Abstract
The Persian Gulf is a semi-enclosed marine system surrounded by eight countries, many of which are experiencing substantial development. It is also a major center for the oil industry. The increasing array of anthropogenic disturbances may have substantial negative impacts on marine ecosystems, but this has received little attention until recently. We review the available literature on the Gulf’s marine environment and detail our recent experience in the United Arab Emirates (U.A.E.) to evaluate the role of anthropogenic disturbance in this marine ecosystem. Extensive coastal development may now be the single most important anthropogenic stressor. We offer suggestions for how to build awareness of environmental risks of current practices, enhance regional capacity for coastal management, and build cooperative management of this important, shared marine system. An excellent opportunity exists for one or more of the bordering countries to initiate a bold and effective, long-term, international collaboration in environmental management for the Gulf.
Keywords: Persian Gulf, Arabian Gulf, Coastal development, Environmental quality, Environmental degradation
Introduction
The Persian Gulf (also know as the Arabian Gulf) is a semi-enclosed marginal sea, connected to the Gulf of Oman through the 56 km wide Strait of Hormuz (Fig. 1). It is a major shipping route for ports in the Middle East and South Asia, and for the global oil transport industry, and its eight bordering countries include several that are experiencing a rapid and very extensive coastal development, chiefly for up-scale residential communities. The Gulf’s geography, climate, and pattern of use put it at risk for various types of environmental degradation, and the current capacity for environmental management is inadequate. This article builds upon several recent reviews of the state of the Gulf (e.g., Khan et al. 2002; Hamza and Munawar 2009; Sheppard et al. 2010), and makes recommendations for suitable steps to improve environmental management in the face of growing anthropogenic pressures.
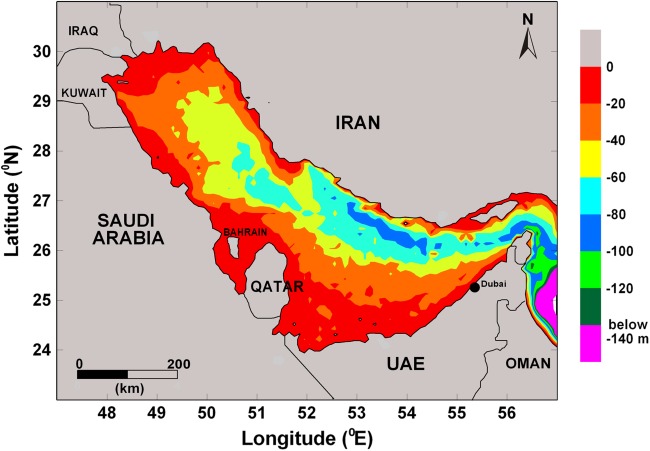
Fig. 1.
The Persian Gulf, 239,000 km2 in area, is a semi-enclosed marginal sea, has an average water depth of 36 m, a maximum internal depth of 94 m, a shallow broad southern margin along the coast of U.A.E., and a relatively narrow and deep north-eastern margin along the coast of Iran
Part 1: Physical and Biological Description
The Gulf’s marine environment is characterized by environmental extremes due to its location and bathymetry (Chao et al. 1992; Kämpf and Sadrinasab 2006). Evaporation is far greater than the combined rainfall and river discharge within the Gulf, leading to an inverse estuarine circulation and a counterclockwise circulation (Reynolds 1993). Surface water flows into the Gulf in the northern part of the Strait of Hormuz as a wedge of less saline water that penetrates deep into the Gulf along the Iranian coast (Brewer and Dyrssen 1985; Reynolds 1993), increasing in salinity and exiting at depth through the Strait of Hormuz (Kämpf and Sadrinasab 2006). Summer surface water temperatures (SST) average 33°C, with an upper maximum reported being 37.7°C (Sheppard and Loughland 2002). Average winter SST, on the other hand, ranges from 22°C near the Strait to 16°C near the head of the Gulf (Brewer and Dyrssen 1985). The lowest reported SST was 11.5°C (Coles and Fadlallah 1991). Salinity observations as high as 43 are common in winter toward the head of the Gulf (Reynolds 1993).
Physical Environment
The bulk of the southern and western portion of the Gulf is characterized by soft-sediment habitats and occasional outcrops of coral-dominated limestone habitats. Subtidal sediments consist of thin layers of relatively pure carbonate sands or mud over consolidated conglomerates (John and George 2006). In areas where currents weaken, sand and shell gravel accumulate and form habitat for infaunal and epibenthic organisms (John and George 2006).
Mudflats are especially dominant intertidal habitats along the coastline of the western Gulf, where water movements are less turbulent. For example, as a result of the discharge of silt from the Tigris and Euphrates Rivers, 57% of the Kuwait coastline is characterized by gently sloping mudflats (Al-Zaidan et al. 2003). Mudflats can be covered by dense, highly productive microbial mats, comprising mainly diatoms and cyanobacteria (Price et al. 1993). These mats can form an essential habitat and food source for intertidal macrofauna (Al-Zaidan et al. 2003).
Sabkhas are coastal flats at or just above the level of normal high tide and are characterized by unconsolidated carbonate or siliciclastic sediments, diagenetic evaporite minerals, aeolian deposits, and a variable association of sedimentary structures (Brown 2006). Sabkhas are either devoid of vegetation or covered with a sparse cover of dwarf shrubs and tussock grasses (Brown 2006).
True coral reefs do not exist within the Gulf. Instead, corals generally form a veneer on areas of exposed limestone cap-rock (pre-Pleistocene reef deposits) that are elevated above the sand substrates that dominate most of this area (Sheppard and Sheppard 1991; Riegl 1999; Burt et al. 2008). Owing to the friable nature of this substratum and repeated mass mortality of corals due to the environmental extremes in this area (Sheppard and Sheppard 1991; Riegl 2002), corals generally do not build true reef framework.
Approximately 60 species of scleractinian coral have been described for the Persian Gulf, representing approximately one tenth of corals known for the Indo-Pacific and 15% of those in the Indian Ocean (Coles 2003; Sheppard et al. 2010). Owing almost certainly to its environmental extremes (Coles 2003), coral communities of the Gulf are depauperate and therefore quite distinct from those in the major biogeographic regions surrounding the Arabian Peninsula (Sheppard 1987; Sheppard and Sheppard 1991).
Ecology and Biogeography
These topics have been well covered (e.g., Khan et al. 2002; Sheppard et al. 2010); we summarize them briefly. Typical Indian Ocean coastal communities are present, although often with depauperate representation, likely because of isolation and the extreme environmental conditions (Coles and Tarr 1990). Although Rhizophora racemosa has been cultivated successfully on the Saudi Arabian coast, the Gulf’s 13,000 ha of naturally occurring mangroves are of a single species, Avicennia marina. They occur principally in khors (lagoons) and on leeward sides of spits, islands, and shoals along the Iranian coast (9000 ha) as well as in Saudi Arabia, Bahrain, Oman, Qatar, and the U.A.E. (Spalding et al. 2010), but are absent in Iraq and Kuwait (Al-Muzaini and Jacob 1996; Al-Zaidan et al. 2003). High salinity is believed to be responsible for the generally small stature of mangrove trees; mangroves are stunted and grow poorly in hypersaline locations.
