Abstract
We compared the European and eastern Chinese waterbird assemblages and checked whether the effects of human disturbance could be detected in the assemblages’ composition. For the different Chinese provinces, we expected to find a negative effect of economic development on the mean bird species mass and on the proportion of bentivorous, piscivorous and insectivorous bird species. We also expected to find relatively fewer large species in the Chinese assemblage. Species rank–abundance curves were relatively similar, but China had significantly more species with smaller body masses. The China assemblage was characterized by relatively higher abundance of heavy-bodied species, contrary to our expectations. Mean bird body mass decreased in China with increasing disturbance and increasing gross domestic product (GDP). For coastal provinces in China the percentage of bentivorous, piscivorous and insectivorous bird species declined with increasing GDP, maybe through the increased use of pesticides or fertilizer.
Keywords: Anatidae, GDP, Disturbance, Body mass, Diet
Introduction
Human disturbance, or the loss or degradation of suitable habitats as a consequence of human activities, is known to affect bird communities. Human disturbance has a major effect on birds by decreasing their foraging success (Rees et al. 2005), reducing breeding success (Beale and Monaghan 2004), modifying distribution (Thiollay 2007), decreasing species richness (Palacio-Núñez et al. 2007) and, therefore, also changing community composition (de Boer 2002; Palomino and Carrascal 2007). Hunting, particularly, can have large impacts on bird population sizes (Ebbinge 1991; Madsen 1995; Madsen 1998) and change the spatial distribution (Ebbinge 1991; Madsen 1995). In particular, the large-bodied geese species have been known to show large fluctuations in abundance in response to differences in hunting pressure (Ankney 1996; Madsen 1998; Madsen et al. 1999), partly because the impact of hunting on population sizes is relatively high for these long-lived species. After the reduction in hunting, numbers of several geese species have increased dramatically in Europe and North America over the last decades (Ankney 1996; Madsen et al. 1999). Other human factors are also important, such as the enormous increase in fertilizer use, favouring grazing birds. Mitigating actions have therefore been taken to minimize the impact that these increased numbers have on agricultural fields, both in Europe (van Eerden et al. 2005) and Asia (Amano et al. 2007).
Europe and China are both situated in the Palearctic and numerous waterbird species occur in both regions. Conservation actions have been taken in Europe over the last century, and similar measures have started recently in China. China is now going through a rapid phase of economic development. When comparing the European and the Chinese waterbird population we therefore expect to find some similarities, but also differences in the community composition. In this article, we analyse and interpret these differences and similarities in waterbird community structure of Europe and China. Similar to the community composition of large herbivores (Prins and Olff 1998), we expect to find a regular pattern in the body mass distributions of birds. Character displacement among sympatric species leads to a stable pattern in which the next species (ranked in order of body mass) is a certain fraction larger than its preceding species. We do not expect to find any difference in species packing of birds between the waterbird populations in Europe and China, as breeding and wintering grounds are relatively similar. Perhaps some bird species have lower abundances, but they have not gone extinct and are still part of the assemblages, and thereby do not affect the species packing. However, the progress of economic development and conservation histories between the two regions differ, and this may be reflected in differences in the relative abundances of the birds.
Wetland conversion is an important threat to wintering waterbirds in China (Li et al. 2007; Zhang et al. 2007), as the Chinese economy is growing quickly (Ren 2003); conversion (60%) is mainly for agriculture (Zhang et al. 2007). For instance, in the Yellow River delta, 67 km2 of wetlands is converted annually to other land use categories (Coleman et al. 2008). Human development in China has been linked to the decrease of certain waterbird species (Ma et al. 2004; Cao et al. 2008a, b). In general, an increase in human density decreases the bird density and heavier species are affected relatively more than smaller species (de Boer and Longamane 1996). Moreover, besides wetland conversion and human development, other disturbance factors also play a role in China, such as hunting, and also with hunting the larger species are the ones affected most (Schmutz and Ely 1999; Milner et al. 2007). Hunting is a severe threat to geese in China (Lu 1993; Tolvanen et al. 2000). Comparing the European and Chinese waterbird assemblages, we therefore expect to find a relatively larger proportion of the heavier-bodied species in Europe compared to China.
The use of fertilizers and pesticides has increased with rising gross domestic product (GDP) worldwide (Shindo et al. 2006), and also in China (Wang 2006). An increase in fertilizer use has been linked with an increase in the local density and population growth of herbivorous bird populations, including several geese species (van Eerden et al. 2005; Amano et al. 2007). However, pesticide use, such as organochlorines, that are widely used in eastern China, have known negative effects on especially bentivorous, piscivorous and insectivorous waterbirds (Rattner et al. 2005; Degernes 2008). Hence, herbivorous species are expected to benefit from intensification of agricultural production, whereas bentivorous, piscivorous and insectivorous birds are expected to be negatively affected.
