Abstract
Life span extending mutations in growth signaling pathways protect against age-dependent DNA damage in yeast and decrease insulin resistance and cancer in mice. To test their effect in humans, we monitored for 22 years Ecuadorian subjects with mutations in the growth hormone receptor gene leading to severe growth hormone receptor (GHR) and IGF-I deficiencies and combined this information with surveys to identify the cause and age of death for subjects who died before this period. The individuals with GHR deficiency (GHRD) exhibited only one non-lethal malignancy and no cases of diabetes, in contrast to 17% cancer and 5% diabetes prevalence in the controls. A possible explanation for the very low incidence of cancer may be revealed by in vitro studies: serum from GHRD subjects reduced DNA breaks but increased apoptosis in human mammary epithelial cells (HMECs) treated with hydrogen peroxide. We also observed reduced insulin concentrations (1.4 μU/ml vs. 4.4μU/ml in unaffected relatives) and a very low homoeostasis model assessment of insulin resistance (HOMA-IR) index (0.34 vs. 0.96 in unaffected relatives) in GHRD individuals, indicating increased insulin sensitivity, which could explain the absence of diabetes in these subjects. Incubation of HMECs with GHRD serum also resulted in reduced expression of RAS, PKA and TOR, and up-regulation of SOD2, changes that promote cellular protection and life span extension in model organisms. These results provide evidence for a role of evolutionarily conserved pathways in promoting aging and diseases in humans and identify a candidate drug target for healthy life span extension.
INTRODUCTION
Reduced activity of growth hormone (GH) and insulin like growth factor-I (IGF-I) signaling proteins or of their orthologs in lower organisms, and the activation of stress resistance transcription factors and antioxidant enzymes, contribute to extended life span and protection against age-dependent damage or diseases (1-15). Pathways that normally regulate growth and metabolism also promote aging and genomic instability, a correspondence that is conserved in simple eukaryotes and mammals (7, 16-18). In yeast, life span extending mutations in genes such as SCH9, the homolog of mammalian S6K, protects against age-dependent genomic instability (19-21). Similarly, mutations in the insulin/IGF-I like signaling (IIS) pathway increase lifespan and reduce abnormal cellular growth in worms, and mice deficient in GH and IGF-I are not only long-lived but also exhibit a delayed occurrence of age-dependent mutations and neoplastic disease (5, 6, 22-26). Among the most frequently detected mutations in human cancers are those that activate the two main signaling proteins downstream of the IGF-I receptor: Ras and Akt, and those in the IGF-1 receptor itself (27, 28). This is in agreement with a potential role for the IGF-I signaling pathway in promoting age-dependent mutations that lead to the generation of oncogenes and for oncogenes in exacerbating the generation of additional mutations and changes required for cancer progression (29). It has been proposed that the growth-promoting and anti-apoptotic functions of the IGF-1 pathway underlie its putative role in cancer development and progression (30). This link is supported by some population studies but not others which instead indicate only a modest association between high IGF-I concentrations and increased risk of certain cancers (30, 31).
GH may also promote insulin resistance. For example, age-dependent insulin resistance is reduced in GH deficient mice (32-35), and GH replacement therapy can exacerbate insulin resistance in GH-deficient individuals (36, 37), apparently because it causes a switch from glucose metabolism to lipolysis (38).
Here, we show that the fundamental link between pro-growth pathways, oxidative stress, age-dependent genomic instability and cellular damage observed in yeast (2, 15, 19-21), worms and mice (5, 6, 22-26) is conserved in humans by reporting on a 22-year monitoring of an Ecuadorian cohort with GHR and IGF-I deficiencies and by investigating the effect of these deficiencies on the cellular response to stress and on markers of cancer and diabetes.
RESULTS
Ecuadorian cohort
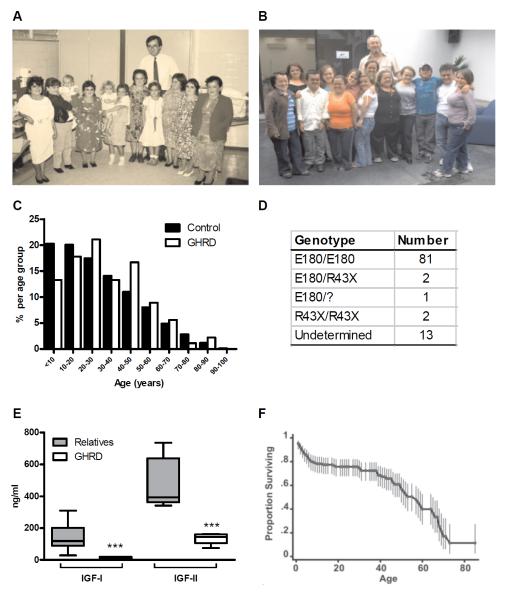
Study subjects were 99 individuals with GHRD who have been followed by one of the authors (J.G-A) at the Institute of Endocrinology, Metabolism and Reproduction (IEMYR) in Ecuador since 1988 (Fig. 1 A, B). Of these, 9 subjects died during the course of monitoring. The age distribution of the 90 living GHRD subjects and the control Ecuador population is shown in Fig. 1 C (39). Using a questionnaire (Table S1), we collected mortality data for 53 additional GHRD subjects who died prior to 1988 and obtained information on illnesses and cause of death for 1606 unaffected first to fourth degree relatives of the GHRD subjects. The GHRD cohort was identified on the basis of the severe short stature of the subjects (Fig. 1 A, B) (40-42) and confirmed by genotyping (Fig. 1 D). The majority of GHRD subjects in this cohort were homozygous for an A to G splice site mutation at position 180 in exon 6 of the growth hormone receptor (GHR) gene (Fig. 1 D). This mutation, termed E180, results in a protein that lacks 8 amino acids in its extracellular domain and is possibly misfolded and degraded (43). Two GHRD subjects were homozygous for the R43X mutation, which results in a truncated GHR protein as a result of a premature stop codon (Fig. 1 D) (44) and two GHRD subjects were E180/R43X heterozygotes (Fig. 1 D).
