Abstract
The objective of this work is to use colloidal gel from alginate-chitosan-PLGA complex to deliver Ac-PLP-BPI-NH2-2 peptide in a controlled-release manner as a vaccine-like therapeutic to suppress experimental autoimmune encephalomyelitis (EAE) in the mouse model. Oppositely charged PLGA nanoparticles were prepared by a solvent diffusion method. The carboxyl group of the alginate and the amine group of the chitosan coated the nanoparticles with negative and positive charges, respectively. The peptide (Ac-PLP-BPI-NH2-2), designed to bind to MHC-II and ICAM-1 simultaneously, was formulated into the colloidal gel by physical mixture. Vaccine-like administration of the peptide-loaded colloidal gel (Ac-PLP-BPI-NH2-2-NP) was achieved by subcutaneous (s.c.) injection to EAE mice. Disease severity was measured using clinical scoring and percent change in body weight. Cytokine production was determined using the splenocytes from Ac-PLP-BPI-NH2-2-NP-treated mice and compared to that of controls. Ac-PLP-BPI-NH2-2-NP suppressed and delayed the onset of EAE as well as Ac-PLP-BPI-NH2-2 when delivered in a vaccine-like manner. IL-6 and IL-17 levels were significantly lower in the Ac-PLP-BPI-NH2-2-NP-treated mice compared to the mice group treated with blank colloidal gel, suggesting that the mechanism of suppression of EAE is due to a shift in the immune response away from Th17 production. The results of this study suggest that a one-time s.c. administration of Ac-PLP-BPI-NH2-2 formulated in a colloidal gel can produce long-term suppression of EAE by reducing Th17 proliferation.
Keywords: Autoimmune disease, immune tolerance, experimental autoimmune encephalomyelitis, colloidal gel, PLGA, chitosan, alginate, bifunctional peptide inhibitor
INTRODUCTION
During the course of multiple sclerosis (MS), the myelin sheath surrounding the nerves becomes demyelinated, exposing multiple proteins that are processed and presented on the surface of antigen-presenting cells (APC).1 The immunodominant epitopes from the myelin sheath that are presented on the surface of major histocompatibility complex class II (MHC-II) include the proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), and myelin basic protein (MBP).2 Modulating the immune response is the goal of many of the available therapies for MS.3 Many of the current treatments fall short because they suppress the general immune system.4 In contrast, bifunctional peptide inhibitors (BPI) are molecules that provide a more selective approach in modulating the immune response by modifying the response of a sub-population of T cells. BPI is a peptide complex in which two functional peptides are tethered together by a linker. The hypothesis is that the antigenic peptide and the adhesion peptide of the BPI simultaneously bind to MHC-II and ICAM-1 on the surface of antigen-presenting cells (APC) suppressing the translocation of signal-1 (MHC-II:T cell interaction) and signal-2 (ICAM-1:LFA-1 interaction) molecules necessary for the formation of the immunological synapse, thereby suppressing T cell activation.5,6
PLP-BPI molecule has been shown to suppress experimental autoimmune encephalomyelitis (EAE) in the mouse model when injected three times in solution in a vaccine-like manner 11, 8, and 5 days prior to disease stimulation. Although we have had success with this treatment in suppressing EAE, translating this into a human model must be simplified. Studies have shown that compliance is an important factor in achieving success in multi-dose vaccinations.7,8 A one-time injection is ideal for achieving a higher degree of compliance. Therefore, it will be beneficial if PLP-BPI can be delivered in a controlled-release manner using a one-time injection. Previously, there have been very few successful applications of protein and peptide controlled-release therapies.9–14 Because nanoparticles containing alginate and chitosan have been shown to be effective for delivering protein antigen and can stabilize ovalbumin, we decided to utilize these polysaccharides to deliver a therapeutic peptide to suppress EAE.15 Poly(D,L-lactic-co-glycolic acid) (PLGA) is a well-established biocompatible and biodegradable polymer.16 Therefore, chitosan and alginate, along with PLGA, were used to deliver PLP-BPI for treating EAE in a vaccine-like manner.
Here, a PLP-BPI molecule called Ac-PLP-BPI-NH2 was formulated into alginate-PLGA/chitosan-PLGA colloidal gels for the controlled-release delivery of peptide in a vaccine-like manner. In this study, the efficacy of Ac-PLP-BPI-NH2 released from colloidal gel to suppress EAE after a single injection was evaluated, and its long-term effect in suppressing EAE was also monitored. The results indicated that vaccine-like injection of Ac-PLP-BPI-NH2-2 in colloidal gel was very effective in suppressing EAE as well as the relapse of EAE in the mouse model. The mechanism of suppression was due to the shift in the immune response away from Th17-mediated pathology of EAE.
MATERIALS AND METHODS
Materials
PLGA (50:50) (Polymer Type: 5050 DLG 2A, inherent viscosity: 0.15–0.25 dl/g) was purchased from Lakeshore Biomaterials (Birmingham, AL). Low molecular weight chitosan was purchased from Sigma Aldrich Co. (St. Louis, MO) (448869, degree of deacetylation: 75%–85%, Brookfield viscosity: 20–200 cps). Sodium alginate (Mv = 1.6 × 105, dynamic viscosity was 39 MPa·s in a 1.0% solution at 20 °C) was obtained from FMC BioPolymer (Philadelphia, PA).
Animals
The female SJL/J mice used in this study were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and were housed under specific pathogen-free conditions at the animal facility at The University of Kansas approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures used in this study were approved protocols reviewed by the Institutional Animal Care and Use Committee (IACUC) at The University of Kansas.
