Abstract
Necrotizing enterocolitis (NEC) is the most common cause of gastrointestinal-related morbidity and mortality in the neonatal intensive care unit (NICU). Its onset is sudden and the smallest, most premature infants are the most vulnerable. Necrotizing enterocolitis is a costly disease, accounting for nearly 20% of NICU costs annually. Necrotizing enterocolitis survivors requiring surgery often stay in the NICU more than 90 days and are among those most likely to stay more than 6 months. Significant variations exist in the incidence across regions and units. Although the only consistent independent predictors for NEC remain prematurity and formula feeding, others exist that could increase risk when combined. Awareness of NEC risk factors and adopting practices to reduce NEC risk, including human milk feeding, the use of feeding guidelines, and probiotics, have been shown to reduce the incidence of NEC. The purpose of this review is to examine the state of the science on NEC risk factors and make recommendations for practice and research.
Keywords: necrotizing enterocolitis, neonatal, nursing, risk assessment, risk profile
Necrotizing enterocolitis (NEC) is the most common and frequently dangerous gastrointestinal emergency in premature infants in the neonatal intensive care unit (NICU).1 Although 90% of infants who develop NEC are born premature, full-term and near-term infants also develop the disease.2 Modern technology and advances in clinical care have improved our ability to sustain and support infants born prematurely, but the prevalence of NEC has not decreased.2,3 It is estimated that nearly 12% of infants born weighing less than 1500 g will develop NEC; of those, about 30% will not survive.2,3
The incidence of NEC is inversely related to an infant’s birth gestation, but marked variability is evident across NICUs and countries.4–6 Because outbreaks continue to occur, the Centers for Disease Control and Prevention recommends that hospitals consider maintaining surveillance for NEC like they do for other nosocomial infections.7,8 The economic cost of NEC is high, accounting for approximately 19% of neonatal expenditures and an estimated $5 billion per year for hospitalizations in the United States alone.9 If the disease can be managed medically, the cost of hospitalization has been estimated at around $73 700 with a length of stay exceeding on average 22 days more than that for other premature infants. However, if surgical care is required, there is at least an additional cost of $186 200, and infants stay an additional 60 days longer than other preterm infants.9
Necrotizing enterocolitis is a multifactorial illness with a poorly understood pathogenesis.1–5 The most important risk factor for NEC is prematurity and the earliest infants are at the greatest risk. Multiple factors, including hypoxia, feeding, sepsis, abnormal colonization of the bowel, and the release of inflammatory mediators stimulated by an ischemic-reperfusion injury in an immature gut, are thought to lead to NEC.2,5 An inflammatory cascade is thought to precipitate NEC as tumor necrosis factor α and platelet-activating factor work synergistically to contribute to mucosal damage in NEC. Yet, this inflammatory cascade is thought to be set off by an inciting event or chain of events. Such events may include hypoxia in utero or sepsis. The release of inflammatory mediators signals neutrophil activation, increased permeability of the vasculature, release of reactive oxygen species, and ultimately vasoconstriction with ischemic-reperfusion injury.2,5 As the mucosal barrier breaks down and NEC becomes severe, it can lead to overwhelming sepsis and death in the worst cases.2,5
Understanding how the combined occurrence of risk factors may lead to the chain of events setting the stage for NEC may lead to heightened vigilance to detect the early symptoms of NEC.5,6 The purpose of this article is to describe and critique the state of the science on NEC risk factors in the context of gestation, feeding practices, and pathophysiology. Preventive treatments will also be discussed. Risk factors will be discussed related to their time of occurrence: before birth (prenatal), during the labor and delivery process (intrapartum), or as part of the clinical course (clinical course factors). This evidence will be used to develop a clinical profile of the infant most at risk to develop NEC.
Review of the Literature
A comprehensive review of literature using the databases PubMed, CINAHL, EBSCO, and Web of Science was conducted using the key words “necrotizing enterocolitis,” “risk,” “clinical profile,” and “risk factors.” Additional relevant research articles were identified from the bibliographies of selected research articles. Literature reviewed included research articles, reviews, and those related to clinical management.
Risk Factors
Difference Between NEC in Full-term Infants and Premature Infants
A different pattern of neonatal susceptibility has been hypothesized between those born early and those born at term.2,3,10 Babies born after 35 weeks’ gestation who develop NEC are more likely than those born before 35 weeks to have experienced low Apgar scores, birth asphyxia, sepsis, or congenital defects (specifically cardiac or gastrointestinal anomalies) leading to a mesenteric ischemia.11 Although NEC predominately affects premature infants, approximately 10% of cases are discovered in infants born after 36 weeks’ gestation.12 Late preterm infants, in particular, are more likely to develop NEC if they have other risk factors, including intrauterine growth retardation, polycythemia, hypoglycemia, sepsis, exchange transfusions, umbilical lines, gestational diabetes, and being born to a mother with chorioamnionitis.11–13 Stout and colleagues14 conducted a retrospective case-control study in multiple centers within a single-hospital system of infants who developed NEC in the first week of life, most of whom were term or late preterm.14 Early-onset sepsis, drug exposure, and respiratory distress were all associated with NEC, and those who developed NEC were significantly less likely to have received breast milk and more likely to have been fed only formula (Table 1).14
TABLE 1.
Necrotizing Enterocolitis (NEC) Risk Differences by Gestation
| Premature Infants | Late Preterm and Full-Term Infants |
|---|---|
| Birth weight < 1000 g13,50 | Cyanotic congenital heart disease4,81 |
| Highest risk with lowest GA13,50 | Polycythemia19 |
| Feeding | Intrauterine growth restriction13–16 |
| Unstandardized approach to feeding and management of feeding intolerance63,65,66 | Formula feeding12,14 |
| Formula feeding41,56–59 | Maternal hypertensive disease17 |
| Breast milk fortifier80 | HIV-positive mother20 |
| H2 blockers31 | Umbilical catheters19 |
| Chorioamnionitis18 | Exchange transfusion19 |
| Sepsis4,24 | Perinatal asphyxia13 |
| Number of infections28 | Mechanical ventilation28 |
| Prolonged (≥5 d) first course of antibiotics70 | Sepsis12,14 |
| Patent ductus arteriosus4,23,24 | Maternal illicit drug use14 |
| Indomethacin treatment23,26,50 | Respiratory distress syndrome14 |
| Glucocorticoids and Indomethacin in first week of life50 | Apgar score < 7 at 5 min13,50 |
| Absence of umbilical arterial catheter50 | |
| Mechanical ventilation4,21,28,50 | |
| Transfusions33,34 | |
| HIV-positive mother20 | |
| Antenatal cocaine use16 | |
| Perinatal asphyxia21 | |
| Apgar score < 7 at 5 min35 | |
| Black race28,50 | |
| Antenatal glucocorticoidsa50 | |
| Morphine infusion80 | |
| Vaginal delivery50 |
Abbreviations: GI, gastrointestinal; HIV, human immunodeficiency virus; NEC, necrotizing enterocolitis.
