Abstract
Investigation of bivalent ligands at μ, δ and κ opioid receptors is now focused on the preparation of ligands containing κ agonist and μ agonist/antagonist pharmacophores at one end joined by a linking chain containing the μ antagonists pharmacophores (naltrexone, naloxone or nalbuphine) at the other end. These ligands were evaluated in-vitro by their binding affinity at μ, δ and κ opioid receptors and their relative efficacy in the [35S]GTPγS assay.
Ligands 6-8 displayed better or the same affinity at κ and μ receptors compared to the monovalent ligands 1-5. Ligands 6-8 generally showed reduced affinity at δ receptor compared to the monovalent ligands 1-5. Functional assays showed that the ligand 6 was a κ agonist/antagonist and μ antagonist whereas ligands 7 and 8 were κ agonists and μ agonists/antagonists.
Introduction
The heterodimerization of G-protein coupled receptors has important implications because it represents another mechanism that could modulate receptor function and suggests additional targets for drug development.1-3 There is now an increasing realization that activity at a single receptor is insufficient for modulating multiple targets for the treatment of a range of disorders.4 Bivalent ligands have been developed for a variety of G-protein coupled receptor targets including opioid 5,6 adrenergic,7 dopamine,8 serotonin,9 muscarinic receptors,10 but also enzymes such as butyrylcholinesterase.11 The methodical combination of pharmacophores from selective ligands that act on specific targets (receptors) is an important technique used for the generation of bivalent ligands. There is the possibility that the development of bivalent ligands in the opioid field which bridge the gap between binding sites on dimerized receptors will lead to a new generation of analgesic drugs that may not cause physical dependence or tolerance with chronic use.12
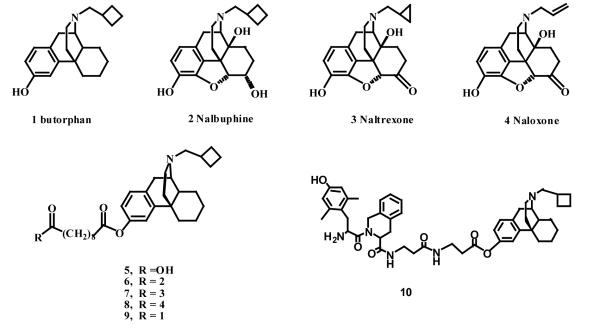
Previous reports from our laboratories indicated that the mixed action of the κ/μ agonist butorphan (1) has a more promising profile of activity than the κ agonist/μ antagonist – cyclorphan.13,14 This finding led to the synthesis of a series of homo-bivalent ligands incorporating butorphan (1) as the pharmacophore connected by linking spacers of varying lengths.15,16 It was observed that the affinity of these ligands was sensitive to the character and length of the spacer. The homobivalent ligand 9 containing butorphan (1) at both ends of the 10-carbon linking ester chain (Figure 1) (Ki=0.09 nM at μ and 0.049 nM at κ) was the most potent ligand in this series.15,16 In the course of the synthesis of a series of hetero-bivalent ligands containing butorphan (1) at one end and other pharmacophores at the other end of the linker, we also found that the stereochemistry of the pharmacophores, the N-substituents of the pharmacophore, ester linkages and the spacer lengths were crucial factors for optimum interactions of such ligands at opioid receptor binding sites.17 The spacer length for these compounds was dictated by the peak potency that was observed when sebacoyl ester (10 carbon) unit was incorporated into the molecule. A multiple ligand 10 (Figure 1) derived from the linkage of a δ selective peptide antagonist Dmt-Tic (2′6′-dimethyl-L-tyrosine-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) and a μ/κ morphinan agonist butorphan (1) through a two-methylene spacer was found to maintain exactly the same characteristics as the two reference compounds.18
Fig. 1.
Structures of opioids and bivalent ligands
Portoghese et al. has also reported a range of homo and hetero dimeric ligands with varying linker lengths designed to investigate pharmacodynamic and organizational features of opioid receptors.19 For example, recently reported heterodimeric ligands containing δ antagonist (naltrindole) and κ agonist (ICI-199,441) pharmacophores joined by variable length oligoglycyl-based linkers were demonstrated to possess significantly greater potency and selectivity when compared to their monomer congeners providing further evidence for the opioid receptor hetero-oligomerization phenomena.20
In order to further investigate opioid bivalent ligands containing pharmacophores that have established κ/μ/δ affinity, a combination of agonist and antagonist pharmacophores was employed in the design of bivalent ligands for exploring the interaction between receptors. Here we report the synthesis of three heterodimeric ligands derived from the linkage via a 10 carbon spacer of the μ antagonists nalbuphine (2), naltrexone (3) or naloxone (4) and a μ / κ agonist butorphan (1).