The seagrass communities of the Gulf are widespread, comprising three euryhaline species, Halodule univervis, Halophila ovalis, and Halophila stipulacea. They are particularly prevalent along southern and western shores (Price and Coles 1992; Sheppard et al. 1992), and are important habitats for a number of fishery species, including many fishes which use them as a nursery habitat, and the commercially important shrimp, Penaeus semisulcatus, and the Pearl oyster, Pinctada radiata (Carpenter et al. 1997). These beds sustain the green turtle, Chelonia mydas, and the world’s second largest population of the endangered dugong, Dugong dugong (Marsh et al. 2002).
Macroalgal beds also occur, frequently mixed in with seagrasses; however, extreme summer conditions allow them only a seasonal presence. During October to May, when bushy stands of Sargassum and Cystoseira are present, they are a vital settlement site and nursery for various fishes, shimp post-larvae, and oyster spat (Price et al. 1993; George and John 2000). Phytoplankton productivity is generally favored by the high nutrient levels, but the plankton communities of the Gulf have not been well characterized and there is considerable disparity among studies (Abdul-Azis et al. 2003).
In addition to green turtles and dugong, the Gulf is occupied by dolphins, both toothed (Odontoceti) and baleen (Mysticeti) whales, and the Whaleshark Rhyncodon typus (Carpenter et al. 1997). The fish fauna is Indian Ocean derived, but fish species diversity is about 60% that of the Gulf of Oman (535 vs. 930 species), and there are few endemic species (Coles and Tarr 1990; Randall 1995; Carpenter et al. 1997). Deeper waters of the northern and eastern Gulf have higher species richness than waters in the southern region (Price et al. 1993). Coastal fish communities show significant seasonality in recruitment and in adult movement, with increases in both juveniles and adults reported on reefs and man-made breakwaters during the warmer summer months in Saudi Arabia and the U.A.E. (Burt et al. 2009b).
The crustacea, mollusca, and echinodermata are possibly the best-known invertebrate groups in the Persian Gulf because of their importance as food species throughout the region. All three groups are depauperate compared to the Gulf of Oman (Carpenter et al. 1997), but there is evidence that the mean abundance of benthic infauna may be relatively high within the Gulf (Coles and McCain 1990). While there may be a tendency for infaunal diversity to track the diversity of better-known faunal groups, decreasing from the wider Indian Ocean and Gulf of Oman to the Arabian Gulf (Sheppard et al. 1992; Price et al. 1993; Carpenter et al. 1997), the paucity of research available may exaggerate these trends. For example, Wehe and Fiege (2002) show that polychaete species diversity and endemicity are higher in the Persian Gulf (231 species, 29 endemics) than in either the Arabian Sea (141 species, 12 endemics) or the Gulf of Oman (60 species, 2 endemics).
Part 2: Human Impacts on the Gulf Ecosystem
Fisheries and Conservation
Throughout the Gulf, fisheries are still predominantly artisanal. Fishing predominantly targets demersal species in the south and west, and shrimp and pelagic fish species in the north (Grandcourt, in press). According to estimates made by the U.N. Food and Agriculture Organization (FAO), potential fishery resources in the Gulf amount to 550,000 tonnes annually, some eight times greater than in the Gulf of Oman (Kardovani 1995). Fisheries represent the second most important natural resource after oil, and the most important renewable natural resource (Carpenter et al. 1997).
Despite existing regulations governing fishing effort (e.g., bans on fish trawling), many fishery species in the Gulf have been heavily exploited, and fishing effort already exceeds optimum levels for most demersal species (Grandcourt et al. 2004, 2009; Grandcourt, in press). Throughout the Gulf, data on stock status are limited, and many catch data are recorded only to the family level, reducing the ability of managers to use statistical catch-at-age methods for conducting species level assessments (Grandcourt, in press). In addition, fisheries regulations are weak, are not rigorously enforced, or are inconsistent among jurisdictions that share stocks (Grandcourt, in press)—all problems common to many developing regions.
About 20 marine protected areas totaling 12,000 km2 in area have been designated for conservation protection (Wood 2007), and there are extensive oil concessions with restricted access in territorial waters. Management regulations governing protected areas routinely forbid commercial fishing other than by artisanal fishermen using traditional gear, and catching of any dugong, turtle, or marine mammal. They may also restrict construction, dredging, filling, or other shore-based development activity. Similar restrictions apply to oil leases. However, while there is little direct information concerning effectiveness with which these regulations are enforced, casual observation suggests that while policing can be effective, management otherwise is quite weak—Palm Jebel Ali and Dubai Waterfront now cover one of the four MPAs in the U.A.E. Effectively managed marine protected areas could significantly lower rates of exploitation of fishery species while also protecting other valued species. Thus, by strengthening enforcement of the existing regulations, Gulf nations could substantially improve the management and conservation of their marine resources.
Hydrocarbon Pollution
The Gulf countries hold the largest hydrocarbon reserve in the world, with over 76 × 109 tonnes of recoverable oil and 32.4 × 1012 m3 of gas reserves (Haapkylai et al. 2007). There are approximately 800 offshore oil and gas platforms and 25 major oil terminals situated in the region. Some 25,000 tankers utilize the Strait of Hormuz annually and transport approximately 60% of all the oil carried by ships globally (Haapkylai et al. 2007).
Oil exploration, production, and transport along with military activities have been the major contributors to pollution levels in the Gulf (Madany et al. 1998). The most significant long-term threat is chronic contamination of coastal waters due to the continuous discharge of oil from harbors, ballast water, terminals, atmospheric fallout, and sewage-plant effluents (Madany et al. 1998). In addition to these routine discharges, during January 1991, Iraqi troops deliberately spilled an estimated 10.8 million barrels of oil from abandoned tankers and several coastal terminals. Second in size worldwide only to the Deepwater Horizon blowout of 2010, it affected over 700 km of coastline from southern Kuwait to northern Saudi Arabia (Price 1998). However, although coastal benthic habitats were seriously impacted, they have shown remarkable recovery (Price 1998). The high hydrocarbon content in this region has been hypothesized to provide an important selective pressure favoring bacterial assemblages capable of mineralizing crude oils (Al-Saleh et al. 2009). These assemblages are further aided in their mineralization rates by the warm and nutrient-rich waters of the Gulf. Overall, there are persistently high levels of hydrocarbon pollution throughout the waters of the Persian Gulf, and most substantially along the Iranian coast (Gevao et al. 2006; Gawad et al. 2008). However, associated toxic compounds related to combustion of crude oils, including carcinogenic polycyclic aromatic hydrocarbons (PAHs), and metal contamination related to oil spills do not appear exceptional relative to impacted sites in northern Europe and North America (Fowler et al. 1993).
