/µL) with absolute lymphocytosis is virtually diagnostic.
In older children (4 months to 9 years), the presence of a worsening paroxysmal, nonproductive cough of ≥7 days’ duration in an afebrile child with coryza that has not become purulent also would indicate high sensitivity and good specificity for pertussis. As noted with current case definitions, the addition of whoop, apnea, and posttussive emesis will each increase specificity. In those persons ≥10 years of age, the same triad listed above for those 4 months to 9 years would also result in high sensitivity with good specificity. In addition, the notation of sweating episodes between paroxysms will significantly increase specificity. In dealing with adult patients, it is important to ask specific questions about productive cough. Adults will often say that the cough is productive, but on further questioning, it is apparent that they actually do not produce purulent sputum.
The case definitions of pertussis delineated here should first be tested in clinical trials to determine their utility to the average clinician and then be compared with existing case definitions to determine whether they confer increased sensitivity and/or specificity. Although retrospective analyses are subject to bias, such analyses could be performed first in a “proof-of-principle” approach. If the new case definitions appear promising, a prospective study should be conducted to evaluate the proposed diagnostic criteria in the 3 different age categories (0–3 months, 4 months to 9 years, ≥10 years).
The protocol we propose would involve the prospective evaluation of all persons in a defined population with cough illnesses of ≥7 days’ duration stratified into the 3 different age categories. The study populations should include geographic regions with different vaccine usage patterns (acellular, whole-cell, or both). Protocols could be adopted from the Adult Pertussis Trial (APERT) and the vaccine efficacy trial in Erlangen, Germany [12, 35]. In both of these studies, investigators contacted participants every 2 weeks, and all subjects with cough illness of ≥7 days that was not improving were evaluated. Intensive education programs will be necessary to prevent observer bias [36]. Studies should be of such duration that they cover the cyclical epidemiologic patterns of pertussis and include populations of sufficient size to allow statistical analysis.
The development and utilization of 3 age-related definitions for pertussis can be expected to increase both the sensitivity and specificity in its diagnosis, which will result in the recognition of pertussis in all age groups, potentially leading to better control of pertussis.
Notes
Acknowledgments. All listed authors were in attendance at the February 2011 meeting. The first draft of this manuscript was written by James Cherry, MSc, MD, with assistance from Tina Tan, MD. All authors then reviewed and commented on the manuscript. Medical writing assistance (ie, assisting with the incorporation of author comments) was provided by Tiffany DeSimone, PhD, of PAREXEL. Editorial assistance (ie, formatting and stylization) was provided by Bari Samson of PAREXEL.
Financial support. This work was supported by an unrestricted educational grant from Sanofi Pasteur. Medical writing and editorial assistance were funded by Sanofi Pasteur.
Potential conflicts of interest: J. C. has received consulting fees and travel expenses from Sanofi Pasteur, and is on the speakers’ bureau of Sanofi Pasteur and GlaxoSmithKline. T. T. has received consulting fees from Sanofi Pasteur, travel expenses from Sanofi Pasteur and Merck, is on the speakers’ bureau of Wyeth/Pfizer, has received payment for educational presentations from Sanofi Pasteur, Merck, and Wyeth/Pfizer, and has grants from Sanofi Pasteur and Merck. C-H. W. von K. has consulted for Sanofi Pasteur, MSD, and is on the speakers’ bureau of Sanofi Pasteur MSD and GlaxoSmithKline Biologicals. K. D. F. has received travel expenses from Sanofi Pasteur. D. G. and D. J. are employed by, and have stock options with, Sanofi Pasteur. C. M. has received travel expenses from Sanofi Pasteur, has consulted for Pfizer, Sanofi Pasteur, and GlaxoSmithKline, has grants from GlaxoSmithKline, Merck, MedImmune, Novartis, and Pfizer, and is on the speakers’ bureau of GlaxoSmithKline and Sanofi Pasteur. S. P. has consulted for, and is on the speakers’ bureau for, Sanofi Pasteur. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cherry JD, Grimprel E, Guiso N, et al. Defining pertussis epidemiology. Pediatr Infect Dis J. 2005;24(5 suppl):S25–4. doi: 10.1097/01.inf.0000160926.89577.3b. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Department of Vaccines and Biologicals. Pertussis Surveillance: A Global Meeting. WH/V&B/01.19. Geneva: World Health Organization; 2001. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Appendix B. CDC and Council of State and Territorial Epidemiologists (CSTE) pertussis case definition. MMWR Recomm Rep. 2006;55(RR03):36. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5503a3.htm. Accessed 20 September 2011. [Google Scholar]
- 4.Guiso N, Wirsing von König CH, Forsyth K, Tan T, Plotkin SA. The Global Pertussis Initiative: report from a round table meeting to discuss the epidemiology and detection of pertussis, Paris, France, 11–12 January 2010. Vaccine. 2011;29:1115–21. doi: 10.1016/j.vaccine.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 5.EU case definition Pertussis: Commission Decision of 28 April 2008 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No. 2119/98/EC of the European Parliament and of the Council, Official Journal of the European Union. Available at: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:159:0046:0090:EN:PDF#page=25. Accessed 20 September 2011. [Google Scholar]
- 6.Australian National Notifiable Diseases Case Definition: Pertussis. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-cd_pertus.htm. Accessed 20 September 2011. [Google Scholar]