Prenatal Risk Factors
Because NEC is hypothesized to result from a reperfusion injury that stimulates an inflammatory cascade with resultant damage to the vasculature and the intestinal mucosa in watershed areas of the intestine, any maternal condition that stimulates such an event may be considered. Possible risk factors present in the prenatal course include maternal drug use (specifically cocaine),15,16 maternal hypertensive disease including pregnancy-induced hypertension,13,17 maternal infections,18,19 and problems related to placental blood flow that may result in a growth-restricted newborn.13,17 Placental disease restricts the quality and quantity of nutrition to the developing fetus, leads to a growth-restricted newborn, and may lead to metabolic compromise if combined with other risk factors.13
Maternal hypertension may lead to placental disease, but it is unclear what impact it has on NEC. In a cross-sectional prospective study (N = 211), Bashiri and colleagues17 found that maternal hypertensive disorders were an independent predictor of NEC in infants weighing less than 1500 g at birth.17 A common treatment for maternal hypertension is magnesium sulfate, and Ghidini and colleagues15 found no reduction in NEC risk from administration of magnesium sulfate during labor to mothers with hypertensive disorders.15 Whether the combination of maternal hypertensive disease with other risk factors may increase an infant’s likelihood of developing NEC remains unknown but appears plausible.
In a case-controlled study in a single NICU spanning 8.5 years of admissions (N = 237), Desfrere and colleagues20 discovered a relationship between maternal human immunodeficiency virus (HIV)–positive status and NEC.20 Mothers positive for HIV were 6.6 times more likely than those without to have babies who developed NEC. This association had not been previously reported and needs to be replicated. Despite the study’s limitations, the authors recommend judicious monitoring for NEC of premature infants born to HIV-positive mothers.
Maternal drug use is a risk factor in full-term infants, but it is unclear whether it leads to NEC in those born prematurely.16 In an epidemiologic study, full-term babies who developed NEC were more likely to have experienced prenatal or intrapartum risks like infection or drug exposure, but premature babies did not experience more NEC when exposed to similar risks.3 Hand and colleagues16 found no difference in NEC incidence between premature babies exposed to cocaine in utero versus those not exposed.16 Conversely, Stout and colleagues14 analyzed infants who developed NEC during the first week of life (mostly term or near-term) and found that those who did were more likely to have positive meconium test for illicit drug exposure (P < .05).14
Intrapartum Risk Factors
Intrapartum risk factors that may contribute to NEC are related to hypoxic-ischemic compromise or bacterial exposure, although further research is needed. High umbilical cord artery base deficit was found to contribute to NEC in growth-restricted infants (P < .01).13 Challenges exist in the communication breakdowns that may occur between the team taking care of the mother and the team taking care of the baby. A lack of available maternal records and ongoing communication of maternal risk factors during the infant’s stay in the NICU may interfere with a nurse’s ability to understand intrapartum factors that may be continuing to influence the infant’s clinical course. Access to, and review of, maternal records is imperative to provide the safest and most appropriate care to the infant and to ascertain NEC risk.
Intrapartum risk factors may include maternal cardiac arrest, umbilical cord prolapse, placental abruption, and chorioamnionitis. Maternal infections that develop during the intrapartum period may also be suspect. Histologic chorioamnionitis with vasculopathy was associated with a 2.5 times higher risk of NEC (OR 2.6, P = .02) in 1 study.18 Because of the high odds ratio reported in this study, it may be important for all placentas to be evaluated by pathology after a premature birth. Pathology findings should be communicated to the neonatal team and not just to the obstetrician or perinatologist.
Clinical Course Factors
The events of the clinical course, including delivery room resuscitation and treatments in the first days of life, undoubtedly play a role in NEC risk. Recent case-control studies have identified increased risk for NEC in infants who had a critical start (ie, required resuscitation at delivery, mechanical ventilation in the first days of life, were born because of placental accidents including placental abruption and cord prolapsed, or were born growth-restricted because of placental insufficiency in utero).3,4,9,21,22
A hemodynamically significant patent ductus arteriosus (PDA) has been shown to put an infant at risk for NEC.4,23,24 Indomethacin has been used to medically close a PDA, but it is recommended that ibuprofen is a better pharmacologic choice to reduce NEC risk.25 In a meta-analysis of 15 studies (n = 865) comparing ibuprofen with indomethacin to close a PDA, the risk for NEC decreased with ibuprofen (risk reduction [RR] = 0.68, 95% confidence interval [CI]: 0.47–0.99).26 One center virtually eliminated NEC in their unit over a 5-year period. They used a standardized feeding protocol, which required feedings to be held when Indomethacin was given to close a hemodynamically significant PDA or the infant was septic.27
Gregory21 evaluated the presence of clinical predictors for NEC in premature neonates (N = 247), using a case-control design in a single center to develop a clinical profile of the infant at risk for NEC.21 Infants who required mechanical ventilation during the neonatal period were 13 times more likely to develop NEC. Furthermore, infants were 6.4 times more likely to develop NEC if they did not receive fortified breast milk and 28.6 times more likely to develop it if they required respiratory support and did not receive fortified breast milk feedings. Additional risks noted were bag-mask ventilation (P < .002), endotracheal tube intubation in the delivery room (P < .001), hemodynamic support/neonatal code (P < .0001), a hypotensive episode (P < .0001), hypothermic episode (P < .002), and receiving fortified breast milk (P = .054). Gregory21 described the infant at most risk for developing NEC as one born at a birth weight of 500 to 1500 g, less than 28 weeks’ gestation, who required resuscitation in the delivery room, demonstrated physiologic instability during the early neonatal period, and was fed (breast milk or formula).21
In a secondary analysis of the Kids Inpatient Data data set, Carter and Holditch-Davis28 identified the number of infections in the neonatal period and length of time on a ventilator as predictors of NEC in babies born early.28 The researchers also found that African-American babies were diagnosed with NEC more often than those of other race. Antibiotic therapy early in the clinical course prevents bacterial colonization with potentially beneficial microbes and predisposes the colonization of antibiotic-resistant bacteria common in the NICU.29
Feeding intolerance and subsequent NEC may result from contaminated enteral feeding tubes.30 In 1 study, infants had an enteral feeding tube in place for 1 week and when the tubes were removed, they were cultured and 57% (N = 125) tubes were found to be contaminated. Infants treated with histamine type 2 (H2) antagonists were more likely to have contaminated tubes (P < .05). H2 antagonists reduce the acidity of the stomach and potentiate the growth of bacteria. When formula was fed via the contaminated tube, an alarming 75% of infants experienced feeding intolerance and 4 infants developed surgical NEC with the causative organism (Enterobacter or Klebsiella) identified as the same bacteria in the contaminated tubes.30