Wastewater
The Gulf region supports major manufacturing industries that produce fertilizers, chemicals, petrochemicals, minerals, and plastics (Gevao et al. 2006), while the availability of capital and the demand for fresh produce have also encouraged agricultural development. These activities have resulted in the delivery via wastewater into coastal marine sediments of a variety of chemicals including heavy metals, oil and petroleum-based compounds, nutrients, and halogenated organics (Gevao et al. 2006).
Despite high standards for wastewater treatment throughout the Gulf region (UNEP 2001; Sheppard et al. 2010), large quantities of domestic and industrial wastewater still enter local waters. Nearly all the locally produced sewage receives secondary or tertiary treatment before discharge some of which is recycled; however, large quantities of nutrients and other pollutants also enter Gulf waters from further inland. Recent estimates suggest that rivers in southern Iran and southeastern Iraq carry 300,000–500,000 m3 days−1 of wastewater to the Persian Gulf (UNEP 2001). Agricultural runoff carrying organic pollutants also enters through river systems throughout the Gulf, which may cause localized eutrophication and lowered oxygen levels in coastal waters (Gawad et al. 2008).
Desalination
Given the shortage of freshwater resources and despite the higher cost, desalination is the principal source of potable water in the region, supplying from 66% (Bahrain) to 90% (Kuwait) of the water used. Countries bordering the Gulf have installed capacity of at least 4 km3 year−1 or 45% of global potable water produced (Lattemann and Höpner 2008), and contaminated effluent brine produced is at least twice this quantity in volume (Hoepner and Lattemann 2002).
While thermal pollution, waste brine, and pre- and post-treatment chemicals from desalination plants may pose a serious threat to the marine environment (Al-Rashed and Sherif 2000), there remain major gaps in the understanding of the repercussions of such high desalination rates within the Gulf. Clear evidence exists that pollution by hyper-saline wastewaters can have serious, detrimental effects on the growth and survivorship of marine fauna such as cuttlefish (Dupavillon and Gillanders 2009); however, few studies have examined the potential effects on Gulf marine fauna (Purnama et al. 2005).
A Growing and Urbanized Population
The countries bordering the Gulf have experienced one of the world’s highest rates of economic growth, because of exploitation of abundant oil and gas reserves (Khan 2007), though tourism is also growing. Such high economic growth has also led to unprecedented levels of population growth, particularly in the U.A.E., Bahrain, and Qatar. Bahrain is one of the ten most densely populated countries in the world with a population that grew from 661 000 in 2001 to 1 million in 2009 and has the fastest growing economy in the Arab world. Qatar’s population grew from 770,000 in 2001 to 1.4 million in 2009, and oil and gas have helped Qatar attain the second highest per-capita income of all countries (following Liechtenstein).
To accommodate expanding industries and rapid population increase, there have been massive changes to large areas of ecologically productive coastal habitats throughout the Gulf (Sheppard et al. 1992; Sheppard et al. 2010). Intertidal flats, mangrove forests, fringing coral reefs, seagrass beds, and sandy embayments, in particular, have all been altered by coastal dredging and development for industrial, commercial, and residential use. There is a lack of capacity in environmental regulatory agencies to help guide this growth, and because development has been too rapid, there have been many negative environmental impacts.
Development in Dubai: Marine Ecosystems of the Nakheel Marine Development Projects
Coastal development projects are expected to have pervasive and long-lasting effects on the health of the Gulf (Sheppard et al. 2010). Major coastal developments are already in place or under construction, particularly in Bahrain, Qatar, and the U.A.E., but there exists little information on their short- and long-term environmental effects. The scale of development is well illustrated by the pattern of growth within the U.A.E., specifically in the Emirate of Dubai. Starting in 2002, the government-owned developer, Nakheel (Arabic for Palm, the shape of their first island developments), commenced construction on a series of large-scale, totally man-made island–lagoon complexes along the entire coast of Dubai (Figs. 2, 3). This immense coastal and offshore development program has increased Dubai’s coastline from approximately 70 to 1500 km. The effects of these coastal developments on marine communities within Dubai provide an example of what may be expected throughout the region.
Fig. 2.
Image of the Dubai coastline showing (L to R) Palm Jebel Ali, Palm Jumeirah, and The World offshore developments (for scale, the outer crescent of Palm Jumeirah is approximately 5 km in diameter. Water depth approximates 10 m at outer limits of Palm Jumeirah and Palm Jebel Ali, and is slightly deeper at The World. All developments are mixed residential, retail, tourism. Image courtesy of the Image Science & Analysis Laboratory, NASA Johnson Space Center. (Image details: NASA, International Space Station (Expedition 18); ISS018-E-41939; “Astronaut Photography of Earth—Display Record.”)
Fig. 3.
The Atlantis Dubai Hotel in final stages of construction. The 1500-roomed hotel, aquarium, and theme park occupies a 45 ha site at the tip of the surrounding crescent directly seaward of the tip of Palm Jumeirah. It is 6 km seaward of the original coastline. Photo: K. Drouillard
With little information available on likely impacts on marine ecosystems, a collaborative project was initiated in 2006 between Nakheel and the United Nations University–Institute for Water, Environment and Health (UNU–INWEH) to develop an environmental management program for the marine ecosystems within and surrounding the constructed island–lagoon complexes. To do this, we initiated an environmental monitoring program and undertook a suite of ecological studies to understand the nature and dynamics of the marine ecosystems present. We anticipate that the results will prove generally useful for evaluating the likely impacts of coastal development throughout the Persian Gulf. The results of this study show that
Prevailing dredge and fill procedures generate sediment plumes that are widespread and very long lasting (Nakheel was dredging in Dubai waters for most of this decade, and has plans for further construction);
Constructed islands permanently modify coastal water movement and sediment transport, and the extent and nature of that modification varies from site to site depending on island shape and orientation; these changes have large-scale and long-term effects on the composition of infauna depending on sediment type;
Constructed islands provide new habitats for a broad range of species adapted to rocky shores, reefs, or shallow, sandy lagoons (Fig. 4). However, there are substantial and long-term (decadal) effects on the composition of the fauna associated with the constructed islands and breakwaters due to successional processes, and complex seasonal variation due to patterns of faunal migration and recruitment;
The type of material used to construct breakwaters can affect some components of the benthic community, including corals, so that similar construction, but the use of different materials can lead to quite different environmental outcomes; and
Specific design aspects of an island development can introduce significant local, physical, and chemical changes that degrade habitat quality and favor undesirable species, or prevent colonization of the site by desired species of aquatic organism.
Fig. 4.
The snapper, Lutjanus ehrenbergii, sea urchins, and oysters living on the Nakheel breakwater at The World. Photo: Nakheel, with permission
We found that the benthic footprint of Palm Jumeirah extended >800 m beyond the outer crescent breakwater. Within 800 m of the breakwater, sediment composition was dominated by silt and finer particles (<63 μm) than typically found as surface sediments on the inner shelf along the Dubai coastline (≥125 μm). As a consequence, infaunal communities were dominated by polychaetes <800 m from the crescent, while more typical communities dominated by small crustacea (Amphipoda, Isopoda, etc.) were present further away (D. Feary, unpublished data). These fine sediments also have a great propensity for re-suspension under moderate sea conditions, so that benthic communities on some parts of the rocky breakwaters are subject to continual high sedimentation rates. No studies have been undertaken to document the impacts of this increased sedimentation on native biota.
