Abstract
The degree of fat deposition in muscle and its implications for obesity-related complications in children and youths are not well understood. One hundred and fifty-nine patients (mean age: 13.3 years; range: 6–20) with a body mass index (BMI) >90th percentile for age and sex were included. Muscle fat content (MFC) was measured in the psoas muscle by proton magnetic resonance spectroscopy. The patients were assigned to two groups: MFC <5% or ≥5%. Visceral adipose tissue volume (VAT) and subcutaneous adipose tissue volume (SAT) were measured by magnetic resonance imaging. The data were analysed to detect associations between MFC and BMI standard deviation scores, VAT and SAT, blood values, pubertal stages, and physical activity scores. The mean BMI standard deviation score (SDS) was 3.04 (range 1.32–5.02). The mean MFC was 8.9% (range 0.8–46.7), and 118 (74.2%) of 159 patients had an MFC ≥5%. Children with an MFC ≥5%, compared with children with an MFC <5%, had a higher BMI SDS (P=0.03), a higher VAT (P=0.04), and elevated intramyocellular lipid (IMCL) and extramyocellular lipid (EMCL) contents (both P<0.0001). SAT, SAT/VAT ratio, blood values, pubertal stages and physical activity scores did not differ between the two groups. Severely obese children and youths tend to have a high MFC, which is associated with elevated VAT, IMCL, and EMCL contents. An increased MFC may be associated with impaired metabolic processes, which may predispose these young people to obesity-related complications.
Key words: child, intramyocellular lipid, magnetic resonance spectroscopy, muscle fat content, obesity.
Introduction
Obesity is strongly associated with the incidence of cardiovascular disease, diabetes, fatty liver disease, and cancer.1 Decades ago, it became evident that ectopic fat deposition is an important predictor of cardiovascular disease and carries more risk than ordinary fat accumulation in subcutaneous fat deposits.2,3 The ectopic fat deposition in non-adipose tissue, including skeletal muscle, has deleterious effects including tissue damage (lipotoxicity) and the development of insulin resistance (IR).4 The cause of ectopic fat distribution seems to be related to the hyperlipolytic activity of the visceral adipose tissue, which induces a lipotoxic state and contributes to the exposure to excess free fatty acids. Excess fat distribution and exposure to excess free fatty acid impair insulin-dependent metabolic processes and lead to biochemical abnormalities, including hyperinsulinaemia and hypertriglyceridaemia.5
Muscle fat content (MFC) has been quantified using methods such as computer tomography scanning6,7 and biochemical extraction,8 which do not distinguish between intramyocellular lipid (IMCL) and extramyocellular lipid (EMCL). These studies showed that a high MFC is associated with IR in non-diabetic individuals,7,8 in people with type 2 diabetes mellitus9,10 or poorly controlled type 1 diabetes,9 in older people,11 and in patients with coronary artery disease.12 The development of proton magnetic resonance spectroscopy (MRS) allows the non-invasive and non-ionizing differentiation and quantification of lipid deposits in skeletal muscle and can distinguish between IMCL and EMCL.13,14 This technology is anticipated to further our understanding of lipid deposition in muscle and its association with complications related to obesity.
In the present study, the content of muscular fat was investigated by MRS in a large group of obese children and youths referred for childhood obesity treatment.15 The hypothesis to be tested was whether the degree of fat in muscles was associated with general or central obesity and/or physical activity/inactivity. The participants were assigned to two groups based on the fat content in their psoas muscle: MFC <5% or ≥5%. The objective of this study was to quantify the MFC percentage and to investigate possible associations with anthropometric data, visceral adipose tissue volume (VAT), subcutaneous adipose tissue volume (SAT), biochemical measurements, pubertal stage, physical activity score (PAS), and physical inactivity score (PIS).
Materials and Methods
Study population
One hundred and ninety-two obese children and youths were enrolled consecutively from August 2009 to August 2010 at The Children's Obesity Clinic, Department of Paediatrics, Copenhagen University Hospital, Holbæk, Denmark.15 The inclusion criteria were 6–20 years of age and a body mass index (BMI) >90th percentile by age- and sex-adjusted reference tables.16 The exclusion criteria were inability to remain calm in the magnetic resonance (MR) scanner for 45 minutes or a body weight >130 kg, which was the maximum capacity for the MR scanner. Eight patients had a body weight >130 kg and where thus not offered an MR scan. Twenty-seven patients did not attend their planned MR examination, leaving 159 patients for the study.
To elucidate the differences between those with a relatively low MFC and those with a relatively high MFC, the study sample was divided in two groups according to the amount of MFC. These groups were arbitrarily defined as: MFC <5% and MFC ≥5%. This limit was chosen because a 5% limit for ectopic fat deposition is also used to study non-alcoholic fatty liver disease17 and since there is no widely accepted limit of fat content in muscles.