Fig. 1.
Ecuadorian cohort. (A, B) Several members of the GHRD cohort with Dr. J-G.A. in (A) 1988 and (B)2009 (C) Age distribution for 90 living GHRD subjects and the Ecuadorian (Control) population (D) Genotypes of the GHRD cohort. All GHRD subjects were identified based on short stature and very low IGF-I levels. Undetermined refers to subjects whose genotypes have not been confirmed (E) Serum IGF-I and IGF-II levels in 13 unaffected relatives and 16 GHRD subjects ***p<0.0001. (F) Survival of the GHRD cohort.
To confirm IGF deficiency in this cohort, we measured IGF-I and IGF-II concentrations (Fig. 1 E) in 13 relatives and 16 GHRD subjects ranging in age from 20 to 50 years, including those whose serum was later used for in vitro studies. Serum IGF-I ranged from 29 to 310 ng/ml (mean 144) among relatives, but was ≤ 20 ng/ml in all GHRD subjects (Fig.1 E). Serum IGF-II ranged from 341-735 ng/ml (mean 473) among relatives , but was below 164 ng/ml in all GHRD subjects (Fig. 1 E). There was no overlap in the range of IGF-I and IGF-II serum values between GHRD subjects and relatives (p<0.0001) (Fig. 1 E).
High mortality from common diseases of childhood has been observed in the GHRD cohort (Fig. 1 F) (45). Because of this, we only considered individuals who survived to at least age 10 for further analysis of diseases in this cohort. Of the 30 deaths among GHRD subjects (data from both monitoring and surveys) over the age of ten, 9 were due to age-related diseases (8 cardiac disease, 1 stroke) and 21 were due to non-age-related causes. Compared to their relatives, GHRD subjects died much more frequently from accidents, alcohol-related causes and convulsive disorders (Fig. 2 A).
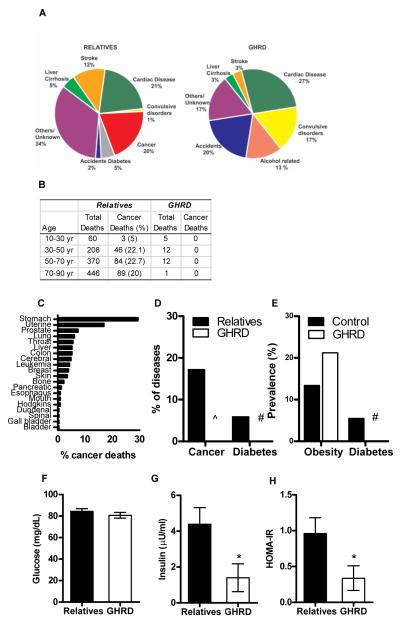
Fig. 2.
Diseases and mortality in the Ecuadorian cohort (A) Causes of death in unaffected relatives and GHRD subjects (B) Percentage of cancers per age group in unaffected relatives and GHRD subjects. (C) Percent distribution of cancer-deaths in the unaffected relatives (D) Percentage of cancer and type II diabetes in unaffected relatives and GHRD subjects. Data are shown as a percentage of all diagnosed/reported diseases. ^ = 1 case of cancer and # = no case of diabetes has been recorded. (E) Percent prevalence of obesity and type II diabetes in Ecuador (control) and GHRD subjects. # = no case of diabetes has been recorded in the GHRDs. Obesity prevalence in Ecuador is based on WHO reports for 2010 (79) while that for GHRD subjects was calculated based on BMI > 30 kg/m2. Prevalence of type II diabetes in Ecuador was obtained from the study by Shaw S.E. et al (47). (F) Fasting glucose (G) fasting insulin and (H) insulin resistance represented by HOMA-IR in relatives and GHRD subjects. Data represent mean ± SEM for 13 control and 16 GHRD samples. * p<0.05.