Because the Nakheel constructions, particularly Palm Jebel Ali, were in close proximity, the majority of dense coral habitat in Dubai has now been buried or heavily impacted by sedimentation. While natural mortality events due particularly to extreme warming events in 1998 and 2003 have taken a toll, coastal development has clearly been responsible for much of the loss and degradation of coral habitat in Dubai (Burt et al. 2008).
At the local scale, breakwater orientation has a substantial effect on the benthic community that develops due to patterns of sediment deposition associated with differences in water movement and wave exposure (Burt et al. 2010). Windward breakwater communities had low levels of sediment deposition and were dominated by coral species, whereas leeward breakwater communities held higher levels of fine sediment, and were dominated by algal turfs and oysters.
The presence of a large-scale development (Palm Jumeirah) also had a substantial effect on the surrounding mid-water communities. On the east side of Palm Jumeirah (where sheltered conditions prevailed), there were significantly higher abundances of phytoplankton than on the west (which faced the prevailing wind). In addition, plankton community composition differed substantially, with the eastern lee dominated by diatoms, while waters west of the development were dominated by a range of dinoflagellate species (E. Marquis unpublished data).
There was a major difference in the flushing rates observed between the western and eastern halves of the large annular lagoon. We found higher discharge and lower residence time (1.2 days) on the eastern side of the main trunk of islands, and slower discharge and longer residence time (42 days) on the western side. Neither this difference nor the average residence times were predicted by modeling done before construction (Cavalcante et al. in press). Conditions for organisms are likely to be substantially different on the two sides, and may become severe on the west side during periods of warm temperatures and calm seas, particularly if there is an accumulation of nutrients in lagoons.
In Dubai, younger (<5.5 year) breakwaters are dominated by algae and oysters, while corals become a dominant member of the benthic community on breakwaters >25 years, developing abundances (>50% cover) that exceed that on natural reefs in this area (37%) (Burt et al. 2010). These breakwater coral communities are relatively depauperate, with just three species comprising over three-quarters of the coral coverage (Burt et al. 2009b).
Seasonality affects benthic and fish communities substantially. Recruitment of corals is highly seasonal, with peaks occurring in late spring and early summer. Differences among species in timing of recruitment may lead to different assemblages developing on breakwaters constructed at different times of the year, if priority effects determine patterns of space allocation (Burt et al. 2009a). Season is also important for fish. Higher densities of reef-associated fish are associated with breakwaters than natural reefs during the warmer summer and autumn seasons primarily as a result of migration of adults of snappers, angelfishes, and cardinal fishes onto these structures, with juvenile fish recruiting at comparable levels to both the natural reefs and the breakwater structures (Burt et al. 2009b).
Cast concrete in various configurations, and quarried gabbro, sandstone, and limestone have been used in the construction of breakwaters in the Gulf. Our study indicates that these materials differentially impact recruitment of corals in areas where coral recruitment is particularly high (Burt et al. 2009a). However, such effects are local, and site-specific differences in larval supply appear to be more important in structuring the wider benthic community on breakwaters in this area (Burt et al. 2009b).
Apart from the breakwater rock, construction of the majority of coastal developments in the Gulf has relied solely on locally dredged marine sediments for material. Quantities have been enormous: 94 million m3 for Palm Jumeirah, 135 million m3 for Palm Jebel Ali, and 330 million m3 for The World to date. The effects of these extensive dredging activities on local marine communities are not well understood (Lindeman and Snyder 1999; Nairn et al. 2004). Construction of Palm Jumeirah also resulted in trench-like borrow pits encircling the project, both outside and inside the crescent breakwater. Most of the trenches are filled with flocculent, suspended fine sediment (D. Feary, unpublished data) that is sometimes anoxic. When organisms were present, they were predominantly species typical of very fine sediments (e.g., Polychaetae). In comparison, within areas of relatively high water exchange (i.e., the apex of the breakwater), the trenches held coarser, sandy sediments and an infaunal community dominated by Crustacea (D. Feary, unpublished data).
All Nakheel projects were designed to be self-contained with on-site desalination and wastewater treatment. Gray water is used for irrigation and black water is tertiary treated before discharge at sea. Few data are yet available to determine whether equipment, procedures, and policies in place will be sufficient to prevent accumulation of nutrients and other pollutants in lagoons and in borrow pit trenches; however, with an anticipated population of 60,000 residents plus 30 hotels on Palm Jumeirah alone, the likelihood of contamination of lagoonal water is not going to be trivial. If there is accumulation, then this will confound efforts to maintain desired water quality.
Harmful Algal Blooms Within the Gulf
Harmful algal blooms (HABs) are formed by phytoplankton species that either express toxicity to the natural community through production of unwanted toxins that can transfer through the food chain, or create levels of biomass that can lead to reduced light penetration or anoxia. Such blooms have shown a global increase in frequency and distribution over the last three decades (Heisler et al. 2008).
Enclosed, shallow inland seas that experience increasing human influence are susceptible to the invasion and establishment of these new algal populations. The critical first step in the movement of HABs into such waters is their global link to HAB source areas through the movement of ballast-laden ships that transfer cells into the new environment, and Hamza (2006) has shown this is happening in the Gulf. The new cells must be capable of both surviving the new environmental conditions and outcompeting the natural phytoplankton communities to make the best use of the available resources.
The Gulf’s physical geography and global shipping traffic make it prone to HAB invasions and outbreaks, yet the historical frequency of outbreaks has been low, and harmful events have not been long lasting. For example, over a two-month period in 1999, a bloom of Gymnodinium sp. resulted in a dramatic fish kill in Kuwait Bay (Heil et al. 2001). The growth and proliferation of this species corresponded to high levels of organic nitrogen (sewage) and a stable warm water mass. However, while ecological damage by this HAB was limited because the cells were consumed by predatory ciliates, the nutrient conditions that initiated the bloom remained.
During August–September 2001, Kuwait Bay experienced a dramatic loss of penned and wild fish populations due to the prolific growth and development of a bloom of the fish killing diatom, Ceratium furca (Glibert et al. 2002). The initiating factors for bloom development were a highly stable water column, created by warmer than usual waters and calm weather, and elevated nutrient levels from either the aquaculture facilities or from sewage inputs (Glibert et al. 2002). Bloom development within the stable water mass depressed oxygen levels, exacerbating the heat stress of the fish community. The loss of fish through stress and infection provided nutrients for a secondary HAB event, a complex co-evolving community of putative toxin-producing dinoflagellates (Gymnodinuium impudicum, G. catenatum, and Pyrodinium bahamense var. compressum) that initiated the contamination of the food chain with several marine biotoxins.