Chemistry
The heterodimeric ligands 6, 7 and 8 were prepared by condensing the acid 5 with either nalbuphine (2), natrexone (3) or naloxone (4) in the presence of DCC and DMAP as previously reported (Figure 1).17
Pharmacological Results and Discussion
Affinity and Selectivity of the Synthesized Ligands
All the novel heterodimer ligands were evaluated for their affinity at and selectivity for μ, δ and κ human opioid receptors with Chinese hamster ovary (CHO) cell membranes stably expressing one of the human opioid receptors. The data are summarized in Table 1. For comparison purposes, opioid binding affinity data for butorphan (1), nalbuphine (2), naltrexone (3) and naloxone (4) are included in Table 1. The monovalent ligand 5 and the homobivalent ligand 9 reported previously17 were also included in order to evaluate the contribution of the spacer itself or the pharmacophores to binding.
Table 1.
Ki Values for the Inhibition of μ, δ and κ Opioid Binding to Chinese Hamster Ovary Membrane by Hetero-dimeric Opioids
| compd | Ki ± SEM (nM) | Selectivity | |||
|---|---|---|---|---|---|
| [3H]DAMGO(μ) | [3H]U69,593(κ) | [3H]Naltrindole(δ) | κ/μ | κ/δ | |
| 1 (Butorphan) | 0.23 ± 0.01 | 0.079 ± 0.003 | 5.9 ± 0.6 | 3 | 70 |
| 2 (Nalbuphine) | 0.89 ± 0.02 | 2.2 ± 0.01 | 240 ± 18 | 0.4 | 109 |
| 3 (Naltrexone) | 0.23 ± 0.05 | 0.25 ± 0.02 | 38 ± 3 | 1 | 152 |
| 4 (Naloxone) | 0.79 ± 0.02 | 1.1 ± 0.03 | 76 ± 2 | 1 | 69 |
| 5a | 0.71 ± 0.02 | 0.29 ± 0.02 | 18 ± 1 | 2 | 62 |
| 6 | 0.46 ± 0.02 | 0.34 ± 0.008 | 28 ± 4 | 1 | 82 |
| 7 | 0.29 ± 0.0007 | 0.12 ± 0.002 | 15 ± 2 | 2 | 125 |
| 8 | 0.43 ± 0.001 | 0.13 ± 0.002 | 39 ± 6 | 3 | 300 |
| 9a | 0.09 ± 0.004 | 0.05 ± 0.001 | 4.2 ± 0.4 | 2 | 84 |
These compounds were reported as compounds (8) and (18) in ref. 17
Heterodimeric compounds such as 6 (butorphan (1) combined with nalbuphine (2)), 7 (butorphan (1) combined with naltrexone (3)) and 8 (butorphan (1) combined with naloxone (4)) with a 10-carbon linking ester, displayed slightly better affinity at μ (around 2 fold) compared to the monovalent ligand 5. Compound 7 (butorphan (1) combined with naltrexone (3)) and 8 (butorphan (1) combined with naloxone (4)) showed lightly better affinity at κ (~ 2 fold) receptor while compound 6 (butorphan (1) combined with nalbuphine (2)) retained same affinity at κ, but all had lower affinity than butorphan (1). From the data shown in table 1, the heterodimer 6 (containing butorphan (1) and nalbuphine (2)) showed increased affinities both at μ (Ki = 0.46 nM) and a 6 fold increase (Ki = 0.34 nM) at κ receptors compared to nalbuphine (2), while the affinity at δ receptor was an average of the two monomeric ligands 1 and 2. Similarly, the heterodimer 8 (containing butorphan (1) at one end and naloxone (4) at the other), displayed a 2 fold increase at μ (Ki = 0.43 nM) and a 10 fold increase at κ receptors (Ki = 0.13 nM) as well as 2 fold increase at δ receptor compared to naloxone (4). It is interesting to note that compound 7 (containing butorphan (1) and naltrexone (3)) displayed almost identical affinities at all three opioid receptors as the monomer naltrexone.