All participants underwent a complete physical examination and provided a detailed medical history, including interview-reported information on physical activity and inactivity. The PAS included organized sports (soccer, tennis, dance, etc.) and unorganized activities (trampoline, walking, cycling, etc.). The PAS was defined as the number of hours spent performing physical activities and was summed to obtain the number of hours per week. The activities being a scout, unorganized play, unorganized walking, and bowling were considered to represent a lower physical activity level than traditional organized sports. The reported estimated time spent on these particular activities was thus reduced by 50% in the calculation of PAS. The PIS was defined as the number of hours spent in front of a computer, television, or game console and was summed to obtain the number of hours per week. The activity scores were calculated to test whether the least physical active exhibited the highest content of muscular fat.
Blood samples were available in 119 of the 159 individuals and were acquired within 122 days from the date of the MR scan. Informed written consent was obtained from the parents of children younger than 18 years and from patients 18 years of age and older. The study was approved by the Ethics Committee of the Region Zealand in Denmark (ID no.: SJ-98 and SJ-104) and the Danish Data Protection Agency, and is registered at ClinicalTrials.gov (ID no.: NCT00823277 and NCT00928473).
Anthropometry
Weight was measured to the nearest 0.1 kg on a Tanita digital medical scale (WB-100 MA; Tanita Corp., Tokyo, Japan). Height was measured by stadiometer to the nearest 1 mm. Weight and height were measured in light indoor clothes with empty pockets and without shoes. BMI was calculated as weight/height squared (kg/m2). The BMI standard deviation score (SDS) was calculated by the least-mean-squares method by converting BMI into a normal distribution by sex and age using the median, coefficient of variation, and a measure of the skewness based on the Box Cox power plot based on Danish BMI charts.16 Tanner stage (development of genitals and pubic hair in boys and development of breasts and pubic hair in girls) and testicular size were determined by a paediatrician according to accepted criteria.18
Magnetic resonance spectroscopy and magnetic resonance imaging
MR measurements were performed on an Achieva 3.0 T MR imaging system (Philips Medical Systems, Best, The Netherlands) using a SENSE cardiac coil. Patients were examined in the supine position.
The MFC and the EMCL/IMCL ratio were measured by MRS. IMCL and EMCL were calculated using the values of MFC and the EMCL/IMCL ratio; MFC equals the sum of the IMCL and EMCL. T2-weighted turbo spin echo (TSE) coronal and axial slices through the abdomen were acquired to position the spectroscopy volumes of interest (VOIs). The parameters for the TSE sequence were: TSE factor=93, repetition time (TR)=2182 ms, echo time (TE)=80 ms, and field of view (FOV)=420 mm. The spectroscopy VOI (11 mm×11 mm×11 mm) was positioned within the psoas muscle. To obtain the spectroscopic MFC, a single voxel spectrum was recorded using the PRESS sequence with TE=75 ms, TR=4000 ms, and 32 averages. The MR scanner's software was used to fit the acquired spectrum to the relative content of water and fat. To determine the EMCL/IMCL ratio, a single voxel spectrum was recorded using the PRESS sequence with water saturation, TE=38 ms, TR=4000 ms, and 80 averages in the same VOI. Spectroscopic MFC was expressed as fat content relative to water and was calculated as: spectroscopic fat (%)=(fat metabolite area/(fat metabolite area+water metabolite area))×100%. The IMCL% expresses the volume fraction of lipid droplets within the myocyte cytoplasm as a percentage of the total muscle volume. The EMCL% expresses the fraction of adipocytes interlaced between the muscle fibres as a percentage of the total muscle volume.
VAT and SAT volumes were measured by MRI. A fast T1-weighted turbo field echo (TFE) MR sequence in the transverse plane was used to obtain images for estimating the adipose tissue volumes (TFE sequence, TFE factor=136, TR=10 ms, TE=2.3 ms, FOV=480 mm, and a respiratory trigger compensation with trigger delay of 1000 ms). A transverse slice of 10 mm thickness was acquired for all subjects in the middle of the third lumbar vertebra (L3). The volumes of visceral and subcutaneous fat at L3 were measured in cm3 using segmentation tool in volume analysis on the Philips ViewForum workstation.
Blood sampling
Blood samples were drawn intravenously from an antecubital vein after 12 hours of fasting. If required, an anaesthetic cream was applied 60 min before venepuncture. The biochemical analyses were performed on a Cobas® 6000 analyser (Roche Diagnostics, Rotkreuz, Switzerland) immediately after the blood samples were obtained. Measurements included the concentrations of triglycerides (TGs), gamma-glutamyl transferase (GGT), alanine transaminase (ALT), lactate dehydrogenase (LDH), and alkaline phosphatase (AP).