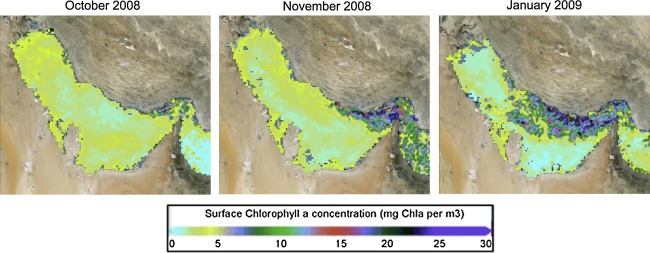
Recently, we monitored a large, prolonged HAB event in the southern reaches of the Gulf. Starting in the Fall of 2008, a HAB that initiated in the Gulf of Oman expanded north along the Musandam coast and into the Gulf as far as the shores of Dubai by the end of 2008, eventually dissipating by August 2009 (Fig. 5). The dominant species in the prolonged bloom was Cochlodinium polykrikoides, a fish-killing species common in other areas throughout the world. This was the first confirmed report of this species in the Gulf (Richlen et al. 2010). While the fish-killing attributes of C. polykrikoides are well known, this bloom also damaged other marine fauna, particularly the hard coral communities and associated fish fauna (Bauman et al. 2010). Through rRNA gene sequence analysis, Richlen et al. confirmed that the outbreak was due to a strain of C. polykrikoides that was commonly found in American and Malaysian waters—indicating the global expansion of this invasive species, probably through ballast water discharge (Richlen et al. 2010). The documented arrival of this invasive species in Gulf waters should be a cause for great concern for the region. The demise of the bloom in Gulf waters may indicate that the physical environment was unsuitable for the natural ecology of the HAB species, or that the natural cycle of the bloom sequence ended and the cells are now present for future bloom opportunities within the region.
Fig. 5.
Progression of the 2008–2009 harmful algal bloom in the Persian Gulf. Distribution of chlorophyll a concentration is shown for dates in October and November 2008, and January 2009. Initially present along the eastern coast of Oman, it developed not only west of the Strait of Hormuz, particularly along the Iranian coast, but also down the coast of the United Arab Emirates
Our understanding of HAB events in the Gulf region is limited, and it is surprising that additional HAB events have not been documented. Factors that lead to new invasions of HAB species are prevalent in the ecology and the oceanography of the Gulf—considerable global ballast discharge into warm stable waters with elevated nutrients. Construction of further protected lagoons and increases in anthropogenic discharge, combined with projected, episodic warming events (Sheppard et al. 2010), may create suitable conditions leading to the expansion of HAB events throughout the Gulf.
Part 3: Building an Effective Management Program for the Gulf Marine Environment
Although our direct experience has been limited to the U.A.E., we believe that there are sufficient similarities with the types of development taking place elsewhere in the Gulf that we are able to support and extend the recommendations made by others concerning the future (Khan et al. 2002; Hamza and Munawar 2009; Sheppard et al. 2010). The oceanography of the Gulf and projections of future climate for the region provide abundant cause for a belief that the Gulf may be particularly sensitive to anthropogenic impacts. It is already remarkably poorly flushed, and anticipated future changes in temperature and rainfall all suggest that this situation will deteriorate further (IPCC 2007). However, the most important impacts are likely to stem from the anticipated future population growth and associated coastal development.
The continued expansion and urbanization of population in the Gulf region will lead to a greater requirement for desalination, more extensive development of coastal regions, and a larger burden of domestic and industrial wastes that are likely be sent ultimately into the Gulf. The addition of greater quantities of warm, hyper-saline desalination effluent and nutrients from domestic sewage can be expected to have major impacts on this very poorly flushed system. More frequent and more extensive HAB events can be anticipated if nutrification becomes a serious issue. Fishing pressure, despite already depleting most fishery stocks, is expected to grow with coastal development. Fisheries management in place is not sufficiently robust for preventing further increases in effort, and lacks tools, such as limited entry or catch shares, which would have to be introduced if curtailment of effort were to be achieved. Because the countries surrounding the Gulf do not always work collaboratively, any curtailment of effort applied to these shared fish stocks is quite unlikely.
The design and general approach to coastal development within the Gulf is expected to introduce and exacerbate environmental risks. The majority of projects (e.g., Palm developments in the U.A.E., the Pearl development in Qatar) have resulted in structures with many blind channels and constrained pathways for water movement (Fig. 2). Given an environment that is already close to the upper limit of temperature and salinity for a broad range of marine species, the creation of environments where water would be impounded and made hotter and saltier is fundamentally a bad idea ecologically. In addition, developments which block or hinder coastal water movement patterns may lead to changes in local sediment deposition and erosion, accumulation of nutrients and other pollutants, and flow-on changes to benthic infauna, plankton, and the communities they support. At the same time, large-scale construction when properly designed can introduce a variety of structurally complex habitat types to sites that were previously uniform and simple, and, if problems of anoxia, high temperatures, and pollution can be avoided, then this could result in more diverse marine communities and populations of desired species of fishes.
The pace of construction within the Gulf has been driven by financial considerations. However, globally there is nothing resembling the large-scale coastal developments that are being built, and their ecological behavior, as a new and complex set of breakwaters and lagoons, cannot be predicted with any certainty. Under these circumstances, it is quite imprudent to commence additional developments before there has been an opportunity to study their ecological performance. For example, the very weak environmental impact assessment (EIA) procedures in place in Dubai meant that the ecological issues relating to trench-like borrow pits in close proximity to Palm Jumeirah did not become apparent until we “discovered” them, long after similar borrow pits were in place around other Nakheel developments. It would have been an almost cost-free adjustment to require that borrow pits be developed away from the island or breakwater being built.
Given the situation now in place, what are the steps that are needed to improve ecological management of the Gulf? There are three major steps needed. The first and most difficult step is to build awareness of the ecological risks of continuing to take the Gulf for granted. Given the nature of governance in this region, it is essential that this awareness be built among the leaders. The common NGO strategy of building grass-roots community support will not be useful on its own, although efforts to raise awareness in the community and galvanize support for improved environmental management need to be encouraged. Unfortunately, the NGO community in the Gulf is weak, and the relative wealth of most of these countries reduces the influence of UN agencies and other multinationals. Building awareness among the leaders may be surprisingly easy if access can be gained, because there does appear to be support for the concept of environmental sustainability, and there are strong economic benefits that accrue from better environmental management. However, the challenge to gain access may be considerable given the current relative weakness of the environmental community and the widespread lack of understanding, even at high levels, of the risks in doing nothing.
The second step needed is to build local capacity to assess and manage environmental risk. This includes a capacity to properly evaluate the environmental risks of new enterprises and the merit of alternative responses to perceived impacts—at present, the tendency is to jump quickly to the technical quick fix without investigating efficacy of, for example, transplantation of sensitive fauna such as corals (usually an unwise action, Edwards and Gomez 2007). Building management capacity is made more difficult by a culture of hiring expertise from outside, because foreign consultants and employees tend not to be vested in building local capacity. It will take a concerted effort, led by a committed leadership, to bring the international research community into effective, long-term collaborative relationships with regional and local universities, to generate a well-educated community of local expertise in environmental science, which does not disappear when a particular foreign worker departs. We do not believe that the need for this improved capacity is fully appreciated in the region.