Peptide Synthesis
PLP (NH2-HSLGKWLGHPDKF-OH) and Ac-PLP-BPI-NH2-2 (Ac-HSLGKWLGHPDKF-(AcpGAcpGAcp)2-ITDGEATDSG-NH2 (Ac = acetyl, Acp = aminocaproic acid)) were synthesized using a 9-fluorenylmethyloxycarbonyl- (FMOC) protected solid-phase peptide chemistry on appropriate polyethylene glycol-polystyrene resins on an automated peptide synthesizer (Pioneer; Perceptive Biosystems, Framingham, MA). Trifluoroacetic acid (TFA) with scavengers was used to remove the amino acid protecting groups as well as the peptides from the resin. A semi-preparative reversed-phase high-performance liquid chromatography (RP-HPLC) with a C18 column was used to purify the crude peptides. A gradient method was used for purification with solvent A (94.9% water, 5% acetonitrile, and 0.1% trifluoroacetic acid) and solvent B (100% acetonitrile). The purity of the peptide was analyzed by analytical RP-HPLC using a C18 column (purity > 96%). The identity of the peptides was confirmed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.
Preparation of Charged PLGA Nanoparticles
Oppositely charged blank PLGA nanoparticles were prepared by a solvent diffusion method. 150 mg of PLGA was dissolved in 10 ml of acetone and then the solution was added into 0.2% of either alginate or chitosan (150 ml) surfactant solution through a syringe pump at a constant rate of 30 ml/h under stirring at 200 rpm. The resulting solution was stirred overnight to evaporate the acetone. Nanoparticles were then centrifuged (10,000 rpm) for 30 min and the resulting PLGA pellet was resuspended in double-distilled water and washed three times to remove excess surfactant. The final resuspended product was then lyophilized for two days.
Preparation of Peptide-loaded Colloidal Gels
Lyophilized nanoparticles (PLGA-alginate or PLGA-chitosan) were dispersed in deionized water at 20% weight/volume followed by mixing of the two dispersions in a 1:1 ratio; then, the mixture was sonicated for 3 minutes to prepare a homogenous colloid mixture. Immediately before injecting into mice, Ac-PLP-BPI-NH2-2 peptide was physically mixed for 2 min with the colloidal gel in a 1:10 ratio of peptide to PLGA.
Characterization of Nanoparticles and Colloidal Gels
The zeta potentials and the sizes of PLGA-alginate and PLGA-chitosan nanoparticles were determined in triplicate using dynamic light scattering (Brookhaven, ZetaPALS). Scanning electron microscopy (SEM) was performed using a Leo 1550 field emission scanning electron microscope at an accelerating voltage of 5 kV.
In Vitro Peptide Release from Colloidal Gel
Peptide release from colloidal gel formulation was measured fluorometrically at excitation λ=280 nm and emission λ=350 nm with a Shimadzu RF5000U spectrofluorophotometer (Shimadzu Co., Kyoto, Japan). Colloidal gel samples containing Ac-PLP-BPI-NH2-2 (n = 3) were placed in a sealed 1.5 ml buffered solution (PBS, pH 7.4) and stirred at 50 rpm at 37 °C. Samples containing the peptide were removed at different time points after centrifugation and replaced with fresh buffer. The concentrations of the peptide were determined using fluorescence spectroscopy as described above.
Induction of EAE and Efficacy Evaluations
Six-to-eight week old mice were immunized subcutaneously (s.c.) on day 0 with 200 μg PLP139–151 peptide in a 0.2 ml emulsion consisting of equal volumes of PBS and complete Freund’s adjuvant (CFA) containing killed mycobacterium tuberculosis strain H37RA (at a final concentration of 4 mg/ml, Difco, Detroit, MI) to induce disease. The emulsion of PLP/CFA was administered to the regions above the shoulder and the flanks (50 μl at each injection site, for a total of 4 injections). Additionally, 200 ng of pertussis toxin was administered intraperitoneally on days 0 and 2.
As a positive control, mice received three s.c. injections of Ac-PLP-BPI-NH2-2 in PBS (100 nmol/injection) at 11, 8, and 5 days prior to stimulation of the disease on day 0. As suggested by the in vitro peptide release profile and to ensure that a majority of the peptide would be released prior to the induction of disease, Ac-PLP-BPI-NH2-2 (300 nmol) in colloidal gel (Ac-PLP-BPI-NH2-2-NP) was injected once via the s.c. route 5 days before induction of the disease on day 0. To determine the effects of the timing of the dose, a different group of mice was treated with one s.c. injection of Ac-PLP-BPI-NH2-2-NP at day 4 after disease stimulation on day 0. To determine the ability of the Ac-PLP-BPI-NH2-2-NP treatment to suppress the relapse of EAE, another group was treated on day 30, after the disease went into remission. As negative controls, two different groups of mice received either PBS on days −11, −8, and −5, or blank colloidal gel (blank-NP) on day −5 prior to disease induction.
Disease progression was evaluated by observing the change in body weight of the mice and clinical scoring: 0—no clinical symptoms of disease; 1—tail weakness or limp tail; 2—paraparesis (weakness or partial paralysis of one or two hind limbs); 3—paraplegia (complete paralysis of two hind limbs); 4—paraplegia with forelimb weakness or paralysis; 5—moribund. When the mice were found to be moribund, they were promptly euthanized.
Determination of Cytokine Levels In Vitro
Spleens from representative mice treated with Ac-PLP-BPI-NH2-2, Ac-PLP-BPI-NH2-2-NP, and blank-NP were isolated during the peak of the relapse, on day 55. The spleen was gently smashed using the coarse end of a 1 ml syringe in a petri dish containing RPMI 1640 medium (10% FBS, 0.05 M BME) to yield the splenocytes of interest. The splenocytes were then filtered through a nylon 40 micrometer strainer. After centrifugation, “ACK lysis buffer” containing 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA was used to lyse the red blood cells followed by washing of the remaining white blood cells with medium. 5 × 106 splenocytes/ml were cultured in parallel in the presence of 20 μM PLP and blank RPMI medium. Supernatants were collected at a 72 h time-point for the measurement of cytokine levels and stored at −80 °C until analysis. The samples were then analyzed using a fully quantitative ELISA-based Q-Plex™ Mouse Cytokine-Screen (Quansys Biosciences, Logan, UT).
Statistical Analysis
Statistical differences among the groups in clinical disease scores were determined by calculating the average score for each mouse from day 12 to day 17, or from day 45 to day 55 by one-way analysis of variance followed by Fisher’s least significant difference. Statistical differences in body weight among groups were also analyzed in the same fashion, but from day 12 to day 24. Comparison of cytokine concentrations was also performed by one-way analysis of variance. All analyses were performed using StatView (SAS Institute, Cary, NC).