H2 blockers were found to be associated with an increased risk for NEC in very low-birth-weight (VLBW) infants (OR = 1.71, 95% CI: 1.34–2.19, P < .0001).31 H2 blockers (eg, ranitidine [Zantac], famotidine [Pepcid], or cimetidine [Tagamet]) alter the pH of the stomach acid and are prescribed to reduce acid reflux. Of note, this study did not control for feeding differences that may also have affected NEC incidence. The National Institutes of Health recommended careful evaluation of giving H2 blockers to premature infants, given the high morbidity and mortality from NEC.32
Caution is recommended when transfusing infants at high risk for NEC.5,33,34 Necrotizing enterocolitis after transfusion is more likely among infants fed full feeding in the 24 hours before a transfusion, particularly if it is a bovine product, and infants are more likely to need surgery.33 Of the babies who developed NEC stage III, 38% did so after a prophylactic blood transfusion in the 48 hours prior to NEC diagnosis.33 The infant at highest risk to develop transfusion-related NEC also is very premature and has a history of being acutely ill.34
In term and late pre-term infants, transfusions and blood product administration have also been linked to NEC. Exchange transfusions to treat high bilirubin in infants with hemolytic disease caused by Rh and ABO incompatibility has been shown to relate to NEC, particularly in term infants.35 High-dose intravenous immunoglobulin was also associated with NEC in late preterm and term infants (OR = 31.66; 95% CI: 3.25–308.57).36 Thrombocytopenia is present in NEC but is also a marker of illness severity. Infants who die from NEC have a lower nadir platelet count (P < .05).37,38
Feeding
The rate and substance of infant feeding have been a major focus of NEC research. Ninety percent of infants who develop NEC have been fed, and more infants fed formula develop NEC than who are fed breast milk.2,39–41 Altering feeding regimens is a powerful measure clinicians can take to reduce the risk for NEC, although the balance between supporting neonatal growth and hedging a baby’s risk of developing NEC is challenging. Few randomized controlled trials (RCTs) are available to determine the optimal rate of feeding advancement. One controversial study found that infants fed at 20 mL/kg per day were more likely to develop NEC than those fed at 10 mL/kg per day.42 The fast advance group did not show improved gut motility over the slow advance group. However, this study has come under scrutiny for methodologic flaws and a nonsignificant difference between groups, given the high background rate of NEC (12%).43 Since that time, others have evaluated fast feeding advance with vigilant exclusion of infants on the basis of NEC risk (birth weight < 1000 g, history of mechanical ventilation, presence of gastrointestinal abnormalities, respiratory distress, Apgar score at 1 minute < 3, presence of umbilical catheter, history of vasopressors) and found faster advance rates to be safe and lead to fewer days to full feedings without an increase in NEC.44 In a recent clinical practice guideline, it is recommended that feedings be advanced at a rate of 15 to 35 mL/kg per day.25 In a meta-analysis of 4 trials (N = 496), slow (15–20 mL/kg per day) versus fast advance (30–35 mL/kg per day) of feedings was evaluated. No statistically significant difference was found between the 2 on the risk of NEC (RR = 1.43, 95% CI: 0.78–2.61).43 Infants fed at slow advance took longer to regain birth weight. It is important to note that few infants who were VLBW or growth restricted were included in this review and more RCTs are recommended to determine the best rate of advance.
Gastric residuals reveal evidence of feeding intolerance and may give clinicians insight into the integrity of the bowel mucosa. Bertino and colleagues24 found a history of bloody gastric residuals at any time during the clinical course to be an early relevant marker for NEC (n = 17). The bloody quality of the residual is hypothesized to be evidence of a disruption in bowel integrity. In this single-center Italian study, the incidence of NEC over a 10-year period was 2%. This center fed VLBW infants exclusively human milk, either provided by the mother or obtained from a donor bank, used standard feeding guidelines, and routinely used probiotics. This study found that the gastric residuals were a marker of intolerance of feedings but not as sensitive as a measure as a history of bloody gastric residuals, indicating a break in the integrity of the bowel layers.24
Delaying feedings for fear of NEC can result in more central catheter days, higher risk for acquired bloodstream infections, and delayed gut development40 and is not recommended as a strategy to reduce NEC.5,25 Following premature birth, an active in utero gut becomes inactive when enteral feeding is delayed. Delaying feeding for 3 days has been shown to lead to gut atrophy in an animal model.45 The National Institute of Child Health and Human Development (NICHD) estimated that the mean time of beginning feedings in US NICUs was 6.4 days.46 In a large retrospective review (n = 385), infants weighing less than 1500 g who started enteral feedings sooner (2.8 days vs 4.8 days) were less likely to develop sepsis from central catheter infections, had fewer days of parenteral nutrition, and showed no significant increase in NEC.47 A meta-analysis of 9 trials showed no effect of early trophic feeding versus fasting on NEC: relative risk (RR = 1.07, 95% CI: 0.67–1.70) and risk difference (RD 0.01, 95% CI: 0.04–0.05) (Table 2).48
TABLE 2.
Definitions of Feeding Intolerance and Criteria to Stop Feedings
| Kuzma-O’Reilly et al82 | Patole et al64 | Krishnamurthy et al44 |
|---|---|---|
| Marked abdominal distention or discoloration | Bile stained gastric aspirate without other symptoms | Gastric residual > 50% of previous feeding |
| Signs of perforation | Bilious gastric aspirate with vomiting and/or abdominal distention | Vomiting > 3 times in 24 h |
| Frank blood in stool | Gastric aspirate ≥ 30% of feeding given over the previous 4 h or bile stained aspiratea | Bile or blood stained emesis |
| Gastric residuals ≥ 25%–50% of volume for 2–3 feedings | Gastric aspirate ≥ 30% of feeding given over the previous 4 h with vomiting and/or abdominal distention | Abdominal tenderness |
| Bilious residual | Abdominal wall erythema | |
| Bilious emesis | Decreased bowel sounds | |
| Significant apnea/bradycardia | Increase > 2 cm in abdominal girth between feedings | |
| Cardiovascular instability | Frank or occult blood in stool | |
| Recurrent apnea | ||
| Neonatal seizure | ||
| Mechanical ventilation | ||
| Vasopressor therapy |
If gastric residual was 30% or more of feeding given over previous 4 hours, then residual was returned to the stomach and the next feeding was held. If the residual before the next feeding was normal, feedings were resumed. If gastric aspirates were large for 4 consecutive feeds, feedings were discontinued for 4 hours and then resumed at 10 mL/kg per day of where they were before the feedings were discontinued.
Risk Reduction Strategies for NEC
Strategies to reduce NEC risk have been demonstrated but not universally adopted. Christensen et al6 claim that implementation of feeding guidelines and increasing the availability and use of human milk feeding alone could cut NEC incidence in half.6 Others have issued specific recommendations to prevent NEC, using strategies that have been demonstrated effective in meta-analyses (Table 3).5
TABLE 3.