Efficacy of Selected Ligands
To characterize the relative efficacy of the ligands, butorphan (1), nalbuphine (2) and mono-valent ligand 5 were selected for the [35S]GTPγS assay. Table 2 showed the agonist and antagonist properties of the ligands in stimulating [35S]GTPγS binding mediated by the κ opioid receptor. Ligand 6 produced similar maximal stimulation of [35S]GTPγS binding (Emax) comparable to that of butorphan (1) and nalbuphine (2), but less than that of selective agonist U50,488. The EC50 value of this ligand is slightly higher than butorphan (1), but much lower than nalbuphine (2). Contrasted to the parent compounds, butorphan (1) and nalbuphine (2), ligand 6 can inhibit U50,488-stimulated [35S]GTPγS binding although it had a high IC50 value, which suggests that this ligand was a κ agonist/antagonist.
Table 2.
Agonist and Antagonist Properties of Compounds in Stimulating [35S]GTPγS Binding Mediated by the κ Opioid Receptora
| compd | Pharmacological properties |
Emax (% maximal stimulation) |
EC50 (nM) |
Imax (% maximal inhibition) |
IC50 (nM) |
|---|---|---|---|---|---|
| (-)-U50,488 | agonist | 110 ± 2 | 46 ± 16 | - - - | - - - |
| 1 (butorphan) | agonist | 80 ± 7 | 1.3 ± 0.4 | - - - | - - - |
| 2 (Nalbuphine) | agonist | 81±4 | 27±3 | NI | NI |
| 3 (Naltrexone) | antagonist | 10 ±2 | NA | 67 ± 4 | 200 ± 13 |
| 4 (Naloxone) | agonist/antagonist | 25 ±2 | 10 ± 1 | 55 ± 3 | 320 ± 2 |
| 5 | agonist | 75±1 | 28 ±0.4 | NI | - - - |
| 6 | agonist/antagonist | 67 ± 2 | 3.2 ± 0.4 | 39 ± 3 | 600 ±170 |
| 7 | agonist | 76 ± 5 | 3.4 ± 0.9 | NI | NI |
| 8 | agonist | 86± 7 | 1.6±0.2 | NI | NI |
Membranes from CHO cells that stably expressed only the κ opioid receptor were incubated with varying concentrations of the compounds. The stimulation of [35S]GTPγS binding was measured as described in the Experimental Section. To determine the antagonist properties of a compound, membranes were incubated with 100 nM of the κ agonist U50,488 in the presence of varying concentrations of the compound. The Imax value is the maximal percent inhibition obtained with the compound. The IC50 value is the concentration of compound needed to produce half maximal inhibition. NI = No inhibition. NA = not applicable. Dashed lines indicate that the compound was not tested for antagonist properties because of its high Emax value.
Ligand 7 produced similar maximal stimulation of [35S]GTPγS binding (Emax) compared to that of butorphan (1), but was higher than that of naltrexone (3). Contrasted with the parent compound naltrexone (3), ligand 7 did not inhibit U50,488-stimulated [35S]GTPγS, suggesting that this ligand was κ agonist.
The agonist and antagonist properties of these ligands in stimulating [35S]GTPγS binding mediated by the μ opioid receptor are shown in Table 3. Ligand 6 produced minimal stimulation of [35S]GTPγS binding mediated by μ receptor while it produced complete inhibition (I ) of the DAMGO stimulated [35 max S]GTPγS binding comparable to that of butorphan (1) and nalbuphine (2). These data indicates that ligand 6 is a μ antagonist. Ligand 7 produced similar maximal stimulation of [35S]GTPγS binding (Emax) and maximal inhibition (I ) of the DAMGO stimulated [35 max S]GTPγS binding mediated by μ receptor comparable to that of butorphan (1), while producing higher maximal stimulation of [35S]GTPγS binding (Emax) and lower maximal inhibition (Imax) of the DAMGO-stimulated [35S]GTPγS binding mediated by μ receptor comparable to that of naltrexone (3). The data indicates that ligand 7 is a μ agonist/antagonist.
Table 3.