A third step, preferably to be taken in parallel with capacity building, will be a concerted effort to strengthen the legal framework that must underlie effective environmental management. Clearly defined governance responsibility is the first step to becoming able to manage effectively. Clearly established regulations and procedures, backed up by enforced law, are also essential and must be put in place. In this case, the strongly centralized decision making that features in governance of Gulf countries may be an asset, permitting a relatively rapid development of effective law. At the same time, the need to build public support for this effort and for the laws put in place will remain essential if management agencies are to have any chance to apply the law effectively.
Central to the legal structures and new research and management capacity to be built must be the use of integrated coastal management (ICM) principles, preferably across neighboring countries, together with EIA mechanisms substantially strengthened to prevent environmentally inappropriate projects leaving the drawing board. An ICM approach should be embedded in the approval process for new coastal construction, so that each new development proposal is evaluated with due recognition of the consequences of already approved projects, and the long-term, community needs for a particular piece of coastline. Advanced nations offer abundant examples of failed, or misused EIA procedures, and ICM is not a panacea, but there are also good examples to learn from including those that are directly relevant to the type of development most common in the Gulf (Peterson and Bishop 2005; Peterson and Lowe 2009). The existing Regional Organization for the Protection of the Marine Environment (ROPME), established in 1978 under the Kuwait Convention and the UNEP Regional Seas Programme, and including all bordering states plus Oman, has the potential to become an important agent in fostering a collaborative management of the Gulf, although it has not been fully effective in the past. By drawing upon the important pockets of marine environmental expertise in Qatar, Kuwait, and other countries, encouraging participation from the global marine research community, and targeting the critical scientific and governance needs of countries in the region, ROPME could play a major part in building a growing capability to ensure the sustainability of this important body of water. Alternatively, the effort to integrate coastal management across international borders could be led by one or more of the bordering states, preferably ones, such as U.A.E. which have been most active in coastal development activity. It would be a substantial confirmation of the stated desire of several Gulf leaders to have environmentally sustainable development, if they were to initiate a bold and effective, long-term, environmental management program for the Gulf.
Biographies
Peter F. Sale
Assistant Director at UNU-INWEH, has research experience in coral reef ecology and coastal management in Hawaii, Australia, various Caribbean sites, and the Middle East. His primary research expertise is in community structure, recruitment, and connectivity of reef fishes.
David Feary
now at URS Corporation, Abu Dhabi, U.A.E., obtained a Ph.D. from James Cook University, and held a postdoctoral research fellowship at UNU-INWEH based in Dubai U.A.E. 2007–2009. His research has focused on the ecological impacts of coral degradation on reef associated fish communities.
John Burt
is Assistant Professor of Biology at New York University in Abu Dhabi. He obtained a Ph.D. from University of Windsor, Canada, and was a member of the UNU-INWEH research team based in Dubai in 2007–2009. His research examines the processes structuring community development on man-made and natural reef systems.
Andrew G. Bauman
is a Ph.D. candidate at James Cook University in Townsville, Australia. He holds a Master’s degree from James Cook University and was a UNU-INWEH research associate based in Dubai 2007–2009. His research focuses on coral reef ecology and disturbance.
Geórgenes H. Cavalcante
is a Senior Marine Scientist at URS Corporation, Doha, Qatar. He obtained a joint Ph.D. in Geosciences from the Universidade Federal Fluminense, Brazil and Texas A&M University, USA, and was a postdoctoral research associate at UNU-INWEH based in Dubai, U.A.E. 2007–2009. His research focuses on oceanographic coastal processes.
Kenneth G. Drouillard
is Associate Professor at Great Lakes Institute for Environmental Research and Department of Biological Sciences, University of Windsor, and adjunct professor with UNU-INWEH. He received his Ph.D. at Trent University, ON Canada. His research focuses on environmental monitoring and management strategies, modeling pollutant fate and bioaccumulation, and use of chemical tracers to track in situ bioenergetics of fish.
Björn Kjerfve
is, since 2009, President and Professor of World Maritime University in Malmö, Sweden, and Professor of Geography and Oceanography at Texas A&M University in College Station, Texas, USA. His research focuses on coastal oceanographic processes and physical-biological coupling.
Elise Marquis
is currently a post-doctoral researcher at the Institute of Oceanography of National Taiwan University. After obtaining a Ph.D. from the University of La Rochelle in France, she worked for UNU-INWEH as a postdoctoral research associate based in Dubai 2007–2009. Her research focuses on plankton ecology and marine pelagic ecosystem functioning.
Charles G. Trick
is the Beryl Ivey Chair for Ecosystem Health at the Schulich School of Medicine and Dentistry, University of Western Ontario. He obtained his Ph.D. in oceanography from The University of British Columbia, Canada. His research focuses on ocean and human health.
Paolo Usseglio
is a Ph.D. candidate at the University of Hawaii. He obtained his M.Sc. at the University of Windsor, Canada, and was a UNU-INWEH research associate based in Dubai 2007–2009. His research focuses on evaluation of success of Marine Protected Areas.
Hanneke Van Lavieren
is Programme Officer, Coastal Zones at UNU INWEH. She has M.Sc. from University of Groningen, and 11 years experience in coastal research, policy, project development and management in East Asia, East Africa, the Middle East and the Wider Caribbean. Her research focus has been on reef fisheries, reef community structure, and MPAs.
References
- Abdul-Azis PK, Al-Tisan IA, Daili MA, Green TN, Dalvi AGI, Javeed MA. Chlorophyll and plankton of the Gulf coastal waters of Saudi Arabia bordering a desalination plant. Desalination. 2003;154:291–302. doi: 10.1016/S0011-9164(03)80044-9. [DOI] [Google Scholar]
- Al-Muzaini S, Jacob PG. Marine plants of the Arabian Gulf. Environment International. 1996;22:369–376. doi: 10.1016/0160-4120(96)00023-2. [DOI] [Google Scholar]
- Al-Rashed MF, Sherif MM. Water resources in the GCC countries: An overview. Water Resources Management. 2000;14:59–75. doi: 10.1023/A:1008127027743. [DOI] [Google Scholar]
- Al-Saleh E, Drobiovaa H, Obuekwea C. Predominant culturable crude oil-degrading bacteria in the coast of Kuwait. International Biodeterioration & Biodegradation. 2009;63:400–406. doi: 10.1016/j.ibiod.2008.11.004. [DOI] [Google Scholar]
- Al-Zaidan ASY, Jones DA, Al-Mohanna SY, Meakins R. Endemic macrofauna of the Sulaibikhat Bay salt marsh and mudflat habitats, Kuwait: Status and need for conservation. Journal of Arid Environments. 2003;54:115–124. doi: 10.1006/jare.2001.0886. [DOI] [Google Scholar]
- Bauman AG, Burt JA, Feary DA, Marquis E, Usseglio P. ropical harmful algal blooms: An emerging threat to coral reef communities? Marine Pollution Bulletin. 2010 doi: 10.1016/j.marpolbul.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Brewer PG, Dyrssen D. Chemical oceanography of the Persian Gulf. Progress in Oceanography. 1985;14:41–55. doi: 10.1016/0079-6611(85)90004-7. [DOI] [Google Scholar]
- Brown G. The sabkha vegetation of the United Arab Emirates. In: Khan MA, Böer B, Kust GH, Barth HJ, editors. Sabkha ecosystems, Volume II: West and central Asia. Dordrecht, The Netherlands: Springer; 2006. pp. 37–51. [Google Scholar]
- Burt J, Bartholomew A, Usseglio P. Recovery of corals a decade after a bleaching event in Dubai, United Arab Emirates. Marine Biology. 2008;154:27–36. doi: 10.1007/s00227-007-0892-9. [DOI] [Google Scholar]
- Burt J, Bartholomew A, Bauman A, Saif A, Sale PF. Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. Journal of Experimental Marine Biology and Ecology. 2009;373:72–78. doi: 10.1016/j.jembe.2009.03.009. [DOI] [Google Scholar]
- Burt J, Bartholomew A, Usseglio P, Bauman A, Sale PF. Are artificial reefs surrogates of natural habitats for corals and fish in Dubai, United Arab Emirates? Coral Reefs. 2009;28:663–675. doi: 10.1007/s00338-009-0500-1. [DOI] [Google Scholar]
- Burt, J.A., D. Feary, P. Usseglio, A. Bauman, and P.F. Sale. 2010. The influence of wave exposure on coral community development on man-made breakwater reefs, with a comparison to a natural reef. Bulletin of Marine Science 28(4). doi:10.5343/bms.2009.1013.