Recommendations to Prevent Necrotizing Enterocolitisa
| Single course of antenatal corticosteroids49,83,84 |
| Early and preferential use of breast milk or donor milk if mother’s milk is unavailable41,56–59 |
| Standardized feeding guidelines with conservative volumes (20–25 mL/kg per day) to start64,65,82 |
| Awareness of potential risk factors (eg, sepsis, intrauterine growth restriction, hemodynamically significant patent ductus arteriosus treated with indomethacin, formula feeds, H2 blocker therapy)6,64,65 |
| Use ibuprofen instead of indomethacin to close a patent ductus arteriosus25,26 |
| Restrict fluid intake, without compromising hydration and nutrition85 |
Antenatal Steroids
Evidence is mixed on the impact of antenatal steroids on NEC. In a recent clinical practice guideline, it was recommended that mothers receive a single course of antenatal corticosteroids if preterm delivery is expected.25 In a meta-analysis, Roberts and Dalziel49 found that corticosteroids reduced the risk for NEC approximately by half (RR = 0.46, 95% CI: 0.29–0.74, 8 studies, N = 1675).49 Treating 32 mothers with steroids strongly reduces the incidence of 1 case of NEC.49 However, others have demonstrated an increased risk for NEC when mothers are treated with steroids.50–52 Guthrie et al50 found steroids to be associated with an increased risk for NEC that was irrespective of birth weight (OR = 1.6, 95% CI: 1.3–2.0, P < .001).50 In the study by Guthrie et al50 (N = 14 878), 59% of mothers had received antenatal steroids but they were unable to determine whether they were given multiple courses. They hypothesized that in utero glucocorticoid exposure selectively stimulates the maturation of the small bowel and skews tissue growth leading to a weak mucosal barrier and increased NEC.
Human Milk Feeding
In 1 large prospective trial, breast milk–fed infants were found to develop NEC 6 to 10 times less often than those fed formula exclusively and 3 times less often if they received a mixture of breast milk and formula.41 The presence of bile acids that induce ileal damage is higher in infants fed formula than in those fed breast milk.53 Epidermal growth factor, a compound that limits ileal damage from bile acids and has been shown to be protective against NEC, is present in breast milk but not in formula.54 Breast milk feeding is clearly the best feeding for premature infants and is rich in immunologic factors including immunoglobulin and growth factors.55 Nurses play an important role in encouraging mothers to provide breast milk for their babies and educating them on how to bring in and sustain their milk supply. Often nursing actions have focused on feeding as a means to prevent NEC without taking into account the other risk factors that may put a baby in danger of developing NEC. It is estimated that feeding 10 babies with breast milk exclusively can prevent 1 case of NEC and treating 8 infants with breast milk can prevent 1 case of surgical NEC or death.56
When mother’s milk is not available, donor milk (DM) is the next best choice for a premature baby’s early feedings.57–60 Boyd et al60 found in a meta-analysis (7 RCTs, n = 471) that DM decreased an infant’s risk for NEC by nearly 80% compared with formula (RR = 0.21, 95% CI: 0.06–0.76).60 Some organizations have made a change in practice to supply DM to all infants at highest risk for NEC (eg, VLBW, < 33 weeks) if mother’s milk is unavailable and dramatically reduced their NEC rates.6,24,57 Using a direct cost-comparison model, Wight61 estimated that for every $1 spent on DM, $11 to $37 in NICU costs might be saved using only reduced length of stay as the outcome. Using a cost-reduction model of DM calculated in 2002 estimating savings from preventing NEC and sepsis, the estimated savings to a hospital approaches $10 000 000 a year (estimated 200 infants fed DM, average cost per day = $3300, average decreased length of stay = 15 days) and a state can save $32 682 000 per year.62 Although DM is more costly than formula, organizations can ultimately save money by reducing length of stay, preventing NEC, and preventing sepsis by using DM.62
Standardized Feeding Guidelines
Some centers have noted a consistent decline in the incidence and severity of NEC following the adoption of feeding guidelines.63–66 Feeding guidelines are written orders that replace the practice of daily feeding orders.5,6 Standard thresholds are set within the feeding guideline for how to manage signs of feeding intolerance and include criteria for discontinuing feedings (Table 2). In a meta-analysis of 6 observational studies, it was estimated that instituting feeding guidelines reduced the risk for NEC by up to 87% for infants weighing less than 2500 g and 29% in infants weighing less than 1500 g.65 Multiple authors have hypothesized that it was the increased vigilance for the early signs of NEC, increased awareness of NEC risk factors, use of a standard approach to management of feeding intolerance, and consistent criteria to advance feedings that ultimately reduced NEC rather than the specific details of the feeding guideline.6,63–66
Probiotics
Probiotics effectively reduce the incidence and mortality from NEC; however, clinical trials are ongoing to determine the safest and most effective preparation of probiotics (see clinicaltrials.gov).67 Probiotics alter the quality of intestinal mucus, improve gut motility, and control the production of inflammatory cytokines.2,68 When the intestine of the preterm infant is colonized with pathogenic bacteria, probiotics compete and may limit the overgrowth of such pathogens. A recent meta-analysis of 11 RCTs (N = 2176) found that probiotics reduce the risk for NEC by approximately 65% and estimated that treating 25 infants would likely prevent 1 case of NEC.67 Most of the RCTs included in the meta-analysis used some combination of bifidobacterium, and trials that did not use bifidobacterium showed no benefit.67,68 Clinicians continue to be cautious about using probiotics because of the risk of sepsis in the extremely vulnerable population of infants, particularly those weighing less than 1000 g. No increased risk for sepsis was noted when probiotics were given. One single-centered RCT demonstrated that in infants weighing 750 to 1499 g at birth (N = 231), bifidobacterium and lactobacillus casei decreased the time to full feeds (P = .02), no NEC cases in those given probiotics and 4 deaths in the control group (RR = 0.00, unable to calculate CI, P < .05).69 Prolonged antibiotic use further disrupts the colonization of the preterm gut and has been shown to increase NEC risk perhaps because of the destruction of good bacteria that compete with pathogenic bacteria.70 Although research is ongoing to identify the safest preparation of probiotics, some argue that future placebo-controlled trials are unnecessary, perhaps unethical, and it is time to change practice and give infants probiotics.68
Disease Presentation
If an infant develops NEC, symptoms may appear insidiously over the few days before a NEC diagnosis or they may appear acutely.71,72 Clinical signs and symptoms are nonspecific and include temperature instability, bradycardia and apnea,73 hypotension, abdominal wall erythema, increased pregavage residuals, abdominal distention, emesis, blood in the stool, absent bowel sounds, abdominal tenderness, and occasionally a right lower quadrant mass.72 When a baby develops symptoms, nurses need strong assessment skills, clinical judgment, and communication skills to advocate for appropriate medical treatment (Table 3). In one study, the most common gastrointestinal symptoms prior to NEC presentation were blood in the stool (32% of cases), new emesis or increased gastric residuals before feeding (48% of cases), and increasing abdominal girth (66% of cases).71 Laboratory indicators of disease include respiratory acidosis, metabolic acidosis, thrombocytopenia, and neutropenia.72
What is known is that if the disease is recognized in its early stages, feedings can be discontinued, the stomach decompressed, and antibiotics initiated to treat possible sepsis.38,72 Serial imaging can be taken to determine whether the disease is advancing.25 Radiologic evidence of NEC includes pneumatosis intestinalis (the presence of air between the layers of the bowel; Figure 1) and advanced NEC is suspected if pneumoperitoneum (indicates perforation; Figure 2) occurs.74
FIGURE 1.