Materials and Methods
The waterbird counts in eastern China were obtained from winter surveys carried out from 2004 to 2007 (Barter et al. 2004, 2005, 2007; L. Cao, unpublished data) of the middle and lower reaches of the Yangtze River, the Huai River and the east coast of China. Winter temperatures are on average above 0°C. The lakes included in the survey were spread over a length of 1,850 km from the Three Gorges Dam to the Yangtze delta near Shanghai, over a total area of approximately 130,000 km2, and a large section of the China east coast (2,800 km). The surveys covered the provinces of Anhui, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shanghai, Zhejiang, Shandong and Fujian, and the mean bird abundance per province was used in the analyses. One Chinese survey was carried out in the wetlands along the Huai River. The counts of this latter survey were spatially positioned close to each other and were therefore treated as one “province” in the analysis.
The waterbird counts from Europe were obtained from Wetlands International, documented in Delany and Scott (2002). Data from 1998 to 1999 were averaged and only records from Belgium, Denmark, France, Germany, and the Netherlands (called the European assemblage) were used in the analyses, as these countries have a similar climate to the area surveyed in China, with winter temperatures slightly above 0°C. This European dataset also comprised inland and coastal waterbird counts. The total area covered by the European counts is far larger than the area in China and the geographic distribution is different, the survey effort is larger, but the survey methods are comparable (Cao et al. 2008b). We, therefore, only used the relative community composition when comparing the European data with the data from China and not absolute bird densities, and avoided using species population size data in the comparison. To minimize the bias created by the use of different years, we analysed species presence/absence data, corrected for the larger sampling effort in Europe by omitting rare species, and analysed the relative contribution of the species to the community by so-called abundance–biomass comparison curves (ABC plots). The presence/absence analysis has been carried out by plotting the body mass of the species (ln BM) on species rank, based on the body mass of the species, with the heaviest species as rank number 1 (Prins and Olff 1998). A linear trend is expected, and deviations in the community composition are apparent from gaps in the body masses of the species. The analysis was carried out for all species, but in order to compensate for the larger sampling effort in Europe as compared to China, also once without those species that contributed <0.01% to the total abundance in Europe and once without those species that contributed <0.001% to the total abundance in Europe, so that about a similar number of species were included. Body masses of the species were obtained from Cramp (1998).
For each dataset we also calculated the relative contribution (%) to the entire community by each species, in terms of biomass and abundance. The abundance analysis was carried out by constructing ABC-plots and calculating the associated W statistics that quantify the cumulative difference between the biomass (B) and abundance (A) values for the total number of species, S, in the assemblage (Clarke 1990):
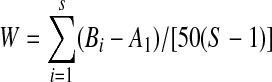 |
Disturbance in the community compositions is generally exposed through the disappearance of the heavier species, thereby lowering the biomass line in the graph in relation to the abundance line, and increasing W (Clarke 1990; de Boer and Longamane 1996). Therefore, W is often used as an indicator of community disturbance, with lower values typically found in more disturbed communities (Warwick and Clarke 1994; de Boer 2002; de Boer and Prins 2002). Differences in the relative composition of the bird assemblages over the Chinese provinces were analyzed using a human disturbance indicator as predictor variable. We reasoned that the disturbance would be larger in provinces with a higher GDP (in million RMB1) as wetland loss and degradation would be expected to be greater; additionally people would be able to hunt and sell waterfowl under better economic conditions (access to boats, hunting equipment, cars or other transport facilities). To test these assumptions we applied a general linear model (GLM) with W as the dependent variable and GDP as the dependent variable.
Changes over time in the China waterbird species composition were analyzed using older surveys carried out from 1990 to 1993 in the same provinces (WSGCOA 1994). These older surveys were probably less reliable in terms of species identification and, therefore, only the mean body mass of the species was used in the analysis.
To test the effect of fertilizer and pesticides on the community composition in China, we first estimated for each of the Chinese species the winter diet in terms of the percentage vegetal and animal matter (totalling 100%), based on Cramp (1998) and personal observations. The metabolic mass of each species was calculated from 0.437M0.729 (Kersten and Piersma 1987), where M is body mass in kg. Multiplying the metabolic mass by the number of birds per province gives the total metabolic mass per species. The total metabolic mass was then multiplied with the percentage of vegetal and animal matter in the diet. These values were summed for all species per province, so that the relative contribution of vegetal and animal matter in the birds’ diet could be calculated, and we tested whether the relative proportion of herbivorous waterbirds increased with increasing economic development. All statistical analyses were carried out with SPSS (v15).
Results
Table 1 shows the relative contribution of the species groups to the two communities. The Anatidae clearly dominated in both Europe and China in terms of number of species, abundance and biomass; goose species were responsible for >40% of the communities’ body mass in both areas. Most important species in Europe were mallard Anas platyrhynchos (abundance: 11%, biomass: 10%), greater white-fronted goose Anser albifrons (8%, 17%), and greylag goose A. anser (3%, 10%); in China the most important species were tundra swan Cygnus columbianus (5%, 23%), bean goose A. fabalis (9%, 21%) and swan goose A. cygnoides (6%, 14%), with dunlin Calidris alpina as the most commonly recorded species (17%, 0.6%). Europe has more geese and gull species, and China more heron and bittern, plover, and rail and coot species.