Cancer was not a cause of death in GHRD subjects of any age group; however, it accounted for 20% of deaths in the relatives (Fig. 2 A, B). Stomach cancer was the predominant cause of cancer-related mortality in the relatives (Fig. 2 C), which is consistent with the high incidence of this cancer in Ecuador (46) Among deaths in each age group, the proportion from cancer was lower in the GHRD subjects than in relatives (based on the exact hypergeometric distribution as implemented in StatXact 7, CytelSoftware Corporation, p=0.003). Cancer accounted for 17% of all diseases in the relatives (Fig. 2 D). Of all the GHRD subjects monitored since 1988, only one was diagnosed with cancer, a papillary serous epithelial tumor of the ovary in 2008. After treatment, she remains cancer free. We did not observe any mortality or morbidity due to Type 2 diabetes in the GHRD cohort, but diabetes was responsible for 5% of the deaths and 6% of all diseases in the relatives (Fig. 2 A, D), in agreement with the 5% prevalence of diabetes in Ecuador (Fig. 2 E) (47). We estimated the prevalence of diabetes in the GHRD cohort as 0/90 = 0%, with 95% exact Clopper-Pearson Confidence Interval: 0% - 4%. To test whether the diabetes prevalence in the GHRD cohort was different from the general population prevalence of 5%, we performed an exact test of the null hypothesis that p=0.05, based on the Binomial distribution, with the type I error rate, α=0.05. The P-value was 0.02, indicating that the prevalence in the GHRD cohort is less than 5%. This is a particularly striking result considering the elevated prevalence of obesity among these GHRD individuals (21% in GHRD subjects vs. 13.4% in Ecuador) (Fig. 2 E) (46). To investigate the mechanisms that could be responsible for the observed lack of diabetes in the GHRD cohort, we measured fasting glucose and insulin concentrations in 13 relatives and 16 GHRD subjects consisting of both male and female subjects between the ages of 20 and 50. We observed no significant difference in fasting glucose concentrations between them (Fig. 2 F). However, the average insulin concentration in the GHRD group was approximately a third of that in the relatives (Fig. 2 G, p<0.05), and the HOMA-IR index (48) indicated that GHRD subjects (HOMA-IR =0.34) were much more insulin sensitive than relatives (HOMA-IR=0.96) (Fig. 2H, p<0.05). These results are consistent with the finding that GHRD mice and other GH deficient mouse models have low serum insulin concentrations and are insulin sensitive (32-35).
Although GHRD subjects may have elevated cardiac disease mortality compared to their relatives (Fig. 2 A), the relative mortality from vascular diseases (combining cardiac disease and stroke) appears to be similar (33% of deaths in relatives vs. 30% of deaths in GHRD subjects) (Fig. 2 A). In agreement with studies of a human population with isolated growth hormone deficiency (49), our data suggest that GHRD does not increase overall vascular disease mortality (Fig. 2 A).
Reduced IGF-1 signaling protects against DNA damage but favors apoptosis of damaged cells
Our studies in S. cerevisiae indicate that homologs of mammalian growth signaling pathway genes, including TOR, S6K, RAS, adenylate cyclase and PKA promote an age-dependent increase in DNA mutations by elevating superoxide production and promoting DNA damage independently of cell growth (20, 21). Notably, the mutation spectrum in p53 from human cancers is similar to that in aging yeast (19, 20, 29). These results raise the possibility that GH and IGF-I signaling may promote mutations and cancer not only by preventing apoptosis of damaged cells but also by increasing DNA damage in both dividing and non-dividing cells. To test this hypothesis, we incubated confluent human mammary epithelial cells (HMECs) in medium supplemented with 15% serum from either relatives or GHRD subjects (50, 51) for 6 hours and then treated them with H2O2 for 1 or 24 hours, followed by comet analysis to detect DNA strand breaks. The cells were cultured in medium devoid of serum. In order to prevent interference from growth factors or insulin, the medium did not contain any growth supplements during the 6-hour incubation period. Because cells were incubated to greater than 90% confluence, cell growth during the pre-incubation and H2O2 treatment periods was minimal. Comet analysis indicated that cells incubated in serum from GHRD subjects had fewer DNA breaks after treatment with 700μM H2O2 for 1 hour (Fig. 3 A, B) or 24 hours (Fig. 3 A, C) compared to cells incubated in serum from relatives. This suggests that serum from GHRD subjects can protect against oxidative DNA damage independently of cell division. We also incubated confluent HMECs in medium supplemented either with serum from relatives, GHRD serum, or GHRD serum supplemented with 200ng/ml IGF-I for 6 hours (normal levels of IGF-I in Ecuadorian adults range between 96-270 ng/ml) (40). Treatment with 700μM H2O2 resulted in higher cytotoxicity in cells incubated in GHRD serum than in control serum (Fig. 3 D). This effect was completely reversed by the addition of 200ng/ml IGF-I to GHRD serum (Fig. 3 D). HMECs also displayed higher caspase activity in response to H2O2 when incubated in GHRD serum rather than serum from relatives (Fig. 3 E), in agreement with the proposed role of IGF-I signaling in increasing cancer incidence by preventing apoptosis (29). We also observed increased cytotoxicity in mouse embryonic fibroblast (MEF) cells in response to H2O2 following incubation in GHRD serum (Fig. 3 F)
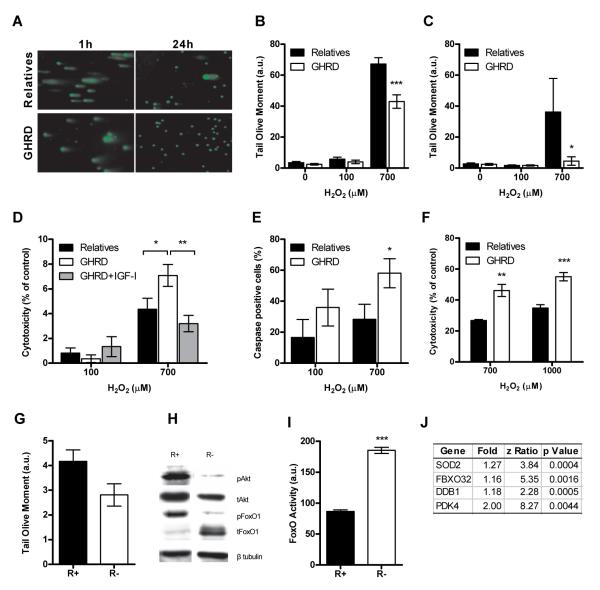
Fig. 3.