Statistical analysis
The two groups of MFC (below 5% and above 5%, respectively) were analysed according to associations with age, BMI SDS, MFC, IMCL, EMCL, EMCL/IMCL ratio, SAT, VAT, SAT/VAT ratio, PAS, PIS, blood values, and pubertal stages using t tests. Values of SAT, VAT, pubertal stages, PAS, PIS, and concentrations of TG, GGT, ALT, LDH, and AP had non-normally distributed residuals and were logarithmically transformed prior to statistical analysis. The results are presented as means with SD and medians with ranges, respectively. Further, we divided each group of MFC into boys and girls and repeated the analyses. By chi-square test we analysed associations between MFC and BMI SDS, associations between IMCL and EMCL and TG concentration, the differences in MFC between sexes, and pubertal stage by menarche for the girls. Logistic regression analysis was used to investigate the associations between MFC and the degree of obesity, SAT, and VAT. P<0.05 were considered significant. Analyses were performed using SAS® (version 9.2; SAS Institute Inc., Cary, NC).
Results
The baseline characteristics of the included obese children and youths are summarized in Table 1. The mean MFC in 159 children and youths was 8.9% (range: 0.8%–46.7%). One hundred and eighteen patients (74.2%), comprising 51 boys and 67 girls, had an MFC ≥5%.
Table 1. Baseline characteristics of all 159 obese children and youths with a muscle fat content <5% and ≥5%, respectively.
| Total | MFC <5% | MFC ≥5% | MFC <5% (n=41) | MFC ≥5% (n=118) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=159) | (n=41) | (n=118) | P | boys | girls | P | boys | girls | P | |
| N (boys/girls) | 159 (71/88) | 41 (20/21) | 118 (51/67) | 0.54 | 20 | 21 | 51 | 67 | ||
| Age (years) | 13.3±2.9 | 13.5±2.6 | 13.2±3.0 | 0.51 | 13.6±2.3 | 13.5±3.0 | 0.93 | 12.8±2.5 | 13.8±3.3 | 0.19 |
| BMI SDS | 3.04±0.6 | 2.82±0.7 | 3.11±0.5 | 0.03 | 3.1±0.8 | 2.6±0.6 | 0.02 | 3.4±0.5 | 2.9±0.5 | <0.0001 |
| MFC (%) | 8.9±7.0 | 3.3±1.1 | 10.9±7.2 | <0.0001 | 3.0±1.1 | 3.6±1.1 | 0.05 | 10.3±6.1 | 11.3±8.0 | 0.47 |
| IMCL (%) | 2.6±1.9 | 1.4±0.8 | 3.1±2.0 | <0.0001 | 2.53±2.3 | 2.3±1.8 | 0.67 | 2.6±2.0 | 2.6±1.7 | 0.56 |
| EMCL (%) | 6.3±6.0 | 1.9±0.9 | 7.8±6.3 | <0.0001 | 4.20±3.37 | 5.3±3.8 | 0.94 | 4.5±3.7 | 8.6±7.7 | 0.88 |
| EMCL/IMCL ratio | 2.9±2.6 | 2.1±1.7 | 3.2±2.8 | 0.003 | 2.1±1.6 | 2.0±1.9 | 0.87 | 3.7±3.7 | 2.5±1.4 | 0.07 |
| VAT (cm3)* | 89 (30-258) | 83 (36-186) | 94 (30-258) | 0.04 | 88.5 (47-186) | 67 (36-171) | 0.06 | 98 (30-258) | 91 (36-217) | 0.16 |
| SAT (cm3)* | 310 (104-752) | 285 (104-647) | 319 (108-752) | 0.12 | 298 (108-570) | 283 (104-647) | 0.47 | 311 (125-628) | 343 (108-752) | 0.77 |
| SAT/VAT ratio* | 3.4 (1.6-10.3) | 3.8 (1.6-9.7) | 3.3 (1.7-10.3) | 0.41 | 3.35 (2-5.9) | 3.9 (1.6-9.7) | 0.26 | 3.7 (1.7-10.3) | 3.4 (1.9-8.3) | 0.05 |
| Tanner ♂G* | 2 (1-5) | 2 (1-4) | 1 (1-5) | 0.67 | ||||||
| Tanner ♂P* | 2 (1-5) | 1 (1-4) | 2 (1-5) | 0.94 | ||||||
| Tanner ♀B* | 3 (1-5) | 3 (1-5) | 2 (1-5) | 0.22 | ||||||
| Tanner ♀P* | 3 (1-5) | 3 (1-5) | 2 (1-5) | 0.17 | ||||||
| PAS (hrs/week)* | 1.0 (0.0-8.0) | 1.5 (0.0-7.0) | 1.0 (0.0-8.0) | 0.47 | 1.13 (0-4.5) | 0 (0-5.25) | 0.87 | 1.0 (0-8) | 1.75 (0-8) | 0.04 |
| PIS (hrs/week)* | 24.5 (3.5-70) | 10.5 (3.5-70) | 28 (14-70) | 0.08 | 21 (7-49) | 24.5 (3.5-70) | 0.92 | 28 (3.5-70) | 24.5 (3.5-70) | 0.16 |
Data are unadjusted mean±standard deviation.