- Carpenter KE, Krupp F, Jones DA, Zajonz U. FAO species identification field guide for fishery purposes: The living marine resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. Rome: Food and Agriculture Organization of the United Nations; 1997. [Google Scholar]
- Cavalcante, G.H., B. Kjerfve, D.A. Feary, A.G. Bauman and P. Usseglio. In press. Currents, water budget and turn-over time within a coastal mega-structure: Palm Jumeirah, Southern Arabian Gulf. Journal of Coastal Research.
- Chao S-Y, Kao TW, AI-Hajri KR. A numerical investigation of circulation in the Arabian Gulf. Journal of Geophysical Research. 1992;7:11219–11236. doi: 10.1029/92JC00841. [DOI] [Google Scholar]
- Coles S. Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: A comparison to the Indo-Pacific region. Atoll Research Bulletin. 2003;507:1–19. [Google Scholar]
- Coles SL, McCain JC. Environmental factors affecting benthic infaunal communities of the western Arabian Gulf. Marine Environmental Research. 1990;29:289–315. doi: 10.1016/0141-1136(90)90024-I. [DOI] [Google Scholar]
- Coles S, Tarr A. Reef fish assemblages in the western Arabian Gulf: A geographically isolated population in an extreme environment. Bulletin of Marine Science. 1990;47:696–720. [Google Scholar]
- Coles SL, Fadlallah YH. Reef coral survival and mortality at low-temperatures in the Arabian Gulf—new species-specific lower temperature limits. Coral Reefs. 1991;9:231–237. doi: 10.1007/BF00290427. [DOI] [Google Scholar]
- Dupavillon JL, Gillanders BM. Impacts of seawater desalination on the giant Australian cuttlefish Sepia apama in the upper Spencer Gulf, South Australia. Marine Environmental Research. 2009;67:207–218. doi: 10.1016/j.marenvres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Edwards, A.J., and E.D. Gomez. 2007. Reef restoration concepts and guidelines: Making sensible management choices in the face of uncertainty. Coral Reef Targeted Research & Capacity Building for Management Program, St Lucia, Australia. Available for download at www.gefcoral.org.
- Fowler SW, Readman JW, Oregioni B, Villeneuve J-P, McKay K. Petroleum hydrocarbons and trace metals in nearshore gulf sediments and biota before and after the 1991 war: An assessment of temporal and spatial trends. Marine Pollution Bulletin. 1993;27:171–182. doi: 10.1016/0025-326X(93)90022-C. [DOI] [Google Scholar]
- Gawad EAA, Al Azab M, Lotfy MM. Assessment of organic pollutants in coastal sediments, UAE. Environmental Geology. 2008;54:1091–1102. doi: 10.1007/s00254-007-0880-x. [DOI] [Google Scholar]
- George, D. and D. John. 2000. The status of coral reefs and associated macroalgae in Abu Dhabi (UAE) after recent coral bleaching events. In: Proceedings of the international symposium on the extent and impact of coral bleaching in the Arabian Region, Riyadh, Saudi Arabia.
- Gevao B, Beg MU, Al-Omair A, Helaleh M, Zafar J. Spatial distribution of polychlorinated biphenyls in coastal marine sediments receiving industrial effluents in Kuwait. Archives of Environmental Contamination and Toxicology. 2006;50:166–174. doi: 10.1007/s00244-005-7070-1. [DOI] [PubMed] [Google Scholar]
- Glibert PM, Landsberg JH, Evans JJ, Al-Sarawi MA, Faraj M, Al-Jarallah MA, Haywood A, Ibrahem S, et al. A fish kill of massive proportion in Kuwait Bay, Arabian Gulf, 2001: The roles of bacterial disease, harmful algae, and eutrophication. Harmful Algae. 2002;1:215–231. doi: 10.1016/S1568-9883(02)00013-6. [DOI] [Google Scholar]
- Grandcourt, E. In press. Reef fish and fisheries. In Coral reefs of the gulf—Arabian and Iranian Waters, ed. B. Riegl and S. Purkis. New York: Springer.
- Grandcourt EM, Al Abdessalaam TZ, Francis F, Al Shamsi AT. Population biology and assessment of representatives of the family Carangidae—Carangoides bajad and Gnathanodon speciosus (Forsskal, 1775), in the Southern Arabian Gulf. Fisheries Research. 2004;69:331–341. [Google Scholar]
- Grandcourt EM, Al Abdessalaam TZ, Francis F, Al Shamsi AT, Hartmann SA. Reproductive biology and implications for management of the orange-spotted grouper Epinephelus coioides in the southern Arabian Gulf. Journal of Fish Biology. 2009;74:820–841. doi: 10.1111/j.1095-8649.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- Haapkylai J, Ramade F, Salvat B. Oil pollution on coral reefs: a review of the state of knowledge and management needs. Vie Milieu. 2007;57:95–111. [Google Scholar]
- Hamza W. Observations on transported exotic plankton species to UAE coastal waters by Gas tankers ballast water. In: Tubielewicz A, editor. Living marine resources and coastal habitats. EUROCOAST-LITTORAL. Poland: Gdansk University of Technology; 2006. pp. 47–53. [Google Scholar]
- Hamza W, Munawar M. Protecting and managing the Arabian Gulf: Past, present and future. Aquatic Ecosystem Health & Management. 2009;12:429–439. doi: 10.1080/14634980903361580. [DOI] [Google Scholar]
- Heil CA, Glibert PM, Al-Sarawi MA, Faraj M, Behbehani M, Husain M. First record of a fish-killing Gymnodinium sp bloom in Kuwait Bay, Arabian Sea: Chronology and potential causes. Marine Ecology Progress Series. 2001;214:15–23. doi: 10.3354/meps214015. [DOI] [Google Scholar]
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008;8:3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepner T, Lattemann S. Chemical impacts from seawater desalination plants—a case study of the northern Red Sea. Desalination. 2002;152:133–140. doi: 10.1016/S0011-9164(02)01056-1. [DOI] [Google Scholar]
- IPCC. 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller, eds.). Cambridge, United Kingdom: Cambridge University Press.