Reduced IGF-1 signaling protects against DNA damage and favors apoptosis of damaged cells. (A,B,C) Comet assay to analyze DNA damage in HMECs incubated in serum from relatives/GHRDs and treated with H2O2 for 1 hour or 24 hours (A) Representative micrographs of cells treated with 700 μM H2O2 (B, C) Tail olive moment in cells treated with H2O2 for (B) 1 hour or (C) 24 hours. Data represent mean ± SEM. At least 6 serum samples were tested per group and 100-200 cells were analyzed per sample. (D) LDH activity in HMECs incubated in serum from relatives/GHRDs and treated with H2O2 for 24 hours. Data represent mean ± SEM. 6 serum samples were tested per group in triplicates. (E) Activation of caspases in HMECs incubated in serum from relatives/GHRDs and treated with H2O2. Data are calculated as percentage of untreated control and represent mean ± SEM. 6 serum samples were tested per group. (F) LDH activity in MEFs incubated in serum from relatives/GHRDs and treated with H2O2 for 24 hours. Data represent mean ± SEM. 6 serum samples were tested per group in triplicates. (G) Tail olive moment to measure basal DNA damage in R+ (IGF-IR overexpression) or R-(IGF-IR deficient) mouse embryonic fibroblasts (MEFs). Data represent mean ± SEM. 100-200 cells were analyzed per sample. (H) Representative western blot showing phosphorylation status of Akt (Ser 473) and FoxO1 (Ser 256) in R+ and R-cells. (I) FoxO activity in R+ and R-cells transfected with a luciferase reporter plasmid (I) List of FoxO target genes significantly upregulated in HMECs incubated in GHRD serum versus serum from relatives, *p<0.05, **p<0.01, ***p<0.0001.
To test whether IGF-I signaling was responsible for the sensitization of cells to oxidative damage, we analyzed DNA damage in MEF cells lacking the IGF-I receptor (R-cells) or overexpressing the human IGF-I receptor (R+ cells) (52). R+ cells had higher basal DNA than did R-cells (Fig. 3 G). Western blot analysis confirmed the anticipated increase in phosphorylation of Akt (Thr 308) and FoxO1 (Ser 256) in R+ cells compared to R-cells, indicating that Akt was activated while FoxO1 was inactivated in the R+ cells (Fig. 3 H) (53-55). The very low level of total FoxO1 protein in R+ cells may be due to the Akt-mediated phosphorylation of FoxO, which results in its ubiquitination and proteasomal degradation (Fig. 3 H) (56). Reduced FoxO activity in R+ cells when compared to R-cells that were transfected with a FoxO luciferase reporter plasmid confirmed that FoxO was inactivated by high IGF-I signaling in these cells (Fig. 3 I). As FoxO transcription factors are known to protect against oxidative stress as well as promote apoptosis (54, 57, 58), we hypothesize that increased FoxO activity could account, in part, for the protective effects observed in HMECs incubated in GHRD serum. In support of this, microarray analysis of HMECs incubated in either control or GHRD serum showed that out of 44 genes that were significantly up-regulated in the GHRD serum-treated group, 4 genes, including SOD2, were FoxO targets (Fig. 3 J).
Protective effects of reduced pro-growth signaling in yeast and mammals
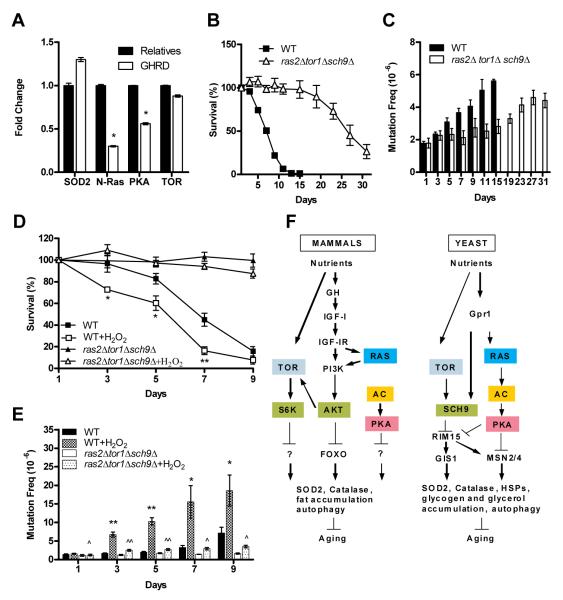
A complete list of genes with significant differential expression in HMECs incubated in either control or GHRD serum is shown in Table S2. Ingenuity Pathways Analysis (IPA) of global gene expression patterns revealed significant differences in pathways involved in cell cycle regulation, gene expression, cell movement and cell death, among others (Fig. S1). IPA also indicated that genes regulating apoptosis were up-regulated (Fig. S2) whereas Ras, PKA and Tor signaling were down-regulated in cells incubated in GHRD serum (Fig. S3). RT-PCR analysis confirmed a 30% higher mRNA level of mitochondrial MnSOD (SOD2) in cells incubated in GHRD serum, and also a 70%, 50% and 20% reduction in N-Ras, PKA and TOR expression, respectively (Fig. 4 A). Ras, PKA and TOR/S6K are central regulators of pro-aging and disease promoting pathways (59) and SOD2 is a key mediator of cellular protection against oxidative stress in organisms ranging from the unicellular yeast to mammals (2, 19, 20, 60-63).
Fig. 4.