RESULTS
Characterization of Coated Nanoparticles and Colloidal Gel
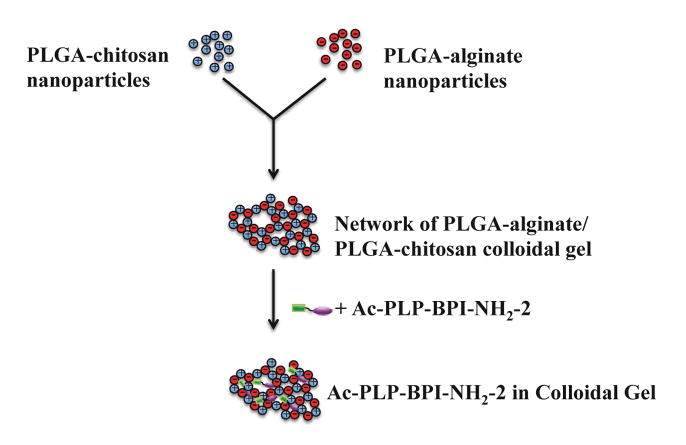
Coated nanoparticles were prepared by a solvent diffusion method where PLGA in acetone was titrated into an alginate- or chitosan-containing water phase. Once in the water phase, PLGA was precipitated and coated with either a negatively charged (alginate) or a positively charged (chitosan) polysaccharide Mixing these two particle types together leads to the self-assembly of dense colloidal gels (Fig. 1). The size of the chitosan-PLGA was 400.1±13.9 nm (polydispersity = 0.092), whereas the alginate-PLGA size was 208.1±7.6 nm (polydispersity = 0.111). The zeta potentials of alginate-PLGA and chitosan-PLGA were found to be −38.85±1.54 mV and 23.79±2.16 mV, respectively. Using scanning electron microscopy, no differences were observed between the structure of the dried blank colloidal gel and dried Ac-PLP-BPI-NH2-2-loaded colloidal gel (Fig. 2A and 2B). Both images suggested a tightly packed colloidal gel formed by the interacting chitosan-alginate nanoparticles.
Figure 1.
Scheme of positively and negatively charged PLGA nanoparticle fabrication to form peptide-loaded colloidal gel.
Figure 2.
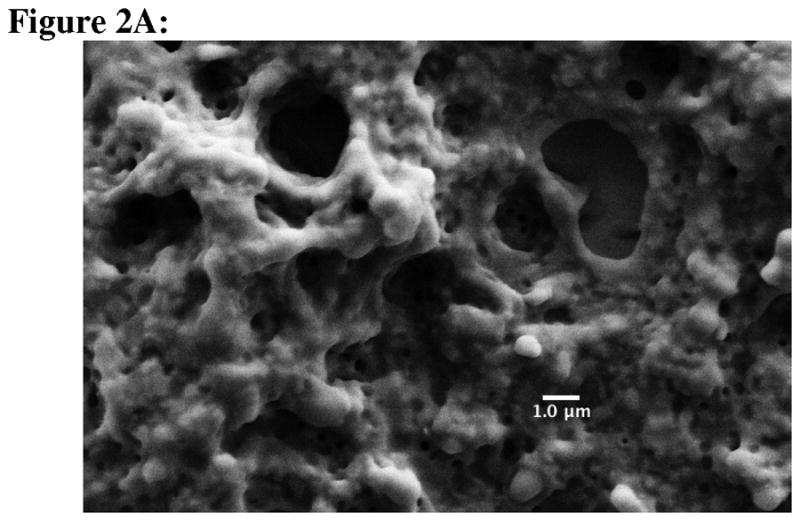
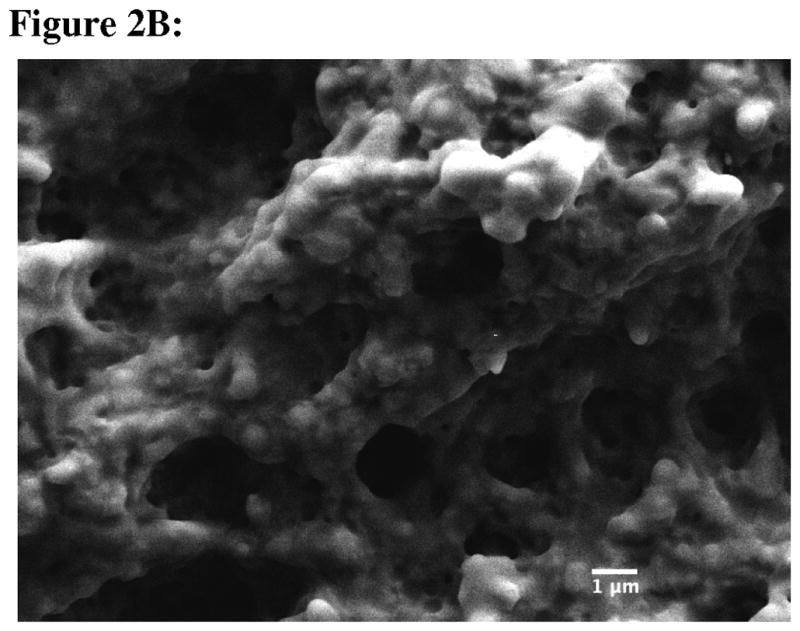
Scanning electron micrographs of (A) blank colloidal gel, and (B) Ac-PLP-BPI-NH2-2-loaded colloidal gel. Scale bar is 1 μm.