Table 1.
The relative contribution of the waterbird communities in China (mean 2004–2007) and Europe (mean 1998–1999) with respect to the number of species, the abundance of the birds (%n) and the body mass (%kg), together with the respective totals
| Europe | China | |||||
|---|---|---|---|---|---|---|
| Species | %n | %kg | Species | %n | %kg | |
| Anatidae | ||||||
| Dabbling ducks | 17 | 22.07 | 16.25 | 11 | 20.25 | 11.05 |
| Diving ducks | 6 | 9.44 | 6.07 | 8 | 1.06 | 0.66 |
| Eiders | 1 | 1.98 | 3.37 | |||
| Geese | 18 | 20.21 | 41.27 | 7 | 19.65 | 43.76 |
| Mergansers | 4 | 0.73 | 0.77 | 4 | 0.66 | 0.59 |
| Scoters | 3 | 0.51 | 0.50 | |||
| Seaducks | 2 | 1.33 | 0.91 | |||
| Shelducks | 3 | 1.78 | 1.52 | 2 | 0.81 | 0.66 |
| Stiff-tailed ducks | 2 | <0.01 | <0.01 | |||
| Swans | 6 | 1.26 | 10.34 | 3 | 4.69 | 24.80 |
| Ardeidae | ||||||
| Herons and Bitterns | 7 | 0.29 | 0.27 | 10 | 3.99 | 2.90 |
| Charadriidae | ||||||
| Plovers | 6 | 5.93 | 1.09 | 11 | 5.14 | 0.44 |
| Ciconiidae | ||||||
| Storks | 2 | <0.01 | 0.01 | 2 | 0.14 | 0.36 |
| Gaviidae | ||||||
| Divers | 4 | 0.01 | 0.02 | 2 | <0.01 | <0.01 |
| Gruidae | ||||||
| Cranes | 1 | 0.14 | 0.61 | 5 | 0.75 | 3.27 |
| Haematopodidae | ||||||
| Oystercatchers | 1 | 6.12 | 2.76 | 1 | 0.03 | <0.01 |
| Laridae | ||||||
| Gulls | 16 | 7.12 | 2.79 | 9 | 11.94 | 3.53 |
| Terns | 4 | 0.01 | <0.01 | 6 | 0.05 | 0.02 |
| Pelecanidae | ||||||
| Pelicans | 1 | 0.01 | 0.06 | |||
| Phalacrocoracidae | ||||||
| Cormorants and Darters | 2 | 1.00 | 1.86 | 1 | 1.60 | 2.71 |
| Phoenicopteridae | ||||||
| Flamingoes | 3 | 0.28 | 0.73 | |||
| Podicipedidae | ||||||
| Grebes | 5 | 1.09 | 0.77 | 5 | 1.10 | 0.33 |
| Rallidae | ||||||
| Rails and Coots | 4 | 7.66 | 5.62 | 8 | 1.38 | 0.87 |
| Recurvirostridae | ||||||
| Stilts and Avocets | 2 | 0.20 | 0.05 | 2 | 2.82 | 0.69 |
| Rostratulidae | ||||||
| Painted Snipes | 0.01 | <0.01 | ||||
| Scolopacidae | ||||||
| Sandpipers | 22 | 10.85 | 2.40 | 21 | 23.31 | 2.66 |
| Threskiornithidae | ||||||
| Ibises and Spoonbills | 2 | 0.01 | 0.01 | 2 | 0.62 | 0.63 |
| Others Identified | 8 | <0.01 | <0.01 | |||
| Unidentified birds | <0.01 | <0.01 | ||||
| Total | 151 spp | 9003625 | 10910 t | 121 spp | 1066013 | 1417 t |
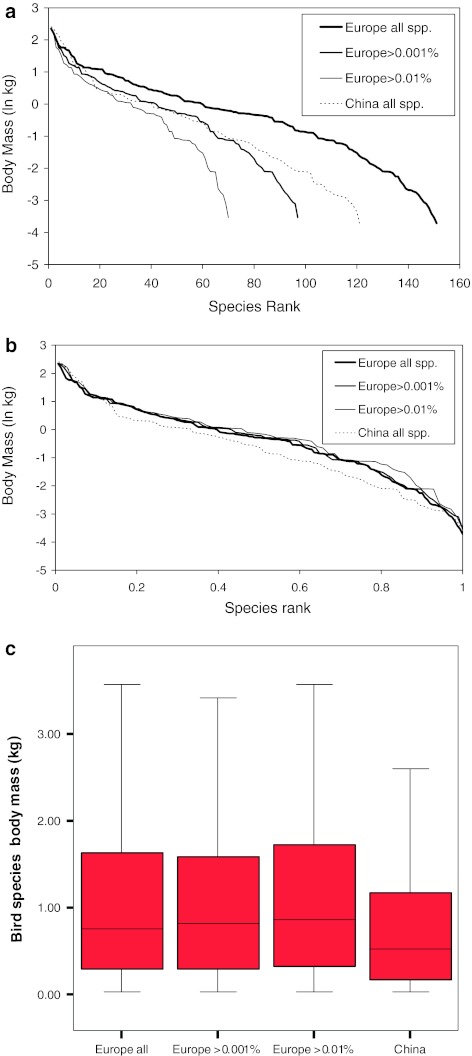
The distribution of the ln-transformed body masses of the species followed the expected negative relationship with species rank (Fig. 1a). The Chinese assemblage comprised 121 species, which lies between the values for the entire European assemblage (151 species) and the European assemblage with all species that contribute >0.001% to the total abundance (97 species). Indeed, the curve for the Chinese species lies between the European lines, but crosses the European 0.001% and even 0.01% threshold lines several times, indicating