Protective effects of reduced pro-growth signaling in yeast and mammals (A) RT-PCR indicating upregulation of SOD2 and downregulation of N-Ras, PKA and TOR in HMECs incubated in GHRD serum compared to cells incubated in serum from relatives (B) Chronological survival of wild-type (WT) yeast cells and ras2Δsch9Δtor1Δ mutants cells (C) Mutation frequency over time in the CAN1 gene (measured as Canr mutants/106 cells). (D) Chronological survival of wild-type yeast cells and ras2Δsch9Δtor1Δ triple mutants treated with H2O2 (E) Mutation frequency over time in the CAN1 gene (measured as Canr mutants/106 cells) in H2O2 –treated yeast cells. Data represent mean ± SEM, n= 5. *p<0.05, **p<0.01 compared to untreated WT cells and ^ p<0.05, ^^p< 0.01 compared to untreated ras2Δ, sch9Δ, and tor1Δ triple mutants. (F) Conserved growth factor signaling pathways in mammals and yeast.
To further test the role of these genes in age and oxidative stress-dependent DNA damage, we generated a yeast triple mutant strain lacking RAS2, TOR1 and SCH9. Our previous studies have shown that yeast sch9Δ mutants exhibit lower age-dependent genomic alterations than wild-type cells in part due to reduced error-prone Polζ-dependent DNA repair (20). We observed a 4-fold life span extension in triple mutants compared to wild-type cells (Fig. 4 B). We analyzed age-dependent DNA genomic instability in the ras2Δtor1Δsch9Δ and wild type cells by measuring the frequency of mutations of the CAN1 gene. Mutations that inactivate the arginine permease encoded by CAN1 can be easily measured since they allow cells to grow in medium containing the normally toxic arginine analog canavanine (64) The frequency of age-dependent mutations in the CAN1 gene, which are mostly point mutations including a high frequency of G to T (transversion) and C to T (transition) base substitutions (19), were much higher in wild type cells compared to the ras2Δtor1Δsch9Δ mutants (Fig. 4 C). Whereas wild-type cells were susceptible to H2O2 treatment, the ras2Δ tor1Δ sch9Δ mutants were almost unaffected at the concentrations tested (Fig. 4 D) and were highly protected against oxidative stress-dependent mutations (Fig. 4 E).
DISCUSSION
Our finding that human GHRD subjects are protected against age-related pathologies is consistent with the elevated cellular protection in both yeast and human cells with reduced expression of specific pro-growth genes and with the effect of serum from GHRD subjects in lowering their expression (Fig. 4 F). Our results also show similarities with GHRD mice, which display lower incidence (49%) and delayed occurrence of fatal neoplasms and increased insulin sensitivity (24, 25, 32). Furthermore, the reduced cancer incidence in GHRD mice is associated with a lower mutation frequency in various tissues (26).
The lack of lifespan extension in GHRD subjects may be explained in large part by the major proportion of deaths (70%) caused by convulsive disorders, alcohol toxicity, accidents, liver cirrhosis and other non-age-related causes. The lack of cancer mortality but normal life span in GHRD subjects, is in agreement with a preliminary study that reported the absence of cancer in a group of 222 patients with congenital IGF-I deficiencies (65) and with the normal lifespan that was observed in 65 GH deficient subjects (66). In contrast to our study of GHRD subjects with specific mutations and their age-matched relatives, Shevah and Laron compared young subjects with IGF-I deficiencies due to many causes, with much older controls, which made it difficult to determine whether cancer incidence was reduced (65). However, taken together these two studies provide strong evidence for reduced cancer incidence in GHR and IGF-I deficient subjects, and indicate that IGF-I could serve as a marker for age-dependent cancer, at least in specific populations. Our results may also provide a partial explanation for the overrepresentation of partial loss-of-function mutations in the IGF-1 receptor gene among Ashkenazi Jewish centenarians (67).
The role of IGF-I in cancer may involve the well-established pro-growth and anti-apoptotic functions of this growth factor(30, 68, 69) as well as an effect on increasing oxidative damage and DNA damage independently of growth as suggested by our studies in yeast (19-21, 29) and mammals (70, 71). In both yeast and mammals, reduction of TOR/S6K, RAS and AC/PKA signaling renders cells and the organism resistant to age- and oxidative stress-dependent mutagenesis (2, 15, 19-21, 70, 72, 73). This effect appears to depend, in part, on increased activity of stress resistance transcription factors and SOD2 (20, 57, 63). In fact, mice lacking Cu/Zn SOD or MnSOD are susceptible to increased DNA damage and cancer (62). The positive effects of serum from GHRD subjects on cellular changes that promote longevity in model organisms, such as reduced levels of RAS, PKA, and TOR and increased expression of SOD2 and other FoxO-regulated genes, suggests that the anti-aging and anti-DNA damage mechanisms promoted by reduced growth signaling are conserved from yeast to humans (Fig. 4F) (7, 16, 74).
The lack of type 2 diabetes in the GHRD cohort, is particularly interesting considering that obesity is one of the clinical phenotype of GHRD. The enhanced insulin sensitivity of GHRD subjects, as indicated by reduced insulin concentrations and a lower HOMA-IR index, could explain the absence of diabetes in this cohort. Although, increased insulin sensitivity has been associated with a longer lifespan, some long-lived mice including the fat insulin receptor knockout (FIRKO) mice, exhibit impaired insulin signaling. In this case however, loss of insulin signaling is restricted to adipose tissue and is not associated with diabetes or glucose intolerance (75). Similarly, male IGF-I receptor heterozygous (IGF 1R +/-) mice show a 16% increase in lifespan although they exhibit impaired glucose tolerance (6). The anti-aging benefits of reduced GHR and IGF-I signaling without the detrimental effects of insulin resistance in GHRD mice may explain, in part, why they display reduced disease incidence and a much longer lifespan compared to IGF 1R +/- mice.