Agonist and Antagonist Properties of Compounds in Stimulating [35S]GTPγS Binding Mediated by the μ Opioid Receptora
| compd | Pharmacological properties |
Emax (% maximal stimulation) |
EC50 (nM) |
Imax (% maximal inhibition) |
IC50 (nM) |
|---|---|---|---|---|---|
| DAMGO | agonist | 120 ± 12 | 110 ± 9 | - - - | - - - |
| 1 (butorphan) | agonist/antagonist | 50 ± 3 | 1.6 ± 0.2 | 50 ± 3 | 20 ± 3 |
| 2 (Nalbuphine) | agonist/antagonist | 47±3 | 14±3 | 74±1 | 110±21 |
| 3 (Naltrexone) | antagonist | 6.7±2 | NA | 79 ± 1 | 17 ± 5 |
| 4 (Naloxone) | antagonist | 13±1 | NA | 92±2 | 23 ± 2 |
| 5 | agonist | 110±8 | 3.0±0.6 | NI | NI |
| 6 | antagonist | 5.1±2 | NA | 94±1 | 18±6 |
| 7 | agonist/antagonist | 43±1 | 4.4±0.3 | 50±2 | 160±44 |
| 8 | agonist/antagonist | 34±1 | 2.0±0.5 | 73±1 | 25±2 |
Membranes from CHO cells that stably expressed only the μ opioid receptor were incubated with varying concentrations of the compounds. The stimulation of [35S]GTPγS binding was measured as described in the Experimental Section. EC50 values were the concentration of compound needed to produce 50% of the Emax value. When the Emax value was 30% or lower, it was not possible to calculate an EC50 value. To determine the antagonist properties of a compound, membranes were incubated with 200 nM of the μ agonist DAMGO in the presence of varying concentrations of the compound. The Imax value is the maximal percent inhibition obtained with the compound. The IC50 value is the concentration of compound needed to produce half maximal inhibition. NI = No inhibition. NA = not applicable. Dashed lines indicate that the compound was not tested.
Conclusions
Heterodimeric ligands were synthesized containing κ agonist and μ agonist/antagonist pharmacophores at one end joined by a 10 carbon linker chain containing μ antagonists pharmacophores (naltrexone, naloxone and nalbuphine) at the other end. These ligands were evaluated in vitro by their binding affinity at opioid receptors. Ligands (6-8) either displayed slightly better or retained the same affinity at κ and μ receptors compared to the monovalent ligands 1-5. Ligands 6-8 showed reduced affinity at δ receptor compared to the monovalent ligands 1 and 5. Functional assays showed that the ligand 6 was a κ agonist/antagonist and μ antagonist, while ligand 7 was a κ agonist and μ agonist/antagonist.
A possible explanation for the lower affinity at the κ receptor displayed by ligand 5 (butorphan (1) with alkyl side chain) in comparison to butorphan 1 would be that the side chain in 5 hinders robust binding of the ligand at the κ and μ receptor site. Similarly the higher affinity at κ receptor for the bivalent ligands 6-8 containing butorphan (1) (a high affinity κ receptor ligand), a 10-carbon linking chain, and a μ antagonist ligand such as nalbuphine, naltrexone or naloxone, could be attributed to the higher binding affinity of both butorphan (1) at the κ site and the μ antagonist (nalbuphine, naltrexone or naloxone) at the μ receptor site.
These ligands either retained or displayed better affinity at κ, μ and δ receptors compared to the reference compounds. These heterodimeric ligands could serve as probes of the opioid receptor-oligomerization phenomena and represent a useful starting point in the synthesis of a new generation of ligands endowed with analgesic effects with minor tolerance and dependence. Potential medications for cocaine abuse requiring both κ agonist and μ antagonist,13,21 require further pharmacological studies to confirm these observations.
Experimental Section
Melting points were determined on a Thomas-Hoover capillary tube apparatus and are reported uncorrected. 1H and 13CNMR spectra were recorded on a Bruker AC300 spectrometer using tetramethylsilane as an internal reference. Element analyses, performed by Atlantic Microlabs, Atlanta, GA, were within 0.4% of theoretical values. Analytical thin-layer chromatography (TLC) was carried out on 0.2 mm Kieselgel 60F 254 silica gel plastic sheets (EM Science, Newark). Flash chromatography was used for the routine purification of reaction products. The column output was monitored by TLC.
General Procedure for the Preparation of Ligands 6-8
The acid 5 (0.6 mmol) and an appropriate opioid (0.5mmol) were dissolved in anhydrous dichloromethane (15 mL) under nitrogen. A catalytic amount of 4-dimethylaminopyridine was added, followed by N, N’-dicyclohexylcarbodiimide (0.6 mmol). The solution mixture was stirred at room temperature overnight, the solid was filtered off and the crude product was purified by column chromatography on silica gel (EtOAc : Et3N, 100 : 1) to afford the corresponding bivalent ligands.