that the heavier species are relatively underrepresented in the assemblage and that the intermediate sized and smaller species are relatively more common in China. This difference is even more visible in Fig. 1b, where the species rank axis has been standardized. Hence, the median body mass of the different species in China is lower than in Europe (Fig. 1c; Mann–Whitney U test, z = 1.984, P < 0.05 for the comparison of the Chinese and European >0.001% body masses). Figure 1c also shows that the two smaller subsets of the European assemblages have no effect on the body mass distribution of the species in the assemblage; by omitting the rarer species in the European samples, we do not create a bias in the species body mass distribution, facilitating the comparison of the Chinese and European bird body masses.
Fig. 1.
The relationship between species body mass and species rank ordered from high to low body masses. a For Europe, two extra curves are depicted with truncation of rare species that vary in their contribution to the total abundance (<0.01 and <0.001%) in order to depict the effect of the larger sample intensity in Europe. b Similar to a, but now with standardized species rank axis. c Distribution of the species’ body masses depicted in a box plot
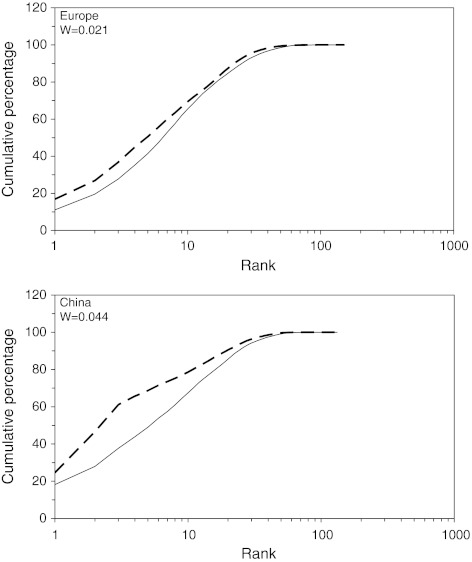
These body mass analyses are based on the presence/absence of the species in the community, but what is the effect of the relative abundances of the species? This question can be addressed by making ABC graphs for the communities. The biomass values were always larger than the abundance values in the ABC graphs for both Europe and China (Fig. 2), but China had relatively more heavier birds in the community, which is confirmed by the larger W value (0.044 for China, compared with 0.021 for Europe). Using the entire European dataset or the selection of species that contributed >0.01% or >0.001% to the total number of birds in Europe did not affect the layout of the graph or W value, but only increased the length of the tail at the cumulative contribution of 100%. In an analysis at species level, we looked at the relative importance of the heavier-bodied species. The 25% of species with the heaviest body mass were responsible for 79% of the total body mass of all birds in China, whereas this figure dropped to 57% in Europe. The Chinese tundra swans (23% of total biomass), bean geese (21%) and swan geese (14%) were responsible for the largest part of this contribution. These three species increased the contribution of the heavy-bodied species to the total bird biomass in China, but the mean (arcsine transformed) percentages of all heavy-bodied species (the 25% criterion) were not different between the Chinese and European assemblages (F1,67 = 0.669, P > 0.05).
Fig. 2.