Our results provide a foundation for further investigation into the role of drugs blocking the GHR and downstream conserved pro-aging pathways to prevent or reduce the incidence of cancer, diabetes and other age-related diseases including inflammatory disorders, stroke, and neurodegenerative diseases.
MATERIALS AND METHODS
Subject Recruitment
GHRD subjects and their relatives were recruited for the study under protocols approved by the Institute for Endocrinology, Metabolism and Reproduction (IEMYR) in Ecuador. All participants signed informed consent forms prior to their participation in the study. Data on deceased GHRD subjects was collected from family members using a detailed questionnaire (Table S1). At least two relatives were required to be present at the time of the interview.
Genotyping
Saliva samples were collected using the Oragene OG-250 DNA collection kit (DNA Genotek Inc., Ontario, Canada) and processed according to the manufacturers protocol. Genotyping of the E180 mutation was performed using the following primers - 5′-CATTGCCCTCAACTGGACTT-3′ Forward 5′-CATTTTCCATTTAGTTTCATTTACT-3′ Reverse (WT) 5′-CATTTTCCATTTAGTTTCATTTAC-3′ Reverse (mutant)
Serum Analysis
Serum IGF-I was measured using an in-house ELISA immunoassay based on paired specific antibodies (R&D systems) and validated against the commercial kit from Diagnostic Systems Laboratories. The assay was performed after acid/ethanol extraction of the samples and has a sensitivity of 0.2ng/ml. IGF-II was measured using paired anti-IGF-II specific antibodies (R&D systems). The sensitivity of the assay is 0.2 ng/ml. Fasting glucose levels were measured with a glucose analyzer from YSI Life Sciences and fasting insulin levels were measured with a human insulin ELISA kit from Millipore. Insulin resistance was assessed using the homeostatic model assessment-insulin resistance (HOMA-IR) index from fasting glucose and fasting insulin values (48).
Cell culture
HMECs were purchased from ScienCell Research Laboratories. Cells were cultured in epithelial cell medium (ScienCell) at 37°C and 5% CO2 on poly-L-lysine (Sigma) coated culture dishes. The epithelial cell medium consisted of basal medium and a proprietary growth supplement supplied by the manufacturer and no serum. Primary mouse embryonic fibroblasts (MEFs) were purchased from ATCC and cultured in DMEM/ F12 (Invitrogen), supplemented with 15% FBS at 37°C and 5% CO2. R+ and R-cells were obtained from Dr. R. Baserga and cultured in DMEM/F12 supplemented with 10% FBS at 37°C and 5% CO2. Cells were seeded at a density of 4×104 per well for comet and apoptosis assays, 8×104 per well for LDH assays or 2×105 per well for microarray analysis and western blots in 24, 96 and 6 well plates respectively. HMECs and MEFs were incubated in basal medium supplemented with 15% serum from relatives or GHRDs for 6 hours followed by treatment with H2O2 for 1 hour (comet and apoptosis assays) or 24 hours (comet and LDH assays). Incubation of HMECs in human serum was done in basal medium without the growth supplement while that for MEFs cells was done in DMEM/F12 without FBS. For microarray analysis, HMECs were incubated in serum from relatives or GHRDs as above for 6 hours and immediately processed for RNA extraction with TRI reagent from Ambion.
Comet Assay
Comet assay was performed according to the method described by Olive et al (76) using the comet assay kit from Trevigen. DNA damage was quantified per cell with the Comet Score software. 100-200 cells were analyzed per sample.
LDH assay
LDH activity was assayed in culture medium with the CytoTox 96 Non-Radioactive Cytotoxicity Assay from Promega according to the manufacturer’s protocol.
Apoptosis assay
Activated caspases were quantified with a fluorescence plate reader with the Fluorescein CaspaTag Pan-Caspase Assay Kit (Chemicon) according to the manufacturer’s protocol.
FoxO activity
50,000 cells/well were transfected with 0.2μg of FoxO luciferase reporter plasmid with the consensus FoxO binding sequence driving firefly luciferase gene expression in 24 well plates. As an internal control cells were co-transfected with 0.02μg of plasmid DNA encoding Renilla luciferase under control of the CMV promoter. 24 hours after transfection, FoxO promoter activity was assayed using the Dual-Luciferase Reporter Assay System from Promega according to the manufacturer’s protocol. Western blot analysis: Cells were lysed in RIPA buffer and total protein was assayed with BCA from Thermo scientific. 15 μg of total protein was loaded on denaturing 10% SDS-PAGE gels. Primary antibodies against phosphorylated and total Akt (Thr 308) as well as phosphorylated and total FoxO1 (Ser 256) were obtained from Cell Signaling Technologies. Anti β-tubulin antibody was obtained from Santa Cruz Biotechnology Inc. Secondary rabbit antibody was obtained from Jackson Immunoresearch Laboratories, Inc. Microarray analysis: RNA was extracted using TRI Reagent (Ambion) and hybridized to BD-103-0603 chips from Illumina Beadchips (San Diego, CA). Raw data were subjected to Z normalization and are available at the gene expression omnibus (GEO) repository, accession number GSE21980. Gene set enrichment was tested with the PAGE method as described (77).