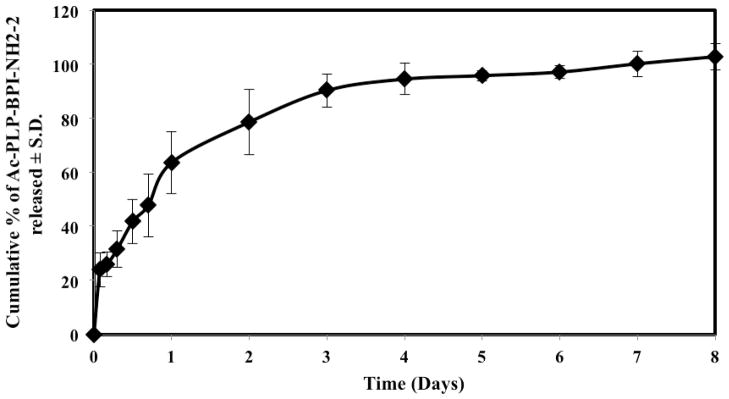
In Vitro Peptide Release from Colloidal Gel
The in vitro peptide release into PBS medium from colloidal gels was measured by detection of fluorescence emission of the tryptophan and phenylalanine side chains in Ac-PLP-BPI-NH2-2 (Fig. 3). Initially, approximately 24% of the peptide was released within the first two hours; this was most likely due to the rapid release of peptide from the surface of the colloidal gel. It was followed by a relatively less pronounced release over a period of 24 hours (~ 64%). Eventually, the amount of peptide released plateaued after day 1, suggesting that the remaining peptide diffused through the gel network into the solution.
Figure 3.
In vitro release of Ac-PLP-BPI-NH2-2 from colloidal gel (in PBS solution, 37 °C, 50 rpm stirring). n = 3 ± S.D.
Efficacy of Ac-PLP-BPI-NH2-2 in Colloidal Gel for Suppression of EAE
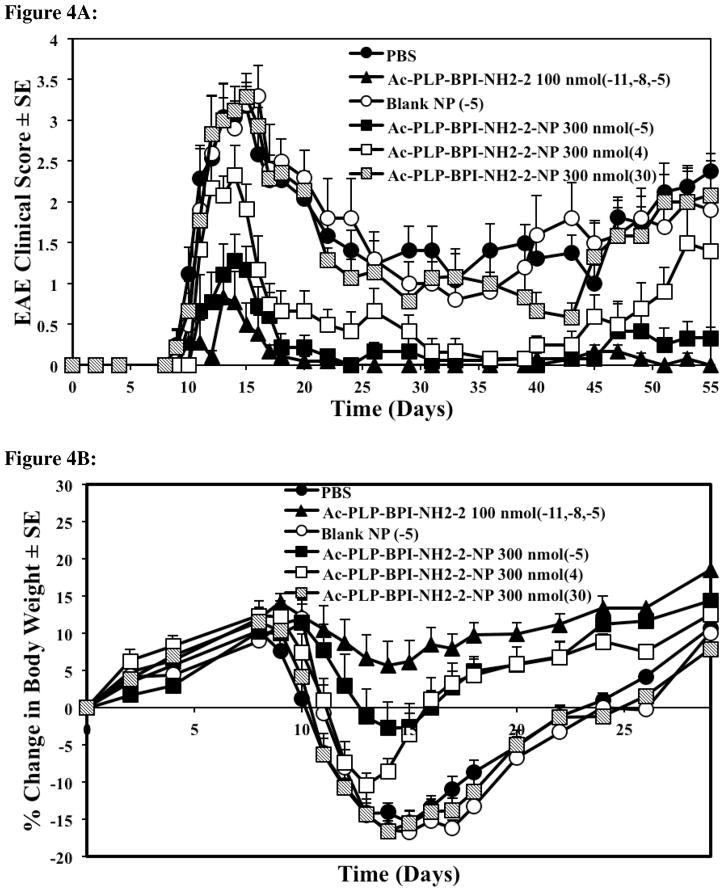
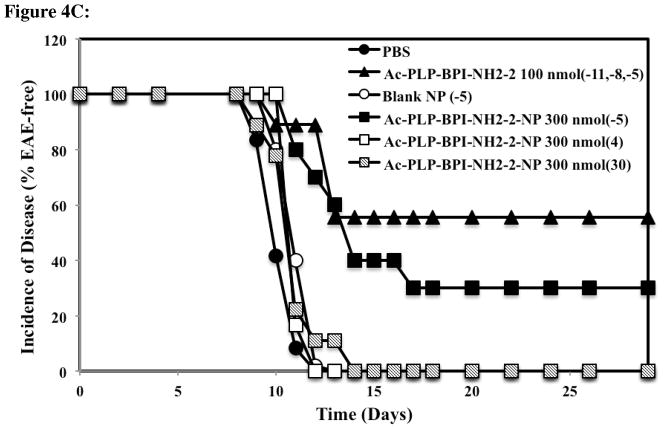
Previously, EAE could be suppressed in mice by administering Ac-PLP-BPI-NH2-2 in buffered solution (i.v. or s.c.) on days 4, 7, and 10, or a one-time injection of Ac-PLP-BPI-NH2-2-loaded microparticles (s.c.) on day 4. Here, Ac-PLP-BPI-NH2-2-NP was used for one-time delivery of the peptide to suppress EAE. Three groups of mice received subcutaneous injections of the peptide-loaded colloidal gel (300 nmol/injection) on day −5, day 4, or day 30 (Fig. 4). The mice treated with Ac-PLP-BPI-NH2-2-NP five days prior to (day −5) or four days after (day 4) the induction of disease had significantly lower clinical scores compared to control, PBS, or blank-NP (p < 0.01, through days 12–17) (Fig. 4A). Furthermore, the changes in body weight were significantly less than in the control groups, suggesting that treating mice with Ac-PLP-BPI-NH2-2-NP prior to the onset of disease is significantly more effective than treatment with PBS or blank-NP in suppressing disease (p < 0.01, through days 12–24) (Fig. 4B). Although the group that received the peptide-loaded gel formulation on day 4 got sick faster, their recovery was quicker than that of the control groups, and the amount of body weight lost was similar to that of the Ac-PLP-BPI-NH2-2-NP (−5) group by day 15 (Fig. 4A and 4B). Compared to PBS and the blank colloidal gel control groups, a delay in the onset of disease was apparent in the mice groups treated with Ac-PLP-BPI-NH2-2-NP on day −5 (Fig. 4C).
Figure 4.