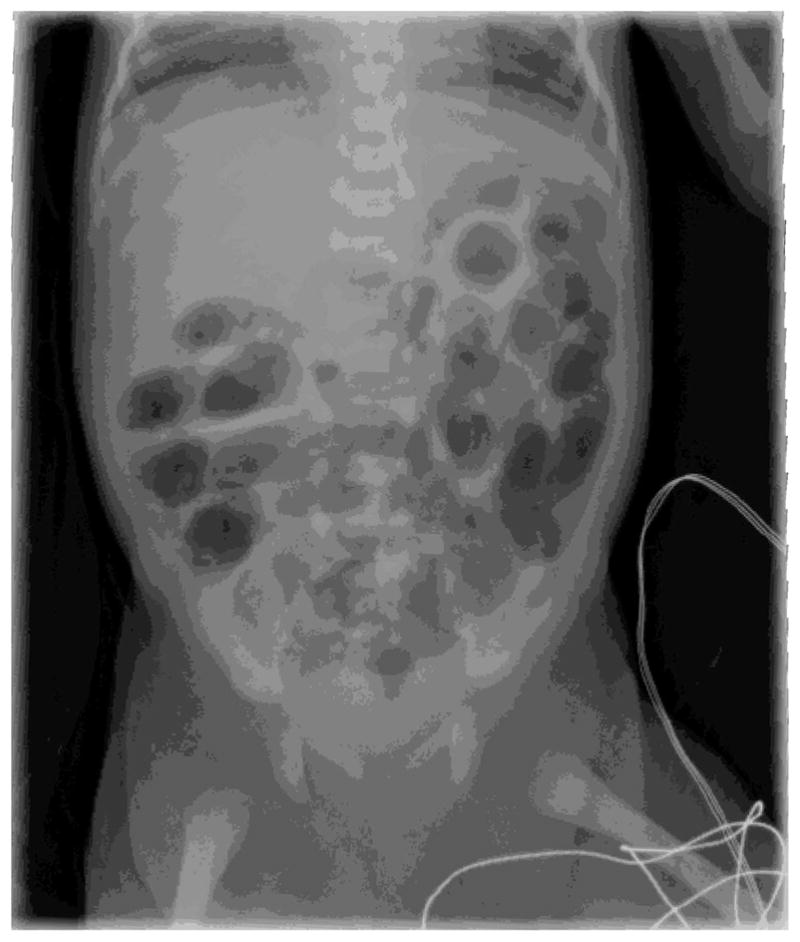
Pneumatosis intestinalis.
FIGURE 2.
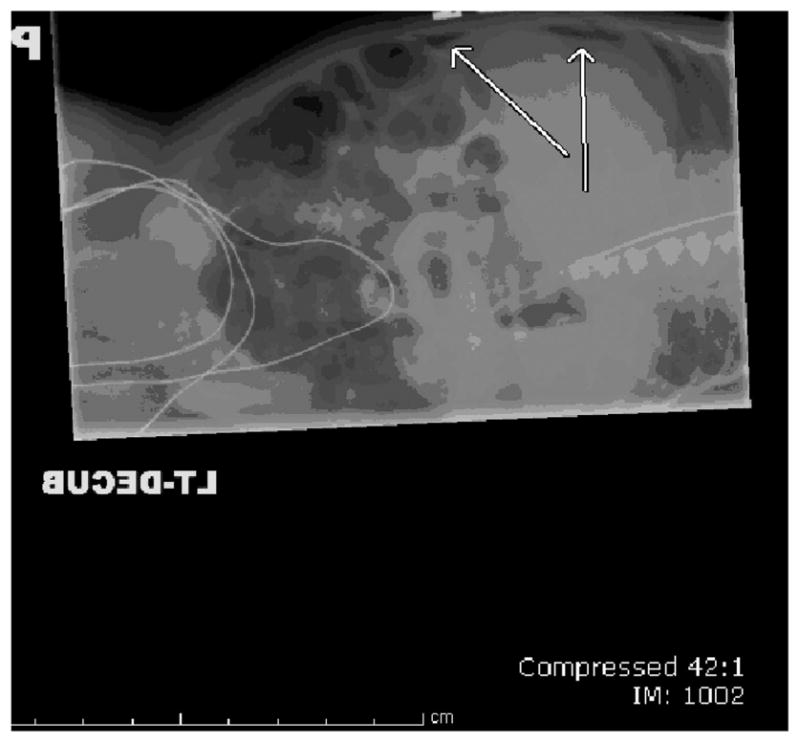
Pneumoperitoneum.
Treatment
Treatment for NEC generally includes bowel rest, gastric decompression, supportive management of hydration and perfusion, correction of hypotension, metabolic acidosis, and hyponatremia.5,72 Frequent laboratory and radiologic assessments are conducted to determine whether the illness has progressed. Antibiotics are given for possible sepsis and an anti-fungal is added if perforation is suspected or confirmed.5,25,72 The infant who develops NEC can rapidly deteriorate as sepsis becomes overwhelming. If air escapes through the bowel into the abdominal cavity, bowel perforation with severe decompensation may occur. Necrotizing enterocolitis is staged using Bell’s Criteria; nursing actions vary, depending on the clinical presentation and severity of the disease (Table 4).75
TABLE 4.
Nursing Actions by Bell’s Stage for Necrotizing Enterocolitisa
| Bell’s Stage | Systemic Signs | Gastrointestinal Signs | Imaging Signs | Nursing Actions | Prognosis |
|---|---|---|---|---|---|
| Pre-NEC: Maintain a high index of suspicion, stay aware of neonatal risk (prenatal, intrapartum, and clinical course factors), and rapidly respond to the infant’s signs of feeding intolerance, nonspecific symptoms, and change in behavior. | |||||
| Stage I (suspected NEC) | Apnea, bradycardia, hypothermia, lethargy | Poor feeding, vomiting, increasing gastric residuals, mild distention of abdomen, fecal occult blood | Distention Mild ileus Inconclusive May advocate for abdominal ultrasound |
Notify physician or NNP, npo, IV fluids, obtain laboratory tests (blood culture, CBC), obtain imaging, place gastric decompression NG tube (vented NG tube to low continuous suction or a nonvented tube to low intermittent suction), continue to monitor closely, consider transport to surgical center, encourage a surgical consult, educate and support parents | Good, may progress to stage II or III |
| Stage II (Definite NEC) | Same as stage I | Marked abdominal distention, consistent occult blood, gross bleeding from GI tract | Ileus, small bowel separation (from peritoneal fluid or bowel wall that is thickened or edematous), fixed bowel loops, pneumatosis intestinalis, portal venous gas | As for stage I, surgical consult, prepare infant for rapid transport to a surgical center, replace gastric losses q4h, serial radiographs, continue vigilant monitoring of clinical worsening, prepare for surgery as indicated | Fair, relates to need for surgery, timely treatment |
| Stage III (Advanced NEC) | Stage I plus deterioration of clinical status as with septic shock, metabolic derangement | As in stage II with possible evidence of significant GI hemorrhage | As in stage II with possibly GI perforation, pneumoperitoneum | Intensive management: transport if needed, prepare for emergency surgery (open laparotomy for resection of necrotic bowel or peritoneal drainage), Postoperative care, critical care management of sepsis | Tenuous, viability of bowel, severity of illness, high mortality |
Abbreviations: CBC, complete blood cell; GI, gastrointestinal; IV, intravenous; NEC, necrotizing enterocolitis; NG, nasogastric; NNP, neonatal nurse practitioner; npo, by mouth.