Figures S6, S7 and S8 were selected based on the names and descriptions provided by Ingenuity Pathways Analysis (Ingenuity Systems; Redwood City, CA) and/or Ariadne Pathway Studio 7 (Ariadne Genomics).
Yeast
Wild type DBY746 (MATα leu2-3 112 his3Δ ,trp1-289 ura3-52 GAL+) and its derivative ras2::LEU2tor1::HIS3sch9::URA3, originated by one-step gene replacement, were grown in SDC containing 2% glucose(2). Chronological life span in SDC medium was monitored by measuring colony forming-units (CFUs), on YPD plates, every other day. The number of CFUs on day one was considered to be the initial survival (100%) and was used to determine the age-dependent mortality (78). Spontaneous mutation frequency was evaluated by measuring the mutation frequency of the CAN1 (YEL063C) gene. Cells were plated onto selective SDC minus Arginine plates in the presence of L-canavanine sulfate (60mg/L). Mutation frequency was expressed as the ratio of Canr colonies over total viable cells (64). Resistance to oxidative stress was also evaluated in yeast cultures chronically treated with 1 mM H2O2 on days 1 and 3.
Statistical analysis
Students t-test, two tailed, was used to analyze insulin, HOMA-IR data, and cellular data from mammalian (comet, LDH, caspase assays, RT-PCR, and FoxO activity) and yeast experiments (survival and mutation frequency) using graph pad prismV.
SUPPLEMENTARY MATERIAL Fig. S1 Functional clustering of genes with significant differential expression in HMECs incubated in either serum from relatives or GHRD serum.
Fig. S2 Ingenuity pathways analysis indicating an upregulation of apoptosis in HMECs incubated in GHRD serum. (Red=Up-regulation, Blue= Down-regulation)
Fig. S3 Ingenuity pathways analysis indicating downregulation of Ras, PKA and RPS6K in HMECs incubated in GHRD serum. (Red=Up-regulation, Blue= Down-regulation)
Table S1 Questionnaire
Table S2 List of genes with significant differential expression in HMECs incubated in either serum from relatives or GHRD serum.
Supplementary Material
Acknowledgements
We thank R. Baserga of Thomas Jefferson University (Philadelphia, PA) for providing R+ and R-cells, William Wood, Elin Lehrmann and Yongqing Zhang for microarray assistance and Rita Thomas for help with glucose measurements.
Funding: This study was funded in part by NIH/NIA grants AG20642 and AG025135 to V.D. Longo, Ted Bakewell (The Bakewell Foundation), the V Foundation for Cancer Research, and a USC CEGS pilot grant to V.D. Longo, grant 1P30 DK063491 to P. Cohen, the Institute of Endocrinology, Metabolism and Reproduction, Ecuador and the Intramural Research Program of the NIH, NIA.
Footnotes
Author Contributions: J.G-A., M.G-A and J.S. interviewed GHRD individuals and their relatives and collected samples for analysis. V.D.L. and J.G. designed and supervised the population studies. V.D.L. designed and supervised in vitro experiments. P.B. designed and performed in vitro experiments. J.G-A, P.B. and V.D.L. analyzed data and wrote the paper. M.W. and F.M. performed genotyping and yeast experiments. C-W.C assisted in performing in vitro experiments. D.H. and P.C. performed IGF analysis in serum samples. A.M-M and R.d.C. performed microarray analysis. S.I. performed statistical analysis of epidemiological data.
Competing interests: J.G-A and V.D.L. have filed patents related to the subject of this manuscript.
REFERENCES AND NOTES
- 1.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993 Dec 2;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 2.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001 Apr 13;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 3.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001 Apr 6;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 4.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001 Apr 6;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 5.Coschigano KT, et al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003 Sep;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 6.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003 Jan 9;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 7.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003 Feb 28;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 8.Longo VD, Gralla EB, Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996 May 24;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 9.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996 Aug 8;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 10.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997 Nov 14;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 11.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994 Feb 25;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 12.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002 Jan;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 13.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996 Nov 7;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 14.Cohen E, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009 Dec 11;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo VD. The Chronological Life Span of S. cerevisiae: Studies of Superoxide Dismutases, Ras and Bcl-2. University of California; Los Angeles: 1997. [Google Scholar]
- 16.Longo VD, Liou LL, Valentine JS, Gralla EB. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999 May 1;365:131–142. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 17.Kenyon C. A conserved regulatory system for aging. Cell. 2001 Apr 20;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 18.Longo VD. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies, and mammalian neuronal cells. Neurobiol Aging. 1999 Sep-Oct;20:479–486. doi: 10.1016/s0197-4580(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 19.Madia F, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008 Jan 14;180:67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madia F, et al. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Rev1/Polzeta-dependent mechanism. J Cell Biol. 2009 Aug 24;186:509–523. doi: 10.1083/jcb.200906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabrizio P, et al. Sir2 blocks extreme life-span extension. Cell. 2005 Nov 18;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006 Aug 18;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- 23.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003 Apr;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 24.Ikeno Y, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009 May;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartke A. Insulin resistance and cognitive aging in long-lived and short-lived mice. J Gerontol A Biol Sci Med Sci. 2005 Jan;60:133–134. doi: 10.1093/gerona/60.1.133. [DOI] [PubMed] [Google Scholar]
- 26.Garcia AM, et al. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008 Sep;129:528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Viciana P, et al. Cancer targets in the Ras pathway. Cold Spring Harb Symp Quant Biol. 2005;70:461–467. doi: 10.1101/sqb.2005.70.044. [DOI] [PubMed] [Google Scholar]
- 28.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006 Apr 15;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 29.Longo VD, Lieber MR, Vijg J. Turning anti-ageing genes against cancer. Nat Rev Mol Cell Biol. 2008 Nov;9:903–910. doi: 10.1038/nrm2526. [DOI] [PubMed] [Google Scholar]
- 30.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004 Jul;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 31.Renehan AG, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004 Apr 24;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 32.Dominici FP, Diaz G. Arostegui, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000 Sep;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- 33.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002 Apr;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 34.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009 May;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JL, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004 Sep;287:E405–413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 36.Carroll PV, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab. 1998 Feb;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 37.Johansson JO, Fowelin J, Landin K, Lager I, Bengtsson BA. Growth hormone-deficient adults are insulin-resistant. Metabolism. 1995 Sep;44:1126–1129. doi: 10.1016/0026-0495(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 38.Bramnert M, et al. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003 Apr;88:1455–1463. doi: 10.1210/jc.2002-020542. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Census Bureau. 2010.