Abundance–biomass comparison (ABC) curves, depicting the cumulative percentage of abundance (solid line) and biomass (broken line) with increasing species rank for the European and Chinese assemblages
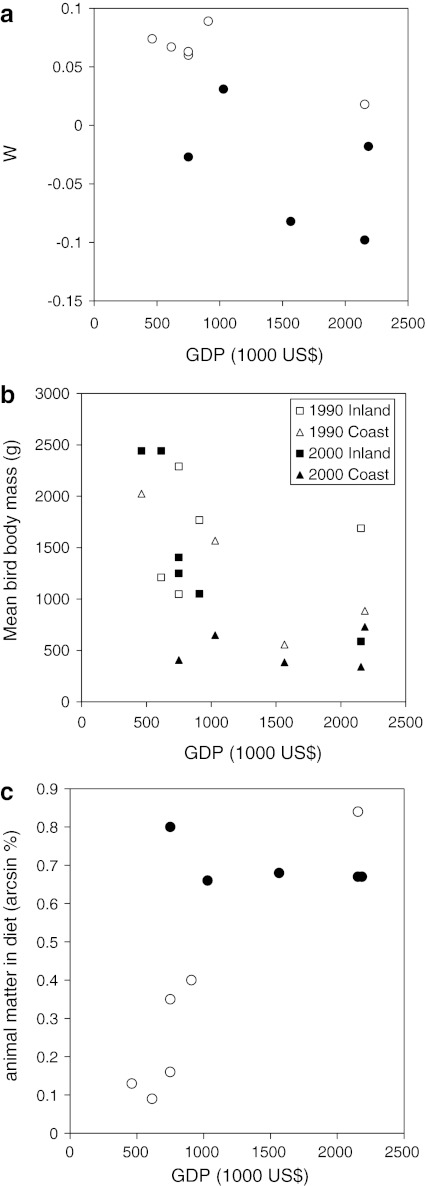
The ABC graphs of the Chinese provinces showed relatively large differences. The W statistic, calculated from the differences between biomass and abundance values, fluctuated between −0.98 and 0.089 from, respectively, Zhejiang to the Huai River. Surveys carried out at the Chinese coast had typically lower W values than inland surveys, caused by the relatively larger contribution of smaller bird species on the coast (e.g., dunlins and Kentish plovers Charadrius alexandrinus) and the lower abundance of heavier-bodied species (e.g., crane, swan and goose species). Assuming that W is an indicator for disturbance (lower values indicate higher disturbance), and taking into account the differences between coastal and inland surveys, we found, as expected, that W decreased with increasing GDP (Fig. 3a; GLM, F1,7 = 10.284, P < 0.05), and that coastal survey counts had lower W values than inland surveys (F1,7 = 16.981, P < 0.01). The total number of species per survey had no significant effect on W (F1,7 = 4.429, P < 0.08). The GLM analysis yielded normally distributed residuals (Kolmogorov–Smirnov statistic = 0.224, n = 11, P = 0.128) with equal error variances (Levene’s test F1,9 = 4.623, P = ns), so that the basic assumptions for the use of the GLM were fulfilled.
Fig. 3.
Relationship between a GDP (in million RMB for 2006) and W statistics, calculated from the ABC curves of the different provinces, for the coastal provinces (closed circles) and the inland provinces (open circles). b Relationship between GDP (in million RMB for 2006) and mean bird body mass calculated for each of the provinces in the China bird surveys for the 1990–1993 surveys (open symbols) and the 2000–2003 surveys (closed symbols), separating the inland counts (squares) from the coastal counts (triangles). c Effects of differences in GDP on the proportion of animal matter in the diet of waterbirds for the coastal provinces (closed circles) and the inland provinces (open circles)
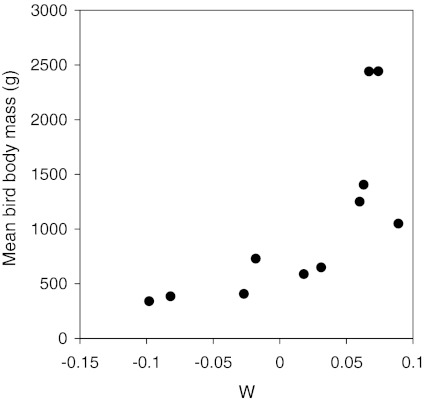
We found that with increasing W (decreasing disturbance) the mean body mass of the species in the survey increased (Fig. 4; Spearman r = 0.882, n = 11, P < 0.001). For these calculations, weighted mean body masses were calculated to incorporate the effect of differences in abundance of the different species. The relationship between human population density and mean bird body mass was, as expected, negative, but this could not be confirmed statistically (r = −0.533, n = 11, P = 0.091). If human disturbance is a factor influencing Chinese wintering bird community composition, then one would expect changes over time under the influence of a changing disturbance regime. We checked this assumption by using older records from extensive surveys carried out during 1990–1993. The mean body mass of the species in 1990–1993 was compared with the data from 2004 to 2007. Indeed, the mean body mass of the bird species per province was negatively correlated with increasing GDP for the 2000–2007 surveys (r = −0.773, n = 11, P < 0.01; Fig. 3b), but no significant relationship was found between GDP and mean bird body mass for the 1990–1993 surveys. Also, the 2000–2007 mean bird body masses were generally lower than the values calculated for the 1990–1993 surveys. Surprisingly, however, the mean bird body mass increased over the study period for the three provinces (Anhui, Hubei and Jiangxi) with the lowest GDP.
Fig. 4.