Adapted from Bell et al.85
Indications for surgical consultation include abdominal wall cellulitis, fixed dilated bowel, tender abdominal mass, or clinical deterioration not responsive to medical management (metabolic acidosis, thrombocytopenia, increasing respiratory support, hypovolemia, oliguria, leucopenia, leukocytosis, hyperkalemia, and increased third-space losses).5,25 A recent clinical practice guideline strongly recommends that all infants who develop NEC should be transferred to a level III NICU, cared for by a surgeon if one or more indications for surgery are present and treated operatively if pneumoperitoneum or portal venous gas are present.25 Primary peritoneal drainage or laparotomy with bowel resection may be used to decompress the gut and/or remove necrotic bowel. One study found a 4 times greater risk of death from NEC if primary peritoneal drainage was used instead of laparotomy with bowel resection when the infant showed signs of metabolic derangement (OR = 4.43; CI: 1.37–14.29; P = .0126).38 Metabolic derangement, a measure of acuity, was defined by assigning 1 point per indicator (thrombocytopenia, metabolic acidosis, neutropenia, left shift of segmented neutrophils, hyponatremia, bacteremia, and hypotension) and calculating a total. When 2 or more indicators of metabolic derangement were present, the odds of mortality dramatically increased (P = .0002).38
Outcomes
To improve NEC outcomes, all clinicians can focus on early recognition, increasing the percentage of infants who receive mother’s milk and considering the use of feeding guidelines. As part of the feeding guideline, careful attention to identifying the infant most at risk may allow the clinician to interpret feeding intolerance in a systematic way. In a meta-analysis of more than 180 studies, extremely strong evidence was identified supporting the poorer long-term developmental outcomes for surgically managed NEC infants compared with those managed medically.76 When gestational age is controlled, the most significant predictor of outcome is whether the infant requires surgery.77 The only persistent predictor of outcome from NEC remains gestational age. However, it is still recommended that babies who develop NEC should be transferred to a tertiary care center early because most of the deaths in the nonsurgical center were presurgery, suggesting that infants who develop NEC outside a surgical center may be more likely to progress to perforation of the bowel during the time required for transport.77 However, it is clear that once NEC advances in severity, if surgery is not done, the infant will die.
Implications for Research and Practice
Research is ongoing to investigate the pathogenesis of NEC and the efficacy and safety of preventative therapies (Table 3).78 The impact of nutritional fortifiers in breast milk on NEC needs more study.40 Most premature babies receive some form of supplementation to support growth, but the impact of such fortifiers on NEC is unclear. More RCTs of feeding protocols and preventive therapies are needed to further substantiate the benefit of such practices. Clinical trials of probiotics are ongoing and scientists continue to look for a genetic predisposition and biomarkers. Finally, as scientists better understand NEC pathogenesis at the cellular level, the development of additives (including epidermal growth factor or heparin-binding growth factor) to prevent the inflammatory cascade that leads to epithelial injury may reduce NEC.
As science advances our knowledge of NEC and the means to prevent it, nurses will continue to play a critical role in recognizing NEC symptoms and ensuring appropriate and timely treatment. Specific changes in practice may include a standard approach to feeding and the management of feeding intolerance, improved awareness of NEC risk factors, and adoption of universal feeding of mother’s milk or DM feedings. Nurses are instrumental in encouraging mothers to provide breast milk. If mother’s milk is unavailable, pasteurized DM should be available and given to all infants less than 32 weeks’ gestation for the first month of life. Although this may seem like a tall order, it puts the burden of action on the organization to secure DM through milk banks as a best practice.6,25 Units can also create positive environments to support mothers who need to pump their breast milk. Nurses are in a privileged position as advocates for these vulnerable infants and their awareness of NEC risk factors will improve their ability to secure the most appropriate treatment when NEC symptoms do present.