Data are median (range). MFC, muscle fat content; BMI, body mass index; SDS, standard deviation score; IMCL, intramyocellular lipid content; EMCL, extramyocellu- Lar lipid content; VAT, visceral adipose tissue volume; SAT, subcutaneous adipose tissue volume; G, genitals; P, pubic hair; B, breast; PAS, physical activity score; PIS, physical inactivity score.
Compared with the group with an MFC <5%, the group with an MFC ≥5% had a higher BMI SDS (3.1±0.5 (SD) vs. 2.8±0.7, P=0.03) and higher VAT (median 94 cm3, range: 30–258 vs. median 83 cm3, range: 36–186, P=0.04) (Table 1). The two groups did not differ on sex distribution (P=0.54), age (mean 13.2 years±3.0, range: 6–20 vs. 13.5 years±2.6, range: 8–18; P=0.51), SAT (P=0.12), SAT/VAT ratio (P=0.47), pubertal stages ((Tanner; 0.17≤ P≤0.94), left testicle size (median 4 mL, range: 1–19 vs. 5 mL, range: 2–20, P=0.18), or right testicle size (median 3 mL, range: 1–18 vs. 5 mL, range: 2–20, P=0.09), and menarche (P=0.24)). The regression analyses between MFC and BMI SDS, VAT, and SAT, respectively, are shown in Figure 1.
Figure 1.
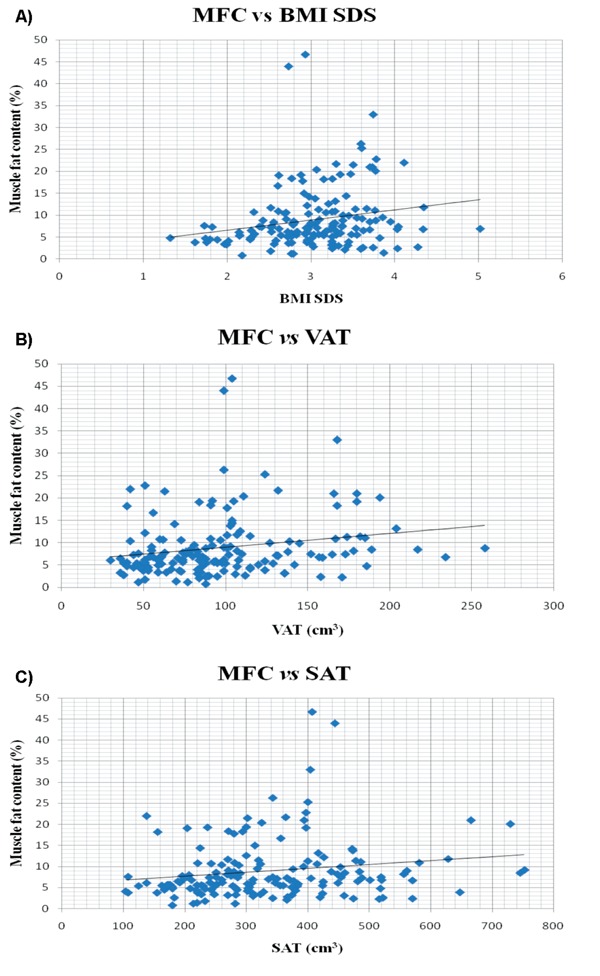
Regression analyses between MFC and BMI SDS, VAT, and SAT in 159 children and youths included in the study. A) Linear regression analysis plot of MFC and BMI SDS with Pearson's correlation coefficient (r2=0.040, 95% CI=[-0.116; 0.194], P=0.012). Equation: y=2.32x+1.90, effect size=2.32, 95% CI=[0.52; 4.12]. B) Linear regression analysis plot of MFC and BMI SDS with Pearson's correlation coefficient (r2=0.039, 95% CI=[-0.117; 0.193], P=0.013). Equation: y=0.03x+5.92, effect size=0.03, 95% CI=[0.01; 0.06]. C) Linear regression analysis plot of MFC and BMI SDS with Pearson's correlation coefficient (r2=0.029, 95% CI=[-0.127; 0.184], P=0.031). Equation: y=0.01x+5.85, effect size: 0.01, 95% CI=[0.00; 0.02]. MFC, muscle fat content; BMI, body mass index; SDS, standard deviation score; VAT, visceral adipose tissue volume; SAT, subcutaneous adipose tissue volume.