Relationship between the W statistics, calculated from the ABC curves of the different provinces, and the mean bird body mass. Lower W values indicate higher disturbance levels
To test for the effect of fertilizer and pesticide use on the waterbird community composition, we first calculated the proportion of vegetal and animal food in the diet. The diet of the Chinese birds is composed of 72% plant material and 28% animal material (insectivorous, bentivorous and piscivorous diets), with large differences between the different areas (Fig. 3c). The species on the coast consumed a significantly larger proportion of animal matter in the diet (GLM, F1,7 = 43.716, P < 0.001), due to the higher proportion of Charadriidae and lower proportion of Anatidae. There was a significant interaction between GDP and the inland/coastal surveys (F1,7 = 28.907, P < 0.001) and, as expected, the proportion of birds with animal matter in the diet decreased for the coastal surveys with increasing GDP. Surprisingly, for the inland surveys GDP had a significantly positive effect on the animal component in the diet.
Discussion
The community compositions of European and Chinese waterbirds are remarkably similar and show a similar decrease in body mass with increasing species rank when analyzing the species’ presence and absence data. The Chinese assemblage had fewer large-bodied species and more smaller-bodied species. There seem to be three potential explanations for these differences. The Chinese assemblage lacks some larger bird species, especially in the range of 1.5–5.5 kg. We have no confirmation of the local extinction of heavier species in the Chinese assemblage, as all expected species were found in the 2000–2007 surveys, and the difference cannot, therefore, be attributed to a sampling artefact. An alternative explanation could be that the European assemblage has accommodated some new, relatively heavy-bodied species. Indeed, some heavier bird species like the Canada goose Branta canadensis (Madsen 1991) or the Egyptian goose Alopochen aegyptiaca (Lensink 1999) have recently invaded Europe, and this could have increased the European species rank curve above the China curve. A third explanation is that the observed differences are natural and have been present in the assemblages for a long time span, maybe as a consequence of differences in habitat composition of the two areas or the species they support.
Contrary to our prediction, heavy-bodied species were not less common in China compared to Europe. Why is this so? Was our prediction wrong and is hunting of little importance in China? Is the effect of hunting in Europe on especially larger species still visible in the community structure, despite the recent strong increase in geese numbers (Ankney 1996; Madsen et al. 1999)? Or maybe human disturbance affected all species evenly in China, or China just holds relatively more heavy-bodied bird species? A potential survey bias could also be responsible for part of this difference; in Europe all wetlands were included in the analysis, including the smaller ones that are maybe not suitable for larger species, and coastal surveys were probably more common in the European assemblage, increasing the relative contribution of the smaller Charadriidae in the European counts. In China, the majority of the Chinese wetlands and lakes are large, and smaller wetlands are probably underrepresented in the surveys. This could have created a survey bias in favour of counting relatively more heavy species in China. However, the data do not provide support for our hypothesis that the heavier-bodied species would be relatively less common in China.
The analysis of the Chinese provinces showed that differences in assemblage structure (mean body mass and W value) could be correlated with GDP, as an indicator of industrial development and hunting pressure. As predicted, the mean bird body mass and W decreased with increasing GDP. This is by no means a causal relationship. We do not know what the exact mechanisms are behind these correlations, although there have been very large changes in land use and fast economic development in China (Zhang et al. 2007; Li et al. 2007), which certainly could have affected wetland availability, habitat quality, or human disturbance, and thereby the occurrence and abundance of certain waterbird species (NWCAPC 2000). The comparison of the 1990–1993 and 2000–2007 data from China also indicates that the heavier species have disappeared, especially from the more developed provinces. Information about the decrease or disappearance of hooded cranes (Grus monacha), tundra swans and swan geese from Chongming Island (Ma et al. 2004) support the latter trend. The increase in average body size in the Yangtze wetlands can also be interpreted as changes in the regional distribution of the species, such as an increase in the herbivorous Anatidae along the Yangtze. Unfortunately, we miss the hard data to investigate the species’ trends over the years in the different areas, although Cao et al. (2008a) showed that the Anatidae population sizes decreased greatly since the 1950s. This calls for future studies, especially long-term annual surveys with a high spatial resolution and experimental studies in which the effect of disturbance on bird populations can be quantified (Madsen 1995; de Boer and Longamane 1996; Desmonts et al. 2009).
The analysis of the diet composition of the birds confirmed our hypothesis that the contribution of bentivorous, piscivorous and insectivorous bird species declined with increasing development in the coastal areas, with a concomitant increase in the proportion of herbivorous bird species. This trend can be explained by either the negative, poisonous effects of pesticides (Rattner et al. 2005; Degernes 2008), or the positive effects of fertilizer application on herbivorous birds (van Eerden et al. 2005; Amano et al. 2007). Herbivorous birds are relatively more abundant in the inland provinces, but the positive relationship between GDP and the contribution of animal matter in the diet (and hence a decrease in the contribution of herbivorous birds) for the inland provinces is difficult to explain, and calls for a more detailed analysis. The abundance of Anatidae has declined during the last 25 years (Cao et al. 2008a) and apparently they have not benefitted very much from the use of fertilizers. This strongly indicates that the driving forces of decline are habitat loss, habitat degradation, or hunting. The impacts of local differences in wetland use are impossible to analyse with the current dataset. For instance, introduction of exotic crab species has led to the disappearance of aquatic vegetation and associated fauna in many wetlands with a consequent impact on bird numbers (personal observation). An increase in fishing pressure might also have contributed to a decrease in piscivorous bird species (Zydelis and Kontautas 2008). Human-induced increases in water turbidity affect the visibility of piscivorous bird species and, therefore, have impacted the bird community composition or affected the growth of aquatic vegetation and, thereby, negatively influenced the abundance of herbivorous water birds (Wu et al. 2007). And, more importantly, we do not know to what extent the differences in wintering bird numbers in China are influenced by factors that operate at the breeding grounds in Russia during summer (Tolvanen et al. 2000; Syroechkovekiy 2006). However, we do know that changes in abundance of geese can trigger large cascading effects, changing the environment completely (Jefferies et al. 2006). It is possible that further economic development could enhance bird conservation when the basic needs of people have been guaranteed (Teel et al. 2007). However, before we can analyse the effects of human impacts or conservation actions, we should prioritize long-term survey data collection and detailed field studies to enable us to evaluate population trends and regional changes in distribution in China.