- 7.PAHO case definition. Available at: http://www.paho.org/english/sha/be994diphtpert.htm. Accessed 20 September 2011. [Google Scholar]
- 8.André P, Caro V, Njamkepo E, et al. Comparison of serological and real-time PCR assays to diagnose Bordetella pertussis infection in 2007. J Clin Microbiol. 2008;46:1672–7. doi: 10.1128/JCM.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanaie RM, Karimi A, Sadeghi H, et al. Sensitivity and specificity of the World Health Organization pertussis clinical case definition. Int J Infect Dis. 2010;14:e1072–5. doi: 10.1016/j.ijid.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Harnden A, Grant C, Harrison T. Whooping cough in school age children with persistent cough: prospective cohort study in primary care. BMJ. 2006;333:174–7. doi: 10.1136/bmj.38870.655405.AE. Epub 2006 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Harnden A. Pertussis-induced cough. Pulm Pharmacol Ther. 2011;24:304–7. doi: 10.1016/j.pupt.2010.10.011. Epub 2010 Oct 27. [DOI] [PubMed] [Google Scholar]
- 12.Strebel P, Nordin J, Edwards K, et al. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995–1996. J Infect Dis. 2001;183:1353–9. doi: 10.1086/319853. [DOI] [PubMed] [Google Scholar]
- 13.Patriarca PA, Biellik RJ, Sanden G, et al. Sensitivity and specificity of clinical case definitions of pertussis. Am J Public Health. 1998;78:833–6. doi: 10.2105/ajph.78.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stehr K, Cherry JD, Heininger U, et al. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics. 1998;101(1 Pt 1):1–11. doi: 10.1542/peds.101.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23:985–9. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- 16.Wendelboe AM, Njamkep E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293–9. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 17.Kowalzik F, Barbosa AP, Fernandes VR, et al. Prospective multinational study of pertussis infection in hospitalized infants and their household contacts. Pediatr Infect Dis J. 2007;26:238–42. doi: 10.1097/01.inf.0000256750.07118.ee. [DOI] [PubMed] [Google Scholar]
- 18.Tondella ML, Carlone GM, Messonnier N, et al. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19–20 July 2007. Vaccine. 2009;27:803–14. doi: 10.1016/j.vaccine.2008.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention Diagnosis Confirmation. Available at: http://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-confirmation.html. Accessed 14 February 2011. [Google Scholar]
- 20.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:323–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mink CM, Cherry JD, Christenson P, et al. A search for Bordetella pertussis infection in university students. Clin Infect Dis. 1992;14:464–71. doi: 10.1093/clinids/14.2.464. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt-Grohé S, Cherry JD, Heininger U, et al. Pertussis in German adults. Clin Infect Dis. 1995;21:860–6. doi: 10.1093/clinids/21.4.860. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Kamachi K, Toyoizumi-Ajisaka H, et al. Marked difference between adults and children in Bordetella pertussis DNA load in nasopharyngeal swabs. Clin Microbiol Infect. 2011;17:365–70. doi: 10.1111/j.1469-0691.2010.03255.x. [DOI] [PubMed] [Google Scholar]
- 24.Guiso N, Wirsing von Koenig CH, Forsyth K, Tan T, Plotkin SA. The global pertussis initiative: report from a round table meeting to discuss the epidemiology and detection of pertussis, Paris, France, 11–12 January 2010. Vaccine. 2011;29:1115–21. doi: 10.1016/j.vaccine.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Yih WK, Lett SM, des Vignes FN, et al. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989–1998. J Infect Dis. 2000;182:1409–16. doi: 10.1086/315863. [DOI] [PubMed] [Google Scholar]
- 26.Marchant CD, Loughlin AM, Lett SM, et al. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis. 1994;169:1297–305. doi: 10.1093/infdis/169.6.1297. [DOI] [PubMed] [Google Scholar]
- 27.Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA. 1995;273:1044–6. [PubMed] [Google Scholar]
- 28.Nenning ME, Shinefield HR, Edwards KM, et al. Prevalence and incidence of adult pertussis in an urban population. JAMA. 1996;275:1672–4. [PubMed] [Google Scholar]
- 29.Vincent JM, Cherry JD, Nauschuetz WF, et al. Prolonged afebrile nonproductive cough illnesses in American soldiers in Korea: a serological search for causation. Clin Infect Dis. 2000;30:534–9. doi: 10.1086/313707. [DOI] [PubMed] [Google Scholar]
- 30.de Melker HE, Versteegh FG, Schellekens JF, et al. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53:106–13. doi: 10.1016/j.jinf.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Kennerknecht N, Riffelmann M, Schmetz J, Wirsing von König CH. Comparison of commercially available immunoblot assays measuring IgG and IgA antibodies to Bordetella pertussis antigens. Eur J Clin Microbiol Infect Dis. 2011;30:1531–5. doi: 10.1007/s10096-011-1256-4. [DOI] [PubMed] [Google Scholar]
- 32.Riffelmann M, Thiel K, Schmetz J, Wirsing von Koenig CH. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. J Clin Microbiol. 2010;48:4459–63. doi: 10.1128/JCM.01371-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guiso N, Berbers G, Fry NK, He Q, Riffelmann M, Wirsing von König CH EU Pertstrain group. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur J Clin Microbiol Infect Dis. 2011;30:307–12. doi: 10.1007/s10096-010-1104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prince HE, Lapé-Nixon M, Matud J. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin Vaccine Immunol. 2006;13:266–70. doi: 10.1128/CVI.13.2.266-270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward JI, Cherry JD, Chang S-J, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353:1555–63. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 36.Cherry JD, Heininger U, Stehr K, Christenson P. The effect of investigator compliance (observer bias) on calculated efficacy in a pertussis vaccine trial. Pediatrics. 1998;102(Pt 1):909–12. doi: 10.1542/peds.102.4.909. [DOI] [PubMed] [Google Scholar]