(5α, 6α)-17-(cyclobutylmethyl)-6, 14-dihydroxy-4, 5-epoxymorphinan-3-yl 17-(cyclobutylmethyl)morphinan-3-yl sebacoylate (6)
colorless oil (40.4%). 1HNMR (300Hz, CDCl3): 7.10(d, J=8.4Hz, 1H), 6.92(d, J=2.1Hz, 1H), 6.85(dd, J=8.1Hz, 2.1Hz, 1H), 6.78(d, J=8.1Hz, 1H), 6.65(dd, J=8.1Hz, 2.4Hz, 1H), 4.64(d, J=5.1Hz, 1H), 4.60(d, J=4.8Hz, 1H), 4.17-4.08(m, 2H), 3.12(d, J=18.9Hz, 1H), 3.02(d, J=18.9Hz, 1H), 2.85-1.05(m, 59H). 13CNMR(75Hz, CDCl3): 172.3, 171.5, 149.2, 148.5, 141.9, 135.1, 132.9, 131.3, 130.7, 128.4, 121.5, 118.7, 118.4, 118.0, 91.6, 69.9, 66.5, 62.9, 61.4, 60.5, 55.8, 46.1, 45.6, 44.8, 43.6, 41.7, 37.7, 36.5, 34.8, 34.3, 33.8, 33.6, 32.6, 32.0, 30.8, 28.9, 28.8, 27.7, 26.8, 26.69, 26.65, 26.4, 26.3, 24.8, 24.7, 24.3, 23.9, 23.3, 22.0, 18.7, 18.6, 14.1. Anal.(C52H70N2O7 · 0.5 H2O) C, H, N.
17-(cyclopropylmethyl)morphinan-3-yl(5α)-17-(cyclopropylmethyl)-14-hydroxy-6-oxo-4,5-epoxymorphiana-3-yl sebacoylate (7)
colorless oil (48.4%). 1HNMR(300Hz, CDCl3): 7.10(d, J=8.1Hz, 1H), 6.92(d, J=2.1Hz, 1H), 6.85(dd, J=8.1Hz, 3Hz, 2H), 6.68(d, J=8.4Hz, 1H), 4.69(s, 1H), 3.21(d, J=5.7Hz, 1H), 3.12-0.84(m, 55H), 0.57(d, J=7.5Hz, 2H), 0.16(d, J=4.8Hz, 2H). 13CNMR(75Hz, CDCl3): 207.6, 172.4, 171.3, 149.2, 147.7, 142.0, 135.2, 132.6, 130.09, 130.07, 128.4, 122.8, 119.2, 118.5, 118.1, 90.6, 70.0, 61.9, 61.5, 59.2, 55.8, 50.6, 45.6, 44.9, 41.8, 37.7, 36.5, 36.0, 34.9, 34.4, 33.9, 31.2, 30.7, 29.03, 29.00, 28.9, 27.8, 26.7, 26.5, 24.8, 24.7, 24.4, 22.9, 22.1, 18.8, 9.3, 4.0, 3.8. Anal.(C51H66N2O7 ·1.5 H2O · 2 HCl) C, H, N.
(5α)-17-allyl-14-hydroxy-6-oxo-4,5-epoxymorphinan-3-yl-17-(cyclobutylmethyl)morphinan-3-yl sebacoylate (8)
pink solid (22.9 %). 1HNMR(300Hz, CDCl3): 7.10(d, J=8.1Hz, 1H), 6.92(s, J=2.1Hz, 1H), 6.85(dd, J=8.1Hz,1.5Hz, 2H), 6.70(d, J=8.4Hz, 1H), 5.86-5.75(m, 1H), 5.26-5.17(m, 2H), 4.69(s, 1H), 3.17-2.82(m, 6H), 2.65-0.93(m, 49H). 13CNMR(75Hz, CDCl3): 183.6, 172.4, 171.3, 149.2, 147.7, 142.0, 135.1, 134.9, 132.6, 130.0, 129.9, 128.4, 122.9, 119.2, 118.4, 118.2, 118.1, 90.5, 70.1, 62.0, 61.4, 57.6, 55.8, 50.5, 45.6, 44.7, 43.1, 41.6, 37.7, 36.5, 36.0, 34.8, 34.3, 33.9, 32.7, 31.1, 30.5, 29.0, 28.9, 27.9, 27.8, 26.7, 26.5, 24.7, 24.6, 24.4, 24.0, 23.0, 22.1, 18.8. Anal. (C50H64N2O7 ·2 H2O) C, H, N.