Acknowledgments
We thank Wetlands International, Alterra/Wageningen UR, and Toon Helmink for supplying the data of the European bird assemblages through the International Waterbird Census. We also thank Han Zhao Guo and Zuo Chen for their assistance during the Chinese surveys. Surveys in Shandong, Jiangsu and Zhejiang, and the Huai River floodplain, were supported by the US Fish and Wildlife Service (Contract No. 70181-6-M440) and the National Natural Science Foundation of China (Grant Nos. 30570253 and 30940010).
Biographies
Willem F. de Boer
is a lecturer at the Resource Ecology Group, Wageningen University. He is responsible for lectures and research in the area of animal ecology, community composition and biodiversity.
Lei Cao
is a Associate Professor teaching ornithology and zoology in the School of Life Sciences at the University of Science and Technology of China.
Mark Barter
is a Visiting Professor in the School of Life Sciences at the University of Science and Technology of China. He is also an Associate Expert of Wetlands International.
Xin Wang
is a PhD student at the University of Science and Technology of China.
Mengmeng Sun
is an undergradute student at the University of Science and Technology of China.
Herman van Oeveren
is a research assistant with an interest in grass and grazers of African savanna at the Resource Ecology Group, Wageningen University.
Jan de Leeuw
contributed to this work as Associate Professor Environmental Science at the International Institute for Geoinformation Science and Earth Observation (ITC) in Enschede, The Netherlands. He recently moved to the International Livestock Research Institute (ILRI), Nairobi, Kenya.
Jeb Barzen
directs the Field Ecology Department at the International Crane Foundation, USA.
Herbert H. T. Prins
is a full Professor and chair holder of the Resource Ecology Group, Wageningen University. His research focuses on understanding herbivory in a spatial context.
Footnotes
http://en.wikipedia.org/wiki/List_of_Chinese_administrative_divisions_by_GDP_per_capita. Accessed 4 Dec 2008.
Contributor Information
Willem F. de Boer, Email: fred.deboer@wur.nl
Lei Cao, Phone: ++86-13966714569, Email: caolei@ustc.edu.cn.
Mark Barter, Email: markbarter@optusnet.com.au.
Xin Wang, Email: wangxin2@mail.ustc.edu.cn.
Mengmeng Sun, Email: smmnancy@mail.ustc.edu.cn.
Herman van Oeveren, Email: herman.vanoeveren@wur.nl.
Jan de Leeuw, Email: j.leeuw@cgiar.org.
Jeb Barzen, Email: jeb@savingcranes.org.