The mean IMCL% was 3.1%±2.0 in patients with an MFC ≥5% and 1.4%±0.8 in those with an MFC <5% (P<0.0001); the respective values for mean EMCL% were 7.8%±6.3 vs. 1.9%±0.9, (P<0.0001) and the EMCL/IMCL ratios were 3.2±2.8 vs. 2.1±1.7 (P=0.003) (Table 1). The mean MFC among the 159 participants did not differ significantly between boys and girls (7.9%±5.4% vs. 9.5%±7.7%, respectively, P=0.28) (Table 1).
PAS was a median of 1.0 hours/week (range 0.0–8.0) and this did not differ significantly between the groups with MFC <5% and ≥5%, respectively, (P=0.47) (Table 1). PIS was a median of 24.5 hours/week (range 3.5–70) and this did not differ significantly between the groups with MFC <5% and ≥5%, respectively, (P=0.08) (Table 1).
Blood values were distributed uniformly in the two MFC groups (Table 2). There were no differences in mean MFC (P=0.08), BMI SDS (P=0.35), or age (P=0.07) between the 119 patients with MRS, anthropometric, and biochemical measurements and the 40 patients who were investigated by MRS and anthropometric measurements alone. We found no associations between the TG concentration and IMCL content (P=0.71) or between the TG concentration and EMCL content (P=0.82). All blood samples were attempted to be performed in close proximity to treatment initiation (median 9 days, range: 0–170). Blood samples were acquired a median of 41 days (range: 0–122) from the MR examination. Ninety-six blood samples were acquired before the MR examination, and 17 blood samples were acquired after the MR examination.
Table 2. Blood values in 119 of the obese children and youths investigated with proton magnetic resonance spectroscopy and magnetic resonance imaging.
| Total | MFC <5% | MFC ≥5% | MFC <5% (n=35) | MFC ≥5% (n=84) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=119) | (n=35) | (n=84) | P | Boys | Girls | P | Boys | Girls | P | |
| N (boys/girls) | 119 (56/63) | 35 (17/18) | 84 (39/45) | 17 | 18 | 39 | 45 | |||
| Age (years) | 13.0±2.8 | 13.5±2.6 | 12.9±2.9 | 0.56 | 13.4±2.4 | 13.4±2.9 | 0.83 | 12.5±2.3 | 13.3±2.3 | 0.19 |
| BMI SDS | 3.0±0.6 | 2.89±0.7 | 3.06±0.6 | 0.17 | 3.1±0.8 | 2.7±0.6 | 0.04 | 3.4±0.6 | 2.8±0.4 | <0.0001 |
| MFC% | 8.4±7.1 | 3.3±1.1 | 10.5±7.5 | <0.0001 | 2.9±1.1 | 3.6±1.1 | 0.08 | 9.7±5.6 | 11.2±8.8 | 0.39 |
| TG (mmol/l)* | 1.1 (0.3-5.8) | 1.1 (0.3-3.2) | 1.1 (0.3-5.8) | 0.80 | 1.1 (0.3-3.2) | 1.1 (0.5-2) | 0.82 | 0.85 (0.3-5.8) | 1.2 (0.5-2.4) | 0.06 |
| GGT (U/l)* | 17.0 (8-134) | 16.5 (8-134) | 17.0 (9-87) | 0.81 | 20 (13-134) | 15 (8-34) | 0.02 | 16.5 (9-87) | 17 (9-61) | 0.78 |
| ALT (U/l)* | 25.0 (12-93) | 14.0 (14-91) | 25.0 (12-93) | 0.68 | 30 (16-91) | 21.5 (14-49) | 0.04 | 25 (13-93) | 24 (12-87) | 0.50 |
| LDH (U/l)* | 220 (112-321) | 218.0 (123-281) | 220.0 (112-321) | 0.56 | 232 (170-271) | 183 (123-281) | 0.02 | 235 (112-321) | 200 (115-297) | 0.0092 |
| AP (U/l)* | 230 (50-432) | 230.5 (50-432) | 230.0 (67-396) | 0.64 | 255 (115-432) | 153 (50-307) | 0.003 | 258 (88-396) | 194 (67-389) | 0.0017 |
Data are unadjusted mean±standard deviation.