Opioid Binding to the Human μ, δ and κ Opioid Receptors
Chinese hamster ovary (CHO) cells stably transfected with the human κ opioid receptor (hKOR-CHO), δ-opioid receptor (hDOR-CHO), and the μ-opioid receptor (hMOR-CHO) were obtained from Drs. Larry Toll (SRI International, Palo Alto, CA) and George Uhl (NIDA Intramural Program, Bethesda, MD), respectively. The cells were grown in 100 mm dishes in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (10 000 units/mL) at 37°C in a 5% CO2 atmosphere. The affinity and selectivity of the compounds for the multiple opioid receptors were determined by incubating the membranes with radiolabeled ligands and 12 different concentrations of the compounds at 25°C in a final volume of 1mL of 50 mM Tris-HCl, pH 7.5. Incubation times of 60 min were used for the μ-selective peptide [3H]DAMGO and the κ-selective ligand [3H]U69,593. A 3h incubation was used with the δ-selective antagonist [3H]naltrindole.
[35S]GTPγS Binding Studies To Measure Coupling to G Proteins
Membranes from CHO cells stably expressing either the human κ or μ opioid receptor were used in the experiments. Cells were scraped from tissue culture plates and then centrifuged at 1000g for 10 min at 4°C. The cells were resuspended in phosphate-buffered saline, pH 7.4, containing 0.04% EDTA. After centrifugation at 1000g for 10 min at 4°C, the cell pellet was resuspended in membrane buffer, which consisted of 50 mM Tris-HCl, 3 mM MgCl2, and 1 mM EGTA, pH 7.4. The membranes were homogenized by with a Dounce homogenizer, followed by centrifugation at 40000g for 20 min at 4°C. The membrane pellet was resuspended in membrane buffer, and the centrifugation step was repeated. The membranes were then resuspended in assay buffer, which consisted of 50 mM Tris-HCl, 3 mM MgCl2, 100 mM NaCl, and 0.2 mM EGTA, pH 7.4. The protein concentration was determined by the Bradford assay using bovine serum albumin as the standard. The membranes were frozen at –80°C until use.
CHO cell membranes expressing either the human κ opioid receptor (15 μg of protein per tube) or μ opioid receptor (7.5 μg of protein per tube) were incubated with 12 different concentrations of the agonist in assay buffer for 60 min at 30°C in a final volume of 0.5 mL. The reaction mixture contained 3 μM GDP and 80 pmol of [35S]GTPγS. Basal activity was determined in the presence of 3 μM GDP and in the absence of an agonist, and nonspecific binding was determined in the presence of 10 μM unlabeled GTPγS. Then, the membranes were filtered onto glass fiber filters by vacuum filtration, followed by three washes with 3 mL of ice-cold 50 mM Tris-HCl, pH 7.5. Samples were counted in 2 mL of Ecoscint A scintillation fluid. Data represent the percent of agonist-stimulation [35S]GTPγS binding over the basal activity, defined as [(specific binding/basal binding) × 100] - 100. All experiments were repeated at least three times and were performed in triplicate. To determine antagonist activity of a compound at the μ opioid receptors, CHO membranes expressing the μ opioid receptor were incubated with the compound in the presence of 200 nM of the agonist DAMGO. To determine antagonist activity of a compound at the κ opioid receptors, CHO membranes expressing the κ opioid receptor were incubated with the compound in the presence of 100 nM of the κ agonist U50,488.
Supplementary Material
Acknowledgment
This work was supported in part by NIH Grants RO1-DA14251 (JLN) and K05-DA 00360 (JMB). Levorphanol tartrate was generously donated by Mallinckrodt Inc.