- 40.Guevara-Aguirre J, Rosenbloom AL, Fielder PJ, Diamond FB, Jr., Rosenfeld RG. Growth hormone receptor deficiency in Ecuador: clinical and biochemical phenotype in two populations. J Clin Endocrinol Metab. 1993 Feb;76:417–423. doi: 10.1210/jcem.76.2.7679400. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbloom AL, Aguirre J. Guevara, Rosenfeld RG, Fielder PJ. The little women of Loja--growth hormone-receptor deficiency in an inbred population of southern Ecuador. N Engl J Med. 1990 Nov 15;323:1367–1374. doi: 10.1056/NEJM199011153232002. [DOI] [PubMed] [Google Scholar]
- 42.Bachrach LK, et al. Bone mineral, histomorphometry, and body composition in adults with growth hormone receptor deficiency. J Bone Miner Res. 1998 Mar;13:415–421. doi: 10.1359/jbmr.1998.13.3.415. [DOI] [PubMed] [Google Scholar]
- 43.Berg MA, Guevara-Aguirre J, Rosenbloom AL, Rosenfeld RG, Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. Hum Mutat. 1992;1:24–32. doi: 10.1002/humu.1380010105. [DOI] [PubMed] [Google Scholar]
- 44.Amselem S, et al. Recurrent nonsense mutations in the growth hormone receptor from patients with Laron dwarfism. J Clin Invest. 1991 Mar;87:1098–1102. doi: 10.1172/JCI115071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guevara-Aguirre J, et al. Growth hormone receptor deficiency (Laron syndrome): clinical and genetic characteristics. Acta Paediatr Scand Suppl. 1991;377:96–103. doi: 10.1111/apa.1991.80.s377.96. [DOI] [PubMed] [Google Scholar]
- 46. http://www.who.int/en/
- 47.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. Jan;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 49.Aguiar-Oliveira MH, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. 2010 Feb;95:714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Cabo R, et al. An in vitro model of caloric restriction. Exp Gerontol. 2003 Jun;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 51.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003 Jun;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 52.Sell C, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993 Dec 1;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999 Jun 11;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 54.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999 Mar 19;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 55.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996 Dec 2;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005 Feb 1;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kops GJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002 Sep 19;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 58.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000 Oct 5;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 59.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. Apr 16;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hlavata L, Nystrom T. Ras proteins control mitochondrial biogenesis and function in Saccharomyces cerevisiae. Folia Microbiol (Praha) 2003;48:725–730. doi: 10.1007/BF02931505. [DOI] [PubMed] [Google Scholar]
- 61.Urban J, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007 Jun 8;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 62.Busuttil RA, et al. Organ-specific increase in mutation accumulation and apoptosis rate in CuZn-superoxide dismutase-deficient mice. Cancer Res. 2005 Dec 15;65:11271–11275. doi: 10.1158/0008-5472.CAN-05-2980. [DOI] [PubMed] [Google Scholar]
- 63.Fabrizio P, et al. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003 Jan;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madia F, Gattazzo C, Fabrizio P, Longo VD. A simple model system for age-dependent DNA damage and cancer. Mech Ageing Dev. 2007 Jan;128:45–49. doi: 10.1016/j.mad.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007 Feb;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Aguiar-Oliveira MH, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. Feb;95:714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008 Mar 4;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parrizas M, LeRoith D. Insulin-like growth factor-1 inhibition of apoptosis is associated with increased expression of the bcl-xL gene product. Endocrinology. 1997 Mar;138:1355–1358. doi: 10.1210/endo.138.3.5103. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy MA, Rakoczy SG, Brown-Borg HM. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol. 2003 Sep;38:997–1008. doi: 10.1016/s0531-5565(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008 Jul;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee C, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. Feb 15;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Longo VD. The Ras and Sch9 pathways regulate stress resistance and longevity. Exp Gerontol. 2003 Jul;38:807–811. doi: 10.1016/s0531-5565(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 73.Hlavata L, Nachin L, Jezek P, Nystrom T. Elevated Ras/protein kinase A activity in Saccharomyces cerevisiae reduces proliferation rate and lifespan by two different reactive oxygen species-dependent routes. Aging Cell. 2008 Mar;7:148–157. doi: 10.1111/j.1474-9726.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 74.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003 Jul 17;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 75.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003 Jan 24;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 76.Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 77.Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003 Apr;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 79. http://www.who.int/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.