- John, D., and D. George. 2006. The shore and shallow seas. In The emirates: A natural history, ed. T.Z. Al Abdessalaam, 123–131. Abu Dhabi: Trident Press.
- Kämpf J, Sadrinasab M. The circulation of the Persian Gulf: A numerical study. Ocean Science. 2006;2:27–41. doi: 10.5194/os-2-27-2006. [DOI] [Google Scholar]
- Kardovani P. Iranian marine ecosystem (The Persian Gulf and the Caspian Sea) Tehran, Iran: Ghomes; 1995. [Google Scholar]
- Khan NY. Multiple stressors and ecosystem-based management in the Gulf. Aquatic Ecosystem Health and Management. 2007;10:259–267. doi: 10.1080/14634980701551168. [DOI] [Google Scholar]
- Khan NY, Munawar M, Price ARG, editors. The gulf ecosystem: Health and sustainability. Leiden: Backhuys Publishers; 2002. [Google Scholar]
- Lattemann S, Höpner T. Environmental impact and impact assessment of seawater desalination. Desalination. 2008;220:1–15. doi: 10.1016/j.desal.2007.03.009. [DOI] [Google Scholar]
- Lindeman KC, Snyder DB. Nearshore hardbottom fishes of southeast Florida and effects of habitat burial caused by dredging. Fishery Bulletin. 1999;97:508–525. [Google Scholar]
- Madany IM, Jaffar A, AI-Shirbini ES. Variations in the concentrations of aromatic petroleum hydrocarbons in Bahraini coastal waters during the period October 1993 to December 1995. Environment International. 1998;24:61–66. doi: 10.1016/S0160-4120(97)00121-9. [DOI] [Google Scholar]
- Marsh, H., H. Penrose, C. Eros and J. Hugues. 2002. Dugong status report and action plans for countries and territories, 1–161. UNEP Early Warning and Assessment Report UNEP/DEWA/RS.02-1.
- Nairn R, Johnson JA, Hardin D, Michel J. A biological and physical monitoring program to evaluate long-term impacts from sand dredging operations in the United States Outer Continental Shelf. Journal of Coastal Research. 2004;20:126–137. doi: 10.2112/1551-5036(2004)20[126:ABAPMP]2.0.CO;2. [DOI] [Google Scholar]
- Peterson CH, Bishop MJ. Assessing the environmental impacts of beach nourishment. BioScience. 2005;55:887–896. doi: 10.1641/0006-3568(2005)055[0887:ATEIOB]2.0.CO;2. [DOI] [Google Scholar]
- Peterson MS, Lowe MR. Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources. Reviews in Fisheries Science. 2009;17:505–523. doi: 10.1080/10641260903171803. [DOI] [Google Scholar]
- Price ARG. Impact of the 1991 Gulf war on the coastal environment and ecosystems: Current status and future prospects. Environment International. 1998;24:91–96. doi: 10.1016/S0160-4120(97)00124-4. [DOI] [Google Scholar]
- Price ARG, Coles SL. Aspects of seagrass ecology along the Western Arabian Gulf. Hydrobiologia. 1992;234:129–141. doi: 10.1007/BF00014245. [DOI] [Google Scholar]
- Price ARG, Sheppard CRC, Roberts CM. The Gulf: Its biological setting. Marine Pollution Bulletin. 1993;27:9–15. doi: 10.1016/0025-326X(93)90004-4. [DOI] [Google Scholar]
- Purnama A, Al-Barwani HH, Smith R. Calculating the environmental cost of seawater desalination in the Arabian marginal seas. Desalination. 2005;185:79–86. doi: 10.1016/j.desal.2005.03.072. [DOI] [Google Scholar]
- Randall J. Coastal fishes of Oman. Honolulu: University of Hawaii Press; 1995. p. 439. [Google Scholar]
- Reynolds MR. Physical oceanography of the Gulf, Strait of Hormuz, and Gulf of Oman—results from the Mt. Mitchell expedition. Marine Pollution Bulletin. 1993;27:35–39. doi: 10.1016/0025-326X(93)90007-7. [DOI] [Google Scholar]
- Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM. The catastrophic 2008–2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae. 2010;9:163–172. doi: 10.1016/j.hal.2009.08.013. [DOI] [Google Scholar]
- Riegl B. Corals in a non-reef setting in the southern Arabian Gulf (Dubai, UAE): Fauna and community structure in response to recurring mass mortality. Coral Reefs. 1999;18:63–73. doi: 10.1007/s003380050156. [DOI] [Google Scholar]
- Riegl B. Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE) Marine Biology. 2002;140:29–40. doi: 10.1007/s002270100676. [DOI] [Google Scholar]
- Sheppard CRC. Coral species of the Indian Ocean and adjacent seas: A synonymized compilation and some regional distribution patterns. Atoll Research Bulletin. 1987;307:1–32. [Google Scholar]
- Sheppard C, Sheppard A. Corals and coral communities of Arabia. Fauna of Saudi Arabia. 1991;12:3–170. [Google Scholar]
- Sheppard C, Loughland R. Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aquatic Ecosystem Health and Management. 2002;5:395–402. doi: 10.1080/14634980290002020. [DOI] [Google Scholar]
- Sheppard C, Price A, Roberts C. Marine ecology of the Arabian region: Patterns and processes in extreme tropical environments. London: Academic Press; 1992. [Google Scholar]
- Sheppard C, Al-Husiani M, Al-Jamali F, Al-Yamani F, Baldwin R, Bishop J, Benzoni F, Dutrieux E, et al. The Gulf: A young sea in decline. Marine Pollution Bulletin. 2010;60:13–38. doi: 10.1016/j.marpolbul.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Spalding M, Kainuma M, Collins L. World Atlas of Mangroves. A collaborative project of ITTO, ISME, FAO, UNESCO-MAB, UNEP-WCMC, UNU-INWEH and TNC. London: Earthscan; 2010. p. 319. [Google Scholar]
- United Nation Environmental Programme (UNEP). 2001. Overview of the socioeconomic aspects related to the management of municipal wastewater in West Asia (including all countries bordering the Red Sea and Gulf of Aden). UNEP/ROWA-GPA SEWAGE.RW.1/5.
- Wehe T, Fiege D. Annotated checklist of the polychaete species of the seas surrounding the Arabian Peninsula: Red Sea, Gulf of Aden, Arabian Sea, Gulf of Oman, Arabian Gulf. Fauna of Arabia. 2002;19:7–238. [Google Scholar]
- Wood, L.J. 2007. MPA global: A database of the world’s marine protected areas. Sea Around Us Project, UNEP-WCMC & WWF. www.mpaglobal.org.