Data are median (range). MRS, proton magnetic resonance spectroscopy; MRI, magnetic resonance imaging; BMI, body mass index; SDS, standard deviation score; MFC, muscle fat content; TG, triglyceride; GGT, gamma-glutamyl transferase; ALT, alanine transaminase; LDH, lactate dehydrogenase; AP, alkaline phosphatase.
Discussion
In the present study, MR spectroscopy was used to non-invasively quantify the intra- and extramyocellular lipid content in 159 obese children and youths included in a multidisciplinary obesity treatment.15 We found that a substantial proportion (74.2%) of these patients had an MFC of ≥5%. A high MFC was correlated with a higher BMI SDS and a higher VAT, but not with sex, age, SAT, SAT/VAT-ratio, pubertal stage, testicular size, menarche, PAS, PIS, or biochemical measures including liver enzymes. As expected, the MFC was strongly associated with both IMCL and EMCL. The IMCL was twice as high and the EMCL four times higher in patients with a high MFC compared with those with an MFC <5%, suggesting that the EMCL accumulates more readily than the IMCL.
An association between MRS-assessed muscular fat deposition and BMI has been reported in two smaller studies on children and adolescents (n=22 and n=29, respectively).19,20 We also found an association between MFC and BMI SDS in this larger group of obese children and youths. However, we used BMI SDS as a measure of obesity that also takes into account the growth and development in boys and girls.
Glycolytic muscles generally contain less fat than oxidative muscles.21,22 In the present study, the amount of fat deposited as IMCL measured in the mainly glycolytic psoas major muscle was higher than that reported by Bredella et al. for the mainly glycolytic tibialis anterior muscle in 21 obese adult women (mean BMI 34 kg/m2) assessed by MRS.23 Compared with studies in children and adolescents, the IMCL was slightly higher in our study than that measured in the mainly oxidative soleus muscle in two groups, each comprising 14 obese youth with normal glucose tolerance (mean BMI 35 kg/m2; age 11–15 years and mean BMI 39 kg/m2; BMI SDS 2.54; age 13.5 years),19,24 and in 14 obese youth with impaired glucose tolerance (mean BMI 37 kg/m2; BMI SDS 2.48; age 13 years).24 The IMCL in the present study was also higher than that reported in studies of 17 and 20 lean adults23,25 and in eight lean adolescents.19 These findings suggest that deposition of fat in skeletal muscle tissue is not related to age, but is promoted by the increased amount of lipids available in the natural course of severe obesity, as indicated by the high MFC in these young, severely obese patients in the present study.
Sinha et al. found a significant association between VAT and both IMCL and EMCL in 14 obese (mean BMI 35 kg/m2; age 11–15 years) and eight lean children.19 Similarly, Saukkonen et al. found increased IMCL and VAT in eight obese children with impaired glucose tolerance and 13 obese children with normal glucose tolerance (mean BMI 36 kg/m2; age 11–15 years and BMI 35 kg/m2; age 11–15 years) compared with eight non-obese siblings.20 These relationships are similar to the association between MFC and VAT found in the present study, suggesting that the development of visceral fat depot and muscular fat content might not be independent of each other. However, MFC was not associated with liver enzyme concentrations in the present study, suggesting that fat deposition in the muscle is independent of the liver status.
Plasma TG concentration correlated positively with IMCL content, but not with EMCL content, in a study of 14 obese (mean BMI 35 kg/m2; age 11–15 years) and eight lean children.19 However, we could not confirm this association between TG concentration and MFC, IMCL or EMCL contents even though we included a large group of children. This finding is intriguing and may suggest a great variability in plasma TG concentration in children and youths, even though blood samples were acquired in relative proximity to the initiation of treatment. A confounding factor is the time gap between blood samples and MR examinations in the present study, which was a median of 41 days in the present study. The MR examination was done after treatment initiation in the majority of the patients (96 of 119), which might tend to show a decreased MFC due to obesity treatment, which may have biased the relationships studied.
Although the role of puberty in accumulation of IMCL and muscular fat is not fully determined, one study has shown elevated IMCL content in 5 lean girls with premature adrenarche (mean BMI SDS 0.65; age 7.8 years) compared with prepubertal controls,26 which may suggest that the IMCL content is affected by pubertal development per se. In the present study we did not find associations between pubertal stages and MFC or IMCL.
An association between physical activity and IMCL has been reported in healthy, lean adult males,27,28 but we did not confirm this association in the present study. However, it is difficult to compare these studies directly because of divergent methods, differences in exercise and workload duration, and the fact that the above-mentioned studies27,28 included only healthy, lean adult males whereas the obese children and youths in the present study may have great difficulty in attaining a high intensity of physical activity. Although the present study did not show a significant association between PIS and MFC (P=0.08), the data might indicate a tendency that children and youths performing high levels of inactivity exhibit a high MFC.