Herbert H. T. Prins, Email: herbert.prins@wur.nl
References
- Amano T, Ushiyama K, Fujita G, Higuchi H. Predicting grazing damage by white-fronted geese under different regimes of agricultural management and the physiological consequences for the geese. Journal of Applied Ecology. 2007;44:506–515. doi: 10.1111/j.1365-2664.2007.01314.x. [DOI] [Google Scholar]
- Ankney CD. An embarrassment of riches: too many geese. Journal of Wildlife Management. 1996;60:217–223. doi: 10.2307/3802219. [DOI] [Google Scholar]
- Barter M, Chen L, Cao L, Lei G. Waterbird Survey of the Middle and Lower Yangtze River Floodplain in Late January and Early February. Beijing: China Forestry Publishing House; 2004. [Google Scholar]
- Barter M, Cao L, Chen L, Lei G. Results of a survey for waterbirds in the lower Yangtze floodplain, China, in January-February 2004. Forktail. 2005;21:1–7. [Google Scholar]
- Barter MA, Yu X, Cao L, Liu BF, Yang ZL, Zheng DT. Wintering waterbird survey of the coastline of Fujian Province, China: 8–27 February 2006. Beijing: China Forestry Publishing House; 2007. [Google Scholar]
- Beale CM, Monaghan P. Human disturbance: people as predation-free predators? Journal of Applied Ecology. 2004;41:335–343. doi: 10.1111/j.0021-8901.2004.00900.x. [DOI] [Google Scholar]
- Cao L, Barter M, Lei G. New Anatidae population estimates for eastern China: implications for current flyway estimates. Biological Conservervation. 2008;141:2301–2309. doi: 10.1016/j.biocon.2008.06.022. [DOI] [Google Scholar]
- Cao L, Barter M, Lewthwaite R. The declining importance of the Fujian coast, China, for wintering waterbirds. Waterbirds. 2008;31:645–650. [Google Scholar]
- Clarke KR. Comparisons of dominance curves. Journal of Experimental Marine Biology and Ecology. 1990;138:143–157. doi: 10.1016/0022-0981(90)90181-B. [DOI] [Google Scholar]
- Coleman JM, Huh OK, Braud D., Jr Wetland loss in world deltas. Journal of Coastal Research. 2008;24:1–14. doi: 10.2112/05-0607.1. [DOI] [Google Scholar]
- Cramp S. The complete birds of the Western Paleartic. Oxford: Oxford University Press; 1998. [Google Scholar]
- Boer WF. The shorebird community structure at an intertidal mudflat in southern Mozambique. Ardea. 2002;90:81–92. [Google Scholar]
- Boer WF, Longamane FA. The exploitation of intertidal food resources in Inhaca Bay, Mozambique, by shorebirds and humans. Biological Conservation. 1996;78:295–303. doi: 10.1016/S0006-3207(96)00050-X. [DOI] [Google Scholar]
- Boer WF, Prins HHT. The community structure of a tropical intertidal mudflat under human exploitation. ICES Journal of Marine Science. 2002;59:1237–1247. doi: 10.1006/jmsc.2002.1287. [DOI] [Google Scholar]
- Degernes LA. Waterfowl toxicology: a review. Veterinary Clinics of North America—Exotic Animal Practice. 2008;11:283–300. doi: 10.1016/j.cvex.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Delany S, Scott D. Waterbird population estimates. Wageningen: Wetlands International; 2002. [Google Scholar]
- Desmonts D, Fritz H, Cornulier T, Maheo R. Rise in human activities on the mudflats and Brent Geese (Branta bernicla) wintering distribution in relation to Zostera spp. beds: a 30-year study. Journal of Ornithology. 2009;150:733–742. doi: 10.1007/s10336-009-0391-5. [DOI] [Google Scholar]
- Ebbinge BS. The impact of hunting on mortality rates and spatial distribution of geese wintering in the western Palearctic. Ardea. 1991;79:197–210. [Google Scholar]
- Jefferies RL, Jano AP, Abraham KF. A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. Journal of Ecology. 2006;94:234–242. doi: 10.1111/j.1365-2745.2005.01086.x. [DOI] [Google Scholar]
- Kersten M, Piersma T. High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea. 1987;75:175–187. [Google Scholar]
- Lensink R. Aspects of the biology of Egyptian Goose Alopochen aegyptiacus colonizing the Netherlands. Bird Study. 1999;46:195–204. doi: 10.1080/00063659909461131. [DOI] [Google Scholar]
- Li ZY, Yang GS, Dong YW. Zoning of the area alongside the Yangtze River in Anhui Province based on the demand for economic development. Journal of Ecology and Rural Environment. 2007;23:12–17. [Google Scholar]
- Lu, J.J., 1993. The utilisation of migratory waterfowl in China. In: Waterfowl and wetlands conservation in the 1990s: a global perspective, eds. M. Moser, and C. Prentice. IWRB Publication 26: 90–92. Slimbridge: IWRB.
- Ma Z, Li B, Zhao B, Jing K, Tang S, Chen J. Are artificial wetlands good alternatives to natural wetlands for waterbirds? A case study on Chongming Island, China. Biodiversity and Conservation. 2004;13:333–350. doi: 10.1023/B:BIOC.0000006502.96131.59. [DOI] [Google Scholar]
- Madsen J. Status and trends of goose populations in the western Palearctic in the 1980s. Ardea. 1991;79:113–371. [Google Scholar]
- Madsen J. Impacts of disturbance on migratory waterfowl. Ibis. 1995;137:67–74. doi: 10.1111/j.1474-919X.1995.tb08459.x. [DOI] [Google Scholar]
- Madsen J. Experimental refuges for migratory waterfowl in Danish wetlands. II. Tests of hunting disturbance effects. Journal of Applied Ecology. 1998;35:398–417. doi: 10.1046/j.1365-2664.1998.00315.x. [DOI] [Google Scholar]
- Madsen J, Cracknell G, Fox AD. Goose populations of the Western Palearctic. A review of status and distribution. Rønde: National Environmental Research Institute; 1999. [Google Scholar]
- Milner JM, Nilsen EB, Andreassen HP. Demographic side effects of selective hunting in ungulates and carnivores. Conservation Biology. 2007;21:36–47. doi: 10.1111/j.1523-1739.2006.00591.x. [DOI] [PubMed] [Google Scholar]
- Palacio-Núñez J, Verdú JR, Galante E, Jiménez-García D