Comparison of the in vivo activity of Ac-PLP-BPI-NH2-2 in solution, Ac-PLP-BPI-NH2-2-NP, blank colloidal gel, and PBS using clinical disease scores. In the controlled-release treatments, different groups of SJL/J mice received a one-time injection of 300 nmol peptide-gel formulation on days −5, 4, or 30. Mice were also treated with a one-time injection of blank colloidal gel 5 days prior to the induction of disease. The positive control group (Ac-PLP-BPI-NH2-2, 100 nmol/injection) was treated on days −11, −8, and −5. The mice group receiving PBS was also injected on days −11, −8, and −5. (A) The mice treated with Ac-PLP-BPI-NH2-2-NP on day −5 and day 4 had significantly lower clinical scores compared to negative control, PBS, or blank-NP (p < 0.01, through days 12–17, and p < 0.0001, through days 45–55). No significant difference was observed between Ac-PLP-BPI-NH2-2-NP (−5) and the positive control (p > 0.05, through days 45–55). No significant difference in clinical scores was observed when comparing the mice treated with Ac-PLP-BPI-NH2-2-NP on day 30 to negative controls (p > 0.05, through days 45–55). (B) The mice treated with Ac-PLP-BPI-NH2-2-NP on day −5 and day 4 had significantly less changes in body weight compared to PBS, or blank-NP (p < 0.01, through days 12–24). (C) Compared to PBS and the blank colloidal gel control groups, a delay in the onset of disease was apparent in the mice group treated with Ac-PLP-BPI-NH2-2-NP on day −5. The results are expressed as the mean ± S.E. (n ≥ 6).
When observing the long-term effect of suppressing the relapse of disease, it was noted that Ac-PLP-BPI-NH2-2-NP (−5) could suppress disease significantly better than the controls and there was no significant difference when compared to the positive control (p > 0.05, through days 45–55) (Fig. 4A). Ac-PLP-BPI-NH2-2-NP, when administered 4 days after the induction of disease, could suppress disease significantly better than the controls (p < 0.0001, through days 45–55) (Fig. 4A).
To test the efficacy of Ac-PLP-BPI-NH2-2-NP to suppress relapse, another group of mice was administered the peptide-loaded colloidal gel on day 30. It was observed that the mice showed signs of recovery with a decreasing clinical score but, upon the time of the relapse of disease (after day 43), no significant difference in clinical scores was observed when compared to the negative controls (p > 0.05, through days 45–55) (Fig. 4A).
In Vitro Cytokine Production
To examine the cytokines produced in response to the different treatments, spleens from three different groups (Ac-PLP-BPI-NH2-2, Ac-PLP-BPI-NH2-2-NP, and blank-NP) were isolated on day 55 and observed for their response to PLP stimulation. The interactions of many different cytokines are complicated, and the ability to determine the role for an individual cytokine is not straightforward. However, one can generalize cytokines into different effector groups. The cytokines that are generally produced during an inflammatory response include IL-6, IL-17, and IFNγ whereas the cytokines that induce suppressor and regulatory responses include IL-2, IL-4, and IL-5.
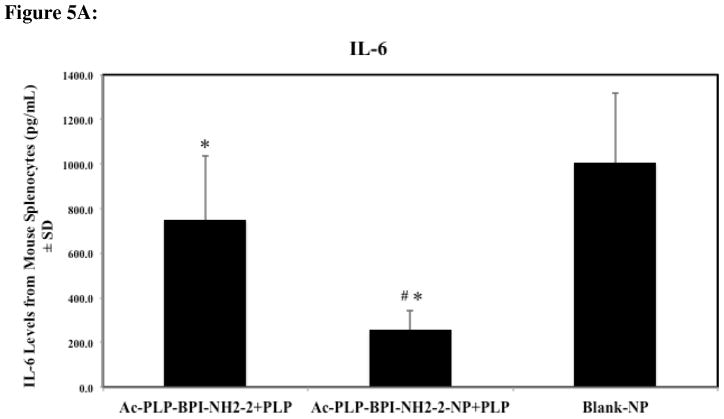
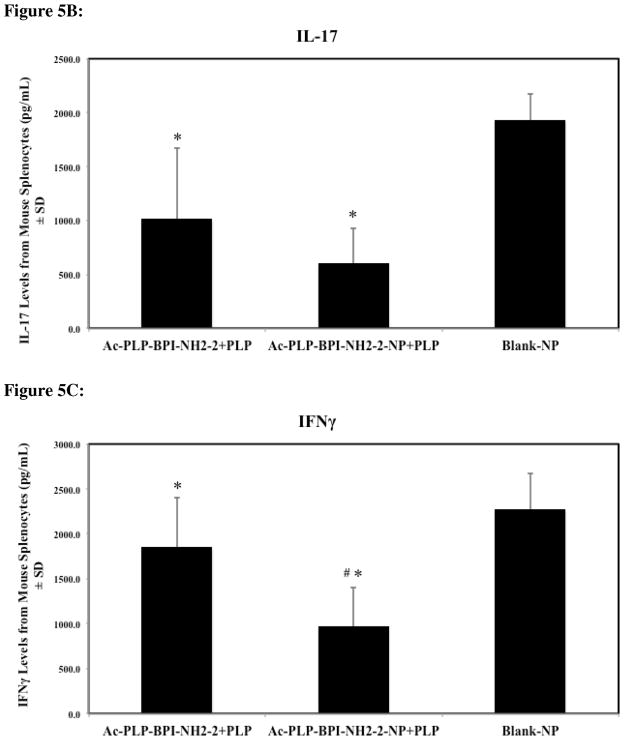