Abbreviations
- Butorphan
(−) 3-hydroxy-N-cyclobutylmethylmorphinan
- U-50488
trans (1s, 2s)-3, 4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide
- U-69593
(+)(5α, 7α, 8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4,5]dec-8-yl]-benzeneacetamide
- DAMGO
[D-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin
References
- (1).Brady LS, Devi LA. Dimerization of G-protein-coupled receptors: Implications for drug design and signaling. Neuropsychopharmacol. 2000;23:S1–S77. doi: 10.1016/S0893-133X(00)00158-5. [DOI] [PubMed] [Google Scholar]
- (2).Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- (3).Gomes I, Jordan BA, Gupta A, Rois C, Trapaidze N, Devi LA. G-Protein coupled receptor dimerization : implications in modulating receptor function. J. Mol. Chem. 2001;79:226–242. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- (4).Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discovery Today. 2004;9:641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- (5)a).Cvejic S, Devi L. Dimerization of the δ Opioid Receptor: Implication for a role in receptor internalization. J. Biol. Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]; b) Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharm. 2000;23:S15–S18. doi: 10.1016/S0893-133X(00)00143-3. [DOI] [PubMed] [Google Scholar]; c) George SR, Fan T, Xie Z, Tse R, Tamni V, Varghese G, O’Dowd BF. Oligomerization of μ- and δ-opioid receptors: generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- (6)a).Portoghese PS, Ronsisvalle G, Larson DL, Yim CB, Sayre LM, Takemori AE. Opioid agonist and antagonist bivalent ligands as receptor probes. Life Sci. 1982;31:1283. doi: 10.1016/0024-3205(82)90362-9. [DOI] [PubMed] [Google Scholar]; b) Erez M, Takemori AE, Portoghese PS. Narcotic antagonistic potency of bivalent ligands which contain beta-naltrexamine. Evidence for bridging between proximal recognition sites. J. Med. Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]; c) Portoghese PS, Larson DL, Sayre LM, Yim CB, Ronsisvalle G, Tam SW, Takemori AE. Opioid agonist and antagonist bivalent ligands. The relationship between spacer length and selectivity at multiple opioid receptors. J. Med. Chem. 1986;29:1855–1861. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]; d) Portoghese PS, Nagase H, Takemori AE. Only one pharmacophore is required for the κ opioid antagonist selectivity of norbinaltorphimine. J. Med. Chem. 1988;31:1344–1347. doi: 10.1021/jm00402a015. [DOI] [PubMed] [Google Scholar]; e) Portoghese PS, Ronsisvalle G, Larson DL, Takemori AE. Synthesis and opioid antagonist potencies of naltrexamine bivalent ligands with conformationally restricted spacers. J. Med. Chem. 1986;29:1650–1653. doi: 10.1021/jm00159a014. [DOI] [PubMed] [Google Scholar]; f) Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. Stereostructure-activity relationship of opioid agonist and antagonist bivalent ligands. Evidence for bridging between vicinal opioid receptors. J. Med. Chem. 1985;28:1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- (7).Lalchandani SG, Lei L, Zheng W, Suni MM, Moore BM, Liggett SB. Yohimbine dimers exhibiting selectivity for the human alpha 2C-adrenoceptor subtype. J. Pharmacol. Exp. Ther. 2002;303:979–984. doi: 10.1124/jpet.102.039057. [DOI] [PubMed] [Google Scholar]
- (8).Abadi AH, Lankow S, Hoefgen B, Decker M, Kassak MU, Lehmann J. Dopamine/serotonin receptor ligands, part III [1]: synthesis and biological activities of 7, 7′-alkylene-bis-6, 7, 8, 9, 14, 15-hexahydro-5H-benz[d]indolo[2, 3-g]azecines—application of the bivalent ligand approach to a novel type of dopamine receptor antagonist. Arch. Pharm. (Pharm. Med. Chem.) 2002;335:367–373. doi: 10.1002/1521-4184(200211)335:8<367::AID-ARDP367>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- (9)a).Halazy S, Perez M, Fourrier C, Pallard I, Pauwels PJ, Palmier C. Serotonin dimers : application of the bivalent ligand approach to the design of new potent and selective 5-HT (1B/1D)agonists. J. Med. Chem. 1996;39:4920–4927. doi: 10.1021/jm960552l. [DOI] [PubMed] [Google Scholar]; b) Perez M, Jorand-Lebrun C, Pauwels PJ, Pallard I, Halazy S. Dimers of 5HT1ligands preferentially bind to 5HT1B/1D receptor subtypes. Bioorg. Med. Chem. Lett. 1998;8:1407–1412. doi: 10.1016/s0960-894x(98)00222-4. [DOI] [PubMed] [Google Scholar]; c) Perez M, Pauwels PJ, Fourrier C, Chopin P, Valentin JP, John GW. Dimerization of sumatriptan as an efficient way to design a potent, centrally and orally active 5-HT1B agonist. Bioorg. Med. Chem. lett. 1998;8:675–680. doi: 10.1016/s0960-894x(98)00090-0. [DOI] [PubMed] [Google Scholar]