We acknowledge that several confounding factors may have biased the results in the present study. One limitation of our study was the lack of a control group, which would have been desirable for comparing the results in obese patients with normal-weight children and youths. A second limitation was that the extremely obese (>130 kg) were excluded from investigation because they could not enter the MR scanner; this precluded the most extreme obese patients from examination, even though they had the highest probability of having a high MFC. MFC and IMCL are dependent of many factors, such as diet,28 morning-to-evening changes in MFC,28 exercise duration,27,28 exercise workload,27,28 pubertal development,26 muscle type,21,22,29 age,30 sex,31,32 and obesity.29,31 In the present study, we did not record dietary details, although the participants were given specific individual guidelines about food groups to eat or avoid as part of the obesity treatment protocol.15 These guidelines included the generally recommended diet low in carbohydrates and fat. The 5% limit in MFC was defined arbitrarily because no studies have defined a widely accepted MFC limit. We used the 5% limit because ectopic fat deposition is thought to be pathological and because this is similar to the limit used in the characterization of liver fat.17 The MRS examinations were not conducted in the fasting condition, which may have caused minor variations in the degree of MFC depending on the diet and time since the last meal consumed. To avoid morning-to-evening changes, the MRS examinations occurred between the hours of 9 and 11 in the morning. We had interview-reported approximate estimates of the duration of physical activity and inactivity, which makes the PAS and PIS rough estimates. Unfortunately, we did not record physical activity and inactivity on a daily basis or the exercise workload or intensity.
Finally, fat deposition in skeletal muscle is a patchy disease as both IMCL and EMCL are distributed inhomogeneously.33,34 Therefore, some variation should be expected, depending on the placement of the spectroscopy VOI, which we did not adjust for by measuring several positions in the psoas muscle at the same time. Instead, measurements by MRS were made in the same muscle (psoas major) on approximately the same anatomical site in all participants. The present MRS data were not corrected for the effect of transverse relaxation time,35 but such adjustments have not traditionally been performed in previous studies.28,33 However, the calculated MFC%, IMCL%, and EMCL% in the present study may deviate somewhat from the objective values. Correction for longitudinal relaxation time was not necessary because of the long TR.
The strength of this study is the relatively large number of participants investigated using a non-invasive, non-ionizing, and precise technology, which showed that most of the patients had increased fat content in their muscles.
Conclusion
Most obese children and youths are prone to having elevated MFC, which is reflected by increased fat deposition in the intra- and extramyocellular lipid compartments and is associated with an elevated VAT. Mounting evidence links general obesity and elevated muscular fat and visceral adipose tissue volume to an increased metabolic risk profile.19,20,24,36,37 It is important to identify and monitor the development of MFC from an early age in obese children.
Acknowledgments:
this study is part of the research activities in The Danish Obesity Research Centre (DanORC, www.danorc.dk) and The Danish Childhood Obesity Biobank; ClinicalTrials.gov ID-no.: NCT00928473. With gratitude to Mrs O. Troest for thorough technical assistance.
References
- 1.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. [Google Scholar]
- 2.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37:841–56. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson B, Svärdsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J. 1984;288:1401–4. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gastaldelli A, Natali A, Vettor R, Corradini SG. Insulin resistance, adipose depots and gut: interactions and pathological implications. Dig Liver Dis. 2010;42:310–9. doi: 10.1016/j.dld.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481–90. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Colberg SR, Simoneau JA, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest. 1995;95:1846–53. doi: 10.1172/JCI117864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–8. [PubMed] [Google Scholar]
- 8.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–8. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 9.Falholt K, Jensen I, Lindkaer Jensen S, et al. Carbohydrate and lipid metabolism of skeletal muscle in type 2 diabetic patients. Diabet Med. 1988;5:27–31. doi: 10.1111/j.1464-5491.1988.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fröberg SO. Muscle triglycerides. Relation to glycogen in muscle and plasma triglycerides in men of different ages. Acta Med Scand. 1973;193:463–8. [PubMed] [Google Scholar]