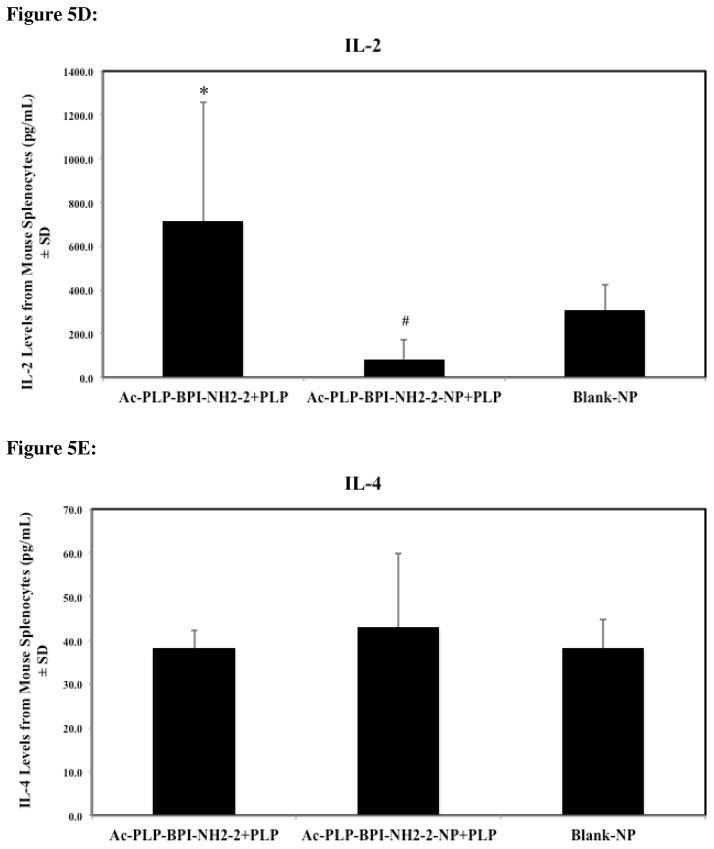
Mice treated with solution Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-NH2-2-NP were observed to have significantly lower levels of IL-6, IL-17, and IFNγ, suggesting that both formulations were effectively suppressing disease by shifting the immune response away from Th17 production (Fig. 5A–C, p < 0.05). It was interesting to observe that IL-6 and IFNγ levels were significantly lower for the Ac-PLP-BPI-NH2-2-NP formulation group than the group treated with solution Ac-PLP-BPI-NH2-2 (p < 0.005).
Figure 5.
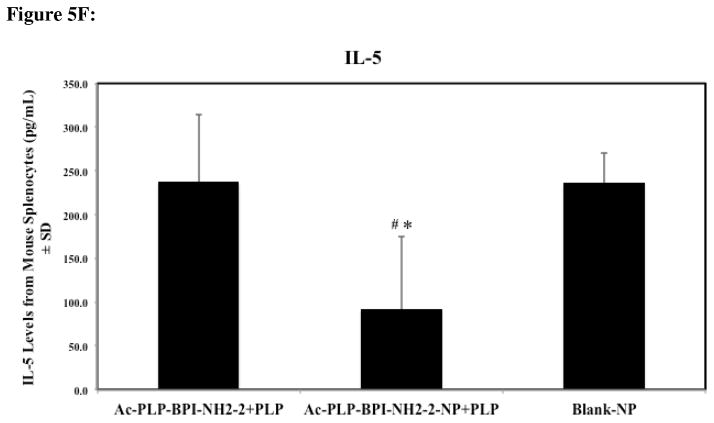
Cytokine levels (day 55) from splenocytes of mice treated subcutaneously with solution Ac-PLP-BPI-NH2-2, Ac-PLP-BPI-NH2-2-NP, or Blank-NP. (A) In the Ac-PLP-BPI-NH2-2-NP-treated group, IL-6 cytokine levels were significantly lower than in the groups treated with Ac-PLP-BPI-NH2-2 (#, p < 0.005) and Blank-NP (*, p < 0.05). (B) In the Ac-PLP-BPI-NH2-2- and Ac-PLP-BPI-NH2-2-NP-treated groups, IL-17 cytokine levels were significantly lower than in the group treated Blank-NP (*, p < 0.05). (C) In the Ac-PLP-BPI-NH2-2-NP-treated group, IFNγ cytokine levels were significantly lower than in the groups treated with Ac-PLP-BPI-NH2-2 (#, p < 0.005) and Blank-NP (*, p < 0.05). (D) In the Ac-PLP-BPI-NH2-2-NP-treated group, IL-2 cytokine levels were significantly lower than in the group treated with Ac-PLP-BPI-NH2-2 (#, p < 0.0005). On the other hand, IL-2 production was higher in the Ac-PLP-BPI-NH2-2-treated group than in the negative control (p < 0.05). (E) No significant difference in IL-4 production was observed between any of the groups (p > 0.05). (F) In the Ac-PLP-BPI-NH2-2-NP-treated group, IL-5 cytokine levels were significantly lower than in the groups treated with Ac-PLP-BPI-NH2-2 (#, p < 0.0005) and Blank-NP (*, p < 0.0005).
Compared to the blank-NP group, IL-2 levels were significantly higher for the Ac-PLP-BPI-NH2-2 treated mice (p < 0.05) whereas no significant difference was observed between the peptide-loaded gel and the blank colloidal gel (p > 0.05) (Fig. 5D). When comparing the two treatment groups, significant differences in IL-2 production were observed (p < 0.0005) (Fig. 5D). No significant difference in IL-4 production was observed between any of the groups on day 55 (Fig. 5E). Whereas no significant difference between Ac-PLP-BPI-NH2-2 and blank-NP control groups was observed, IL-5 levels were significantly lower for the Ac-PLP-BPI-NH2-2-NP group (p < 0.0005) (Fig. 5F).
DISCUSSION
Previously, BPI molecules such as PLP-BPI, GAD-BPI, and CII-BPI have been shown to suppress the progression of autoimmune diseases in the animal models of diseases such as MS, type 1 diabetes, and rheumatoid arthritis, respectively.6,17–20 Furthermore, GAD-BPI was found to simultaneously bind to MHC-II and ICAM-1, co-localizing them on the surface of B cells that were isolated from a non-obese diabetic (NOD) mouse.6 It is proposed that blocking the formation of the immunological synapse that is necessary for T-cell activation will alter the immune response toward a regulatory and suppressor phenotype and away from a Th17 and/or Th1 inflammatory response.