Acknowledgments
The project described was supported by grant number F31NR012333 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Caplan MS. Neonatal necrotizing enterocolitis [Introduction] Semin Perinatol. 2008;32(2):69. doi: 10.1053/j.semperi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep. 2008;10(5):450–457. doi: 10.1007/s11894-008-0084-x. [DOI] [PubMed] [Google Scholar]
- 3.Luig M, Lui K NSW ACT NICUS Group. Epidemiology of necrotizing enterocolitis—part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41(4):174–179. doi: 10.1111/j.1440-1754.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 4.Gagliardi L, Bellu R, Cardilli V, De Curtis M Network Neonatale Lombardo. Necrotising enterocolitis in very low birth weight infants in Italy: incidence and non-nutritional risk factors. J Pediatr Gastroenterol Nutr. 2008;47(2):206–210. doi: 10.1097/MPG.0b013e318174e855. [DOI] [PubMed] [Google Scholar]
- 5.Patole SK. Prevention and treatment of necrotizing enterocolitis in preterm neonates. Early Hum Dev. 2007;83:635–642. doi: 10.1016/j.earlhumdev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half—today? Fetal Pediatr Pathol. 2010;29:185–198. doi: 10.3109/15513815.2010.483874. [DOI] [PubMed] [Google Scholar]
- 7.Hentschel J, de Veer I, Gastmeier P, Ruden H, Obladen M. Neonatal nosocomial infection surveillance: incidences by site and a cluster of necrotizing enterocolitis. Infection. 1999;27(4/5):234–238. doi: 10.1007/pl00012176. [DOI] [PubMed] [Google Scholar]
- 8.Boccia D, Stolfi I, Lana S, Moro ML. Nosocomial necrotizing enterocolitis outbreaks: epidemiology and control measures. Eur J Pediatr. 2001;160:385–391. doi: 10.1007/s004310100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109(3):423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 10.McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106(5):1080–1087. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 11.Lambert DK, Christensen RD, Henry E, et al. Necrotizing enterocolitis in term neonates: data from a multi-hospital health-care system. J Perinatol. 2007;27:437–443. doi: 10.1038/sj.jp.7211738. [DOI] [PubMed] [Google Scholar]
- 12.Maayan-Metzger A, Itzchak A, Mazkereth R, Kuint J. Necrotizing enterocolitis in full-term infants: case-control study and review of the literature. J Perinatol. 2004;24(8):494–499. doi: 10.1038/sj.jp.7211135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manogura AC, Turan O, Kush ML, et al. Predictors of necrotizing enterocolitis in preterm growth-restricted neonates. Am J Obstetr Gynecol. 2008;198(6):638.e1–638.e5. doi: 10.1016/j.ajog.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Stout G, Lambert DK, Baer VL, et al. Necrotizing enterocolitis during the first week of life: a multicentered case-control and cohort comparison study. J Perinatol. 2008;28(8):556–560. doi: 10.1038/jp.2008.36. [DOI] [PubMed] [Google Scholar]
- 15.Ghidini A, Espada RA, Spong CY. Does exposure to magnesium sulfate in utero decrease the risk of necrotizing enterocolitis in premature infants? Acta Obstet Gynecol Scand. 2001;80(2):126–129. [PubMed] [Google Scholar]
- 16.Hand IL, Noble L, McVeigh TJ, Kim M, Yoon JJ. The effects of intrauterine cocaine exposure on the respiratory status of the very low birth weight infant. J Perinatol. 2001;21(6):372–375. doi: 10.1038/sj.jp.7210552. [DOI] [PubMed] [Google Scholar]
- 17.Bashiri A, Zmora E, Sheiner E, Hershkovitz R, Shoham-Vardi I, Mazor M. Maternal hypertensive disorders are an independent risk factor for the development of necrotizing enterocolitis in very low birth weight infants. Fetal Diagn Ther. 2003;18(6):404–407. doi: 10.1159/000073132. [DOI] [PubMed] [Google Scholar]
- 18.Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Mat-Fetal Neonatal Med. 2003;13(2):102–109. doi: 10.1080/jmf.13.2.102.109. [DOI] [PubMed] [Google Scholar]
- 19.Ruangtrakool R, Laohapensang M, Sathornkich C, Talalak P. Necrotizing enterocolitis: a comparison between full-term and pre-term neonates. J Med Assoc Thail. 2001;84(3):323–331. [PubMed] [Google Scholar]
- 20.Desfrere L, de Oliveira I, Goffinet F, et al. Increased incidence of necrotizing enterocolitis in premature infants born to HIV-positive mothers. AIDS. 2005;19(14):1487–1493. doi: 10.1097/01.aids.0000183123.09206.07. [DOI] [PubMed] [Google Scholar]
- 21.Gregory KE. Clinical predictors of necrotizing enterocolitis in premature infants. Nurs Res. 2008;57(4):260–270. doi: 10.1097/01.NNR.0000313488.72035.a9. [DOI] [PubMed] [Google Scholar]
- 22.Kamoji VM, Dorling JS, Manktelow B, Draper ES, Field DJ. Antenatal umbilical Doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatr. 2008;97(3):327–331. doi: 10.1111/j.1651-2227.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 23.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40(2):184–188. doi: 10.1097/00005176-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2009;48(4):437–442. doi: 10.1097/mpg.0b013e31817b6dbe. [DOI] [PubMed] [Google Scholar]
- 25.Necrotizing Enterocolitis Guideline Team, Cincinnati Children’s Hospital Medical Center. Pediatric Evidence-Based Care Guidelines. Vol. 28. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center Guideline; 2010. Evidence-Based Care Guideline for Necrotizing Enterocolitis Among Very Low Birth Weight Infants; pp. 1–10. [Google Scholar]
- 26.Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010;(4):CD003481. doi: 10.1002/14651858.CD003481.pub4. [DOI] [PubMed] [Google Scholar]
- 27.Patole S, McGlone L, Muller R. Virtual elimination of necrotizing enterocolitis for 5 years—reasons? Med Hypothesis. 2003;61(5/6):617–622. doi: 10.1016/s0306-9877(03)00251-2. [DOI] [PubMed] [Google Scholar]
- 28.Carter BM, Holditch-Davis D. Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv Neonat Care. 2008;8(5):285–290. doi: 10.1097/01.ANC.0000338019.56405.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42(suppl 2):S46–S52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 30.Mehall JR, Kite CA, Saltzman DA, Wallett T, Jackson RJ, Smith SD. Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. J Pediatr Surg. 2002;37(8):1177–1182. doi: 10.1053/jpsu.2002.34467. [DOI] [PubMed] [Google Scholar]
- 31.Gantz M, Roy J, Guillet R. Analyzing retrospective data with time-varying exposure: a cautionary tale of H2 blockers in ELBW neonates. Am J Perinatol. 2008;25:93–100. doi: 10.1055/s-2007-1004835. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Child Health and Human Development. [Accessed June 22, 2011];Common reflux treatment linked to life threatening bowel infection in premature infants. http//: www.nih.gov/news/pr/feb2006/nichd-08.htm. Published February 8, 2006.