- (10)a).Christopoulos A, Grant MK, Ayoubzadeh N, Kim ON, Sauerberg P, Jeppesen L. Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J. Pharmacol. Exp. Ther. 2001;298:1260–1268. [PubMed] [Google Scholar]; b) Rajeswaran WG, Cao Y, Huang XP, Wroblewski ME, Colclough T, Lee S. Design, synthesis, and biological characterization of bivalent 1-methyl-1,2,5,6-tetrahydropyridyl-1,2,5-thiadiazole derivatives as selective muscarinic agonist. J. Med. Chem. 2001;44:4563–4576. doi: 10.1021/jm0102405. [DOI] [PubMed] [Google Scholar]; c) Messer WS, Rajeswaran JR, Cao WG, Zhang Y, El-Assadi HJ, Dochery AA. Design and development of selective muscarinic agonists for the treatment of Alzheimer’s disease: characterizaiton of tetrahydropyrimidine derivatives and development of new approaches for improved affinity and selectivity for M1 receptors. Pharm. Acta. Helv. 2000;74:135–140. doi: 10.1016/s0031-6865(99)00026-6. [DOI] [PubMed] [Google Scholar]
- (11).Decker M. Homobivalent quinazolinimines as novel nanomolar inhibitors of cholinesterases with dirigible selectivity toward butyrylcholinesterase. J. Med. Chem. 2006;49:5411–5413. doi: 10.1021/jm060682m. [DOI] [PubMed] [Google Scholar]
- (12).Owens J. Bridging the GPCR gap. Nature Rev. Drug Discov. 2006;5:105. [Google Scholar]
- (13).Neumeyer JL, Bidlack JM, Zong R, Bakthavachalam V, Gao P, Cohen DJ, Negus SS, Mello NK. Synthesis and opioid receptor affinity of morphinan and benzomorphan derivatives: mixed kappa agonists and mu agonists/antagonists as potential pharmacotherapeutics for cocaine dependence. J. Med. Chem. 2000;43:114–122. doi: 10.1021/jm9903343. [DOI] [PubMed] [Google Scholar]
- (14).Gates M, Montzka TA. Some morphine antagonists possessing high analgesic activity. J. Med. Chem. 1964;7:127–131. doi: 10.1021/jm00332a002. [DOI] [PubMed] [Google Scholar]
- (15).Neumeyer JL, Zhang A, Xiong W, Gu X, Hilbert JE, Knapp BI, Negus SS, Mello NK, Bidlack JM. Design and synthesis of novel dimeric morphinan ligands for κ and μ opioid receptors. J. Med. Chem. 2003;46:5162–5170. doi: 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- (16).Mathews JL, Peng X, Xiong W, Zhang A, Negus SS, Neumeyer JL, Bidlack JM. Characterization of a novel bivalent morphinan possessing κ agonist and μ agonist/antagonist properties. J. Pharmacol. Exp. Ther. 2005;315:821–827. doi: 10.1124/jpet.105.084343. [DOI] [PubMed] [Google Scholar]
- (17).Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Synthesis and preliminary in vitro investigation of bivalent ligands containing homo- and heterodimeric pharmacophores at μ, δ and κ opioid receptors. J. Med. Chem. 2006;49:256–262. doi: 10.1021/jm050577x. [DOI] [PubMed] [Google Scholar]
- (18).Neumeyer JL, Peng X,M, Knapp BI, Bidlack JM, Lazarus LH, Salvadori S, Trapella C, Bolboni G. New opioid designed multiple ligand form Dmt-Tic and morphinan pharmacophores, J. Med. Chem. 2006;49:5640–5643. doi: 10.1021/jm0605785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Portoghese PS. From Models to Molecules: Opioid Receptor Dimers, Bivalent Ligands, and Selective Opioid Receptor Probes. J. Med. Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- (20).Daniels JD, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A Bivalent Lignd(KDAN-18) Containing δ-Antagonist and κ-Agonist Pharmacophores Bridges δ2 and κ1 Opioid Receptor Phenotypes. J. Med. Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- (21).Archer S, Glick SD, Bidlack JM. Cyclozocine revisited. Neurochem. Res. 1996;21:1369–1373. doi: 10.1007/BF02532378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.