There are two parameters of colloidal gel to consider in delivering Ac-PLP-BPI-NH2-2 in a controlled-release manner; these parameters are the particle size and zeta potential.21 The particles were manufactured to a size of approximately 200–400 nm because the small size allowed a stronger cohesiveness of the colloidal gel.21 For larger particles (in the μm range), the number of particle-particle contacts decreases, thus leading to a less stable colloidal gel. The large zeta potential of the coated nanoparticles also yields stronger electrostatic interactions, leading to tighter packing of the colloidal gel. Using SEM, the formation of a tight network structure of the colloidal gel was observed, which suggested that oppositely charged particles are interacting and forming ring-like structures.22 Grooves and pores are also observed, which further suggested that there is also a repulsive force among similarly charged nanoparticles.22
In vitro studies showed that Ac-PLP-BPI-NH2-2 could be delivered in a controlled-release manner, where the full dose was delivered in a period of a little over a week. One advantage of using a controlled-release system over the traditional dosing method is that Ac-PLP-BPI-NH2-2 was being continually released, permitting a persistent exposure of the peptide to APC, whereas the control method required three separate injections, delivering bolus doses at every injection. In another study, a hepatitis B vaccine that required three injections was delivered effectively using one injection in an alginate-chitosan microsphere formulation to produce a robust immune response.23
Another advantage of using the colloidal gel system was the ease of formulating the peptide by simple mixing without losing peptide. To improve the release profile, such as slowing the release of the peptide, future studies will be done where the colloidal gel will be formulated to have higher amounts of positively charged PLGA nanoparticles to improve electrostatic adsorption,24 due to the net charge of −2 for Ac-PLP-BPI-NH2-2. Peptide release may also be delayed or increased by adjusting the loading.
Vaccine-like delivery of Ac-PLP-BPI-NH2-2 (day −5) from the colloidal gel was shown to be effective in suppressing disease throughout the study, including relapse. Administration of Ac-PLP-BPI-NH2-2 after the induction of disease on day 4 was able to suppress disease better than the controls during both the initial phase of the disease and the relapse. However, administration of Ac-PLP-BPI-NH2-2-NP on day 4 did not suppress disease as well as when given on day −5. This is possibly due to the timing of the delivery of the Ac-PLP-BPI-NH2-2-NP, which is too close to the time of disease onset (day ~10). In this case, the peptide may not be completely released by the onset of the disease. In vitro release studies suggest that it takes approximately eight days for the peptide to completely release for the mice to receive the full dose. Therefore, for the peptide to be fully effective, Ac-PLP-BPI-NH2-2-NP would need to be administered anywhere between day −5 and day 4. It is also plausible that the difference in the efficacy of the two treatments was due to the production of regulatory and suppressor cells that shift the balance of the immune system to a non-immunogenic phenotype in the group treated on day −5. Cytokine studies need to be done in the future to confirm this proposal.
Most of the initial cases of MS are characterized as relapsing-remitting.25 To test for the ability to suppress relapse after remission, Ac-PLP-BPI-NH2-2-NP was administered on day 30. We observed that while the group initially showed clinical signs of improvement, the treatment was not able to fully suppress relapse. Because MS is an autoimmune disease that generally involves multiple epitopes from different myelin sheath proteins, it is possible that the peptide is suppressing a subpopulation of T cells that are responsible for the recognition of PLP; however, the MOG- and MBP-specific T cells may be responsible for the relapse in disease.2,26 The reason a relapse was not observed in the Ac-PLP-BPI-NH2-2-NP (day −5) group was possibly because the peptide completely suppressed any immunogenic T cells in the early stage, and this resulted in a shift in the immune balance towards a regulatory response before any epitope spreading occurred.26
Th17 is thought to play a large role in the pathogenesis of both EAE and MS.27 Through cytokine studies, it was revealed that IL-6, a proinflammatory marker that is a precursor for Th17 production, was significantly lower in both Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-NH2-2-NP treatment groups.27 Furthermore, IL-6 is important in the breakdown of the blood brain barrier (BBB), a hallmark of MS and EAE.28 Our previous work had shown that Ac-PLP-BPI-NH2-2 prevented the destruction of the BBB (unpublished data). The lower IL-6 production by the Ac-PLP-BPI-NH2-2-NP group compared to the Ac-PLP-BPI-NH2-2 group suggested that continual exposure to the peptide dose may be better at suppressing Th17 production. It could also be that the higher initial dose followed by a more sustained release provided by the colloidal gel formulation might be a more optimal way to deliver this peptide. Lower levels of IL-17 by both treatments compared to blank-NP on day 55 corroborate the IL-6 findings, suggesting that suppression of Th17 production was the major mechanism of the peptide. The other inflammatory marker, IFNγ, was also significantly lower in the treated groups than in the blank-NP group. Just as in IL-6, IFNγ was significantly lower in the Ac-PLP-BPI-NH2-2-NP group compared to the mice group treated with bolus injections of Ac-PLP-BPI-NH2-2, suggesting that delivering the peptide using a colloidal gel formulation is more advantageous at suppressing the Th1 immunogenic response.
Th2 cells generally function as suppressor cells and usually develop in the presence of IL-4.29 IL-4 production was not significantly different in the treatment groups compared to the negative control. This suggests that Th2 involvement, especially during the peak of the relapse (day 55), may not be as important as Th17. IL-5, also considered to be a Th2 marker, was significantly lower in the group treated with Ac-PLP-BPI-NH2-2-NP compared to the controls. IL-2 levels in the Ac-PLP-BPI-NH2-2 treatment group were also significantly higher. These results suggested that, although the peptide used for treatment is the same, the dosing schedule and dose availability play vital roles in developing an immunotolerant state.