- 33.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50(5):1106–1112. doi: 10.1111/j.1537-2995.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 34.Josephson CD, Wesolowski A, Bao G, et al. Do red cell transfusions increase the risk of necrotizing ente rocolitis in premature infants? J Pediatr. 2010;157(6):972, 978.e1–3. doi: 10.1016/j.jpeds.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruangtrakool R, Laohapensang M, Sathornkich C, Talalak P. Necrotizing enterocolitis: a comparison between full-term and pre-term neonates. J Med Assoc Thailand. 2001;84(3):323–331. [PubMed] [Google Scholar]
- 36.Figueras-Aloy J, Rodriguez-Miguelez JM, Iriondo-Sanz M, Salvia-Roiges MD, Botet-Mussons F, Carbonell-Estrany X. Intravenous immunoglobulin and necrotizing enterocolitis in newborns with hemolytic disease. Pediatrics. 2010;125(1):139–144. doi: 10.1542/peds.2009-0676. [DOI] [PubMed] [Google Scholar]
- 37.Ververidis M, Kiely EM, Spitz L, Drake DP, Eaton S, Pierro A. The clinical significance of thrombocytopenia in neonates with necrotizing enterocolitis. J Pediatr Surg. 2001;36(5):799–803. doi: 10.1053/jpsu.2001.22964. [DOI] [PubMed] [Google Scholar]
- 38.Tepas JJ, Sharma R, Hudak ML, Garrison RD, Pieper P. Coming full circle: an evidence-based definition of the timing and type of surgical management of very low-birth-weight (<1000 g) infants with signs of acute intestinal perforation. J Ped Surg. 2006;41:418–422. doi: 10.1016/j.jpedsurg.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan M, Henderson G, McGuire W. Enteral feeding for very low birth weight infants: reducing the risk of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F162–F166. doi: 10.1136/adc.2007.115824. [DOI] [PubMed] [Google Scholar]
- 40.Schurr P, Perkins EM. The relationship between feeding and necrotizing enterocolitis in very low birth weight infants. Neonatal Network J Neonatal Nurs. 2008;27(6):397–407. doi: 10.1891/0730-0832.27.6.397. [DOI] [PubMed] [Google Scholar]
- 41.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 42.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111(3):529–534. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 43.Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2011;(3):CD001241. doi: 10.1002/14651858.CD001241.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy S, Gupta P, Debnath S, Gomber S. Slow versus rapid enteral feeding advancement in preterm infants 1000–1499 g: a randomized controlled trial. Acta Paediatr. 2010;99(1):42–46. doi: 10.1111/j.1651-2227.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 45.Hughes C, Dowling R. Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clin Sci. 1980;59:317–327. doi: 10.1042/cs0590317. [DOI] [PubMed] [Google Scholar]
- 46.Stoll BJ, Hansen N, Fanaroff A, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2008;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 47.Flidel-Ramon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell E. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis Child, Fetal Neonatal Ed. 2004;89:F289–F292. doi: 10.1136/adc.2002.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bombell S, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2008;(2):CD001970. doi: 10.1002/14651858.CD001970.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23(4):278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 51.Kamitsuka MD, Horton MK, Williams MA. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics. 2000;105(2):379–384. doi: 10.1542/peds.105.2.379. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence D, Brewer D, Hornung R, Mersmann M, Donovan D. Antenatal glucocorticoids use, not perinatal antibiotics, may result in increased risk of necrotizing enterocolitis in very-low-birthweight infants. Pediatr Res. 2001;49:Abstract 1798. [Google Scholar]
- 53.Halpern MD, Dvorak B. Does abnormal bile acid metabolism contribute to NEC? Semin Perinatol. 2008;32(2):114–121. doi: 10.1053/j.semperi.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halpern MD, Holubec H, Saunders TA, et al. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130(2):359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11(5):369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lo wer rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mother’s own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 58.Quigley M, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2011;93(3):81–86. doi: 10.3945/ajcn.2010.29799. [DOI] [PubMed] [Google Scholar]
- 59.McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotizing enterocolitis in preterm infants: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F11–F14. doi: 10.1136/fn.88.1.F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F169–F175. doi: 10.1136/adc.2005.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wight NE. Donor human milk for preterm infants. J Perinatol. 2001;21:249–254. doi: 10.1038/sj.jp.7200533. [DOI] [PubMed] [Google Scholar]
- 62.Arnold LD. The cost-effectiveness of using banked donor milk in the neonatal intensive care unit: prevention of necrotizing enterocolitis. J Hum Lact. 2002;18:17–24. doi: 10.1177/089033440201800210. [DOI] [PubMed] [Google Scholar]
- 63.Scmolzer G, Urlesberger B, Haim M, et al. Multi-modal approach to prophylaxis of necrotizing enterocolitis: clinical report and review of literature. Pediatr Surg Int. 2006;22:573–580. doi: 10.1007/s00383-006-1709-5. [DOI] [PubMed] [Google Scholar]
- 64.Patole SK, Kadalraja R, Tuladhar R, Almonte R, Muller R, Whitehall JS. Benefits of a standardized feeding regimen during a clinical trial in preterm neonates. Int J Clin Pract. 2000;54(7):429–431. [PubMed] [Google Scholar]
- 65.Patole SK, de Klerk N. Impact of standardized feeding regimens on incidence of neonatal necrotizing enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Feral Neonatal Ed. 2005;90:F147–F151. doi: 10.1136/adc.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiedmeier SE, Henry E, Baer VL, et al. Center differences in NEC within one health-care system may depend on feeding protocol. Am J Perinatol. 2008;25:5–11. doi: 10.1055/s-2007-995220. [DOI] [PubMed] [Google Scholar]
- 67.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 68.Tarnow-Mordi WO, Wilkinson D, Trivedi A, Brok J. Probiotics reduce all-cause mortality and necrotizing enterocolitis: is it time to change practice. Pediatrics. 2010;125:1068–1070. doi: 10.1542/peds.2009-2151. [DOI] [PubMed] [Google Scholar]
- 69.Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of bifidobacterium breve and lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. 2010;93(1):81–86. doi: 10.3945/ajcn.2010.29799. [DOI] [PubMed] [Google Scholar]
- 70.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christensen RD, Wiedmeier SE, Baer VL, et al. Antecedents of Bell stage III necrotizing enterocolitis. J Perinatol. 2010;50:54–57. doi: 10.1038/jp.2009.93. [DOI] [PubMed] [Google Scholar]
- 72.Gregory KE, DeForge CE, Natale KM, Phillips M, Van Marter LJ. Necrotizing enterocolitis in the premature infant. Adv Neonat Care. 2011;11(3):155–164. doi: 10.1097/ANC.0b013e31821baaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blond MH, Chavet MS, Lecuyer AI, et al. [Necrotizing enterocolitis and apnoeasbradycardias of the preterm newborn] Arch de Pediatr. 2003;10(2):102–109. doi: 10.1016/s0929-693x(03)00305-1. [DOI] [PubMed] [Google Scholar]
- 74.Buonomo C. The radiology of necrotizing entero colitis. Radiol Clin North Am. 1999;37(6):1187–1198. doi: 10.1016/s0033-8389(05)70256-6. [DOI] [PubMed] [Google Scholar]
- 75.Bell M, Ternberg J, Feigin R, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F193–F198. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2008;17(2):98–109. doi: 10.1053/j.sempedsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Loh M, Osborn DA, Lui K. NSW Neonatal Intensive Care Unit Study Group. Outcome of very premature infants with necrotising enterocolitis cared for in centres with or without on site surgical facilities. Arch Dis Child Fetal Neonatal Ed. 2001;85(2):F114–F118. doi: 10.1136/fn.85.2.F114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62(4):510–504. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 80.Hallstrom M, Koivisto AM, Janas M, Tammela O. Frequency of and risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatr. 2003;92(1):111–113. doi: 10.1111/j.1651-2227.2003.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 81.Bolisetty S, Lui K, Oei J, Wojtulewicz J. A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr. 2000;89(10):1226–1230. doi: 10.1080/080352500750027619. [DOI] [PubMed] [Google Scholar]
- 82.Kuzma-O’Reilly B, Duenas ML, Greecher C, et al. Evaluation, development and implementation of potentially better practices in neonatal intensive care unit nutrition. Pediatrics. 2003;111:e461–e470. [PubMed] [Google Scholar]
- 83.Bauer CR, Morrison JC, Poole WK, et al. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoids therapy. Pediatrics. 1984;73:682–688. [PubMed] [Google Scholar]
- 84.Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence of controlled trials. Br J Obstet Gynaecol. 1990;97:11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 85.Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;(3):CD000503. doi: 10.1002/14651858.CD000503. [DOI] [PubMed] [Google Scholar]