- 12.Fröberg SO. Concentration of cholesterol and triglycerides in skeletal muscle of healthy men and myocardial infarction patients. Acta Med Scand. 1973;194:553–8. doi: 10.1111/j.0954-6820.1973.tb19491.x. [DOI] [PubMed] [Google Scholar]
- 13.Schrauwen-Hinderling VB, Hesselink MKC, Schrauwen P, Kooi ME. Intramyocellular lipid content in human skeletal muscle. Obesity. 2006;14:357–67. doi: 10.1038/oby.2006.47. [DOI] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 15.Holm JC, Gamborg M, Bille DS, et al. Chronic care treatment of obese children and adolescents. Int J Pediatr Obes. 2011;6:188–96. doi: 10.3109/17477166.2011.575157. [DOI] [PubMed] [Google Scholar]
- 16.Nysom K, Mølgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45-y-old Danes: reference values and comparison with published European reference values. Int J Obes Relat Metab Disord. 2001;25:177–84. doi: 10.1038/sj.ijo.0801515. [DOI] [PubMed] [Google Scholar]
- 17.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 18.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R, Dufour S, Petersen KF, et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–7. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 20.Saukkonen T, Heikkinen S, Hakkarainen A, et al. Association of intramyocellular, intraperitoneal and liver fat with glucose tolerance in severely obese adolescents. Eur J Endocrinol. 2010;163:413–9. doi: 10.1530/EJE-10-0186. [DOI] [PubMed] [Google Scholar]
- 21.Alasnier C. Lipid characteristics associated with oxidative and glycolytic fibres in rabbit muscles. Meat Science. 1996;43:213–24. doi: 10.1016/s0309-1740(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 22.Malenfant P, Joanisse DR, Thériault R, et al. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1316–21. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- 23.Bredella MA, Torriani M, Thomas BJ, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94:3995–4002. doi: 10.1210/jc.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–7. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz-Nieto F, Johansson L, Ahlström H, Weis J. Quantification of lipids in human lower limbs using yellow bone marrow as the internal reference: gender-related effects. Magn Reson Imaging. 2010;28:676–82. doi: 10.1016/j.mri.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Leibel N, Shen W, Mao X, et al. Body composition in premature adrenarche by structural MRI, 1H MRS and DXA. J Pediatr Endocrinol Metab. 2009;22:301–7. doi: 10.1515/jpem.2009.22.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brechtel K, Niess AM, Machann J, et al. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS) Horm Metab Res. 2001;33:63–6. doi: 10.1055/s-2001-12407. [DOI] [PubMed] [Google Scholar]
- 28.Machann J, Etzel M, Thamer C, et al. Morning to evening changes of intramyocellular lipid content in dependence on nutrition and physical activity during one single day: a volume selective 1H-MRS study. MAGMA. 2011;24:29–33. doi: 10.1007/s10334-010-0233-8. [DOI] [PubMed] [Google Scholar]
- 29.Cui M-H, Hwang J-H, Tomuta V, et al. Cross contamination of intramyocellular lipid signals through loss of bulk magnetic susceptibility effect differences in human muscle using (1)H-MRSI at 4 T. J Appl Physiol. 2007;103:1290–8. doi: 10.1152/japplphysiol.01088.2006. [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugaard SB, Mu H, Vaag A, Madsbad S. Intramyocellular triglyceride content in man, influence of sex, obesity and glycaemic control. Eur J Endocrinol. 2009;161:57–64. doi: 10.1530/EJE-08-0931. [DOI] [PubMed] [Google Scholar]
- 32.Zehnder M, Ith M, Kreis R, et al. Gender-specific usage of intramyocellular lipids and glycogen during exercise. Med Sci Sports Exerc. 2005;37:1517–24. doi: 10.1249/01.mss.0000177478.14500.7c. [DOI] [PubMed] [Google Scholar]
- 33.Vermathen P, Kreis R, Boesch C. Distribution of intramyocellular lipids in human calf muscles as determined by MR spectroscopic imaging. Magn Reson Med. 2004;51:253–62. doi: 10.1002/mrm.10721. [DOI] [PubMed] [Google Scholar]
- 34.Weis J, Courivaud F, Hansen MS, et al. Lipid content in the musculature of the lower leg: evaluation with high-resolution spectroscopic imaging. Magn Reson Med. 2005;54:152–8. doi: 10.1002/mrm.20518. [DOI] [PubMed] [Google Scholar]
- 35.Chabanova E, Bille DS, Thisted E, et al. MR spectroscopy of liver in overweight children and adolescents: Investigation of (1)H T(2) relaxation times at 3T. Eur J Radiol. 2011 Mar 3; doi: 10.1016/j.ejrad.2011.02.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Cali AMG, Caprio S. Ectopic fat deposition and the metabolic syndrome in obese children and adolescents. Horm Res. 2009;71(Suppl1):2–7. doi: 10.1159/000178028. [DOI] [PubMed] [Google Scholar]
- 37.Savgan-Gurol E, Bredella M, Russell M, et al. Waist to hip ratio and trunk to extremity fat (DXA) are better surrogates for IMCL and for visceral fat respectively than for subcutaneous fat in adolescent girls. Nutr Metab. 2010;7:86–86. doi: 10.1186/1743-7075-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]