In conclusion, a one-time injection given in a vaccine-like manner with the colloidal gel formulation loaded with Ac-PLP-BPI-NH2-2 suppressed EAE as well as the three-times injection of the positive control, Ac-PLP-BPI-NH2-2. Even though treatment with Ac-PLP-BPI-NH2-2-NP on day 4 could suppress EAE, the timing of the dose was found to be an important factor in suppressing disease. Ac-PLP-BPI-NH2-2-NP was also found to suppress disease by downregulating production of IL-6 and IL-17, cytokines associated with Th17 production. Future studies using Ac-PLP-BPI-NH2-2-NP will aim to further elucidate its mechanism of action. The effect of epitope spreading will be studied by co-administering other immunodominant epitopes, such as MOG-BPI.26 Finally, a colloidal gel was found to be an effective way of delivering Ac-PLP-BPI-NH2-2 in a controlled-release manner without the loss of peptide during formulation.
Acknowledgments
We gratefully acknowledge the assistance of the University of Kansas Microscopy and Analytical Imaging Laboratory. We also thank the National Institutes of Health (R01-AI-063002 and R56-AI-063002), Institute for Advancing Medical Innovation (The University of Kansas Cancer Center), and National Multiple Sclerosis Society for supporting this work. BB was also supported by Pharmaceutical Aspects of Biotechnology Training Grant (NIGMS, T32-GM008359). We are grateful for the help of Nancy Harmony in proofreading the manuscript.
References
- 1.Lassmann H. Classification of demyelinating diseases at the interface between etiology and pathogenesis. Curr Opin Neurol. 2001;14(3):253–8. doi: 10.1097/00019052-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurol Clin. 2011;29(2):257–78. doi: 10.1016/j.ncl.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markowitz CE. The current landscape and unmet needs in multiple sclerosis. Am J Manag Care. 2010;16(8 Suppl):S211–8. [PubMed] [Google Scholar]
- 4.Manikwar P, Kiptoo P, Badawi AH, Buyuktimkin B, Siahaan TJ. Antigen-specific blocking of CD4-specific immunological synapse formation using BPI and current therapies for autoimmune diseases. Med Res Rev. 2011 doi: 10.1002/med.20243. DOI: 10.1002/med.20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295(5559):1539–42. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 6.Murray JS, Oney S, Page JE, Kratochvil-Stava A, Hu Y, Makagiansar IT, Brown JC, Kobayashi N, Siahaan TJ. Suppression of type 1 diabetes in NOD mice by bifunctional peptide inhibitor: modulation of the immunological synapse formation. Chem Biol Drug Des. 2007;70(3):227–36. doi: 10.1111/j.1747-0285.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 7.Heron L, Selnikova O, Moiseieva A, Van Damme P, van der Wielen M, Levie K, Hoet B, Stoffel M. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine. 2007;25(15):2817–22. doi: 10.1016/j.vaccine.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Delivery Rev. 2008;60(8):915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mata E, Igartua M, Patarroyo ME, Pedraz JL, Hernandez RM. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur J Pharm Sci. 2011;44(1–2):32–40. doi: 10.1016/j.ejps.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, Qie Y, Wang J, Zhang Y, Wang Q, Xu Y, Wang H. Chitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosis. Eur J Pharm Biopharm. 2007;66(3):318–26. doi: 10.1016/j.ejpb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura K, Nishimura S, Nishi N, Numata F, Tone Y, Tokura S, Azuma I. Adjuvant activity of chitin derivatives in mice and guinea-pigs. Vaccine. 1985;3(5):379–84. doi: 10.1016/0264-410x(85)90127-6. [DOI] [PubMed] [Google Scholar]
- 12.Otterlei M, Ostgaard K, Skjak-Braek G, Smidsrod O, Soon-Shiong P, Espevik T. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother (1991) 1991;10(4):286–91. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Brodbeck KJ, Pushpala S, McHugh AJ. Sustained release of human growth hormone from PLGA solution depots. Pharm Res. 1999;16(12):1825–9. doi: 10.1023/a:1018943107688. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Borges O, Borchard G, Verhoef JC, de Sousa A, Junginger HE. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int J Pharm. 2005;299(1–2):155–66. doi: 10.1016/j.ijpharm.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Kiptoo P, Kobayashi H, Ridwan R, Brocke S, Siahaan TJ. Prophylactic and therapeutic suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. Clin Immunol. 2008;129(1):69–79. doi: 10.1016/j.clim.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi N, Kobayashi H, Gu L, Malefyt T, Siahaan TJ. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. J Pharmacol Exp Ther. 2007;322(2):879–86. doi: 10.1124/jpet.107.123257. [DOI] [PubMed] [Google Scholar]
- 19.Ridwan R, Kiptoo P, Kobayashi N, Weir S, Hughes M, Williams T, Soegianto R, Siahaan TJ. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor: structure optimization and pharmacokinetics. J Pharmacol Exp Ther. 2010;332(3):1136–45. doi: 10.1124/jpet.109.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Kiptoo P, Williams TD, Siahaan TJ, Topp EM. Immune response to controlled release of immunomodulating peptides in a murine experimental autoimmune encephalomyelitis (EAE) model. J Controlled Release. 2010;141(2):145–52. doi: 10.1016/j.jconrel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Wang J, Lu Q, Detamore MS, Berkland C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials. 2010;31(18):4980–6. doi: 10.1016/j.biomaterials.2010.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Jamal S, Detamore MS, Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J Biomed Mater Res, Part A. 2011;96(3):520–7. doi: 10.1002/jbm.a.33000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Huang Y, Zheng C, Dong S, Liang W. Alginate-chitosan-PLGA composite microspheres enabling single-shot hepatitis B vaccination. AAPS J. 2010;12(4):519–24. doi: 10.1208/s12248-010-9213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Irvine DJ. Engineering chemoattractant gradients using chemokine-releasing polysaccharide microspheres. Biomaterials. 2011;32(21):4903–13. doi: 10.1016/j.biomaterials.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox EJ. Alemtuzumab in the treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother. 2010;10(12):1789–97. doi: 10.1586/ern.10.135. [DOI] [PubMed] [Google Scholar]
- 26.Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164(2):670–8. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 27.Strzepa A, Szczepanik M. IL-17-expressing cells as a potential therapeutic target for treatment of immunological disorders. Pharmacol Rep. 2011;63(1):30–44. doi: 10.1016/s1734-1140(11)70396-6. [DOI] [PubMed] [Google Scholar]
- 28.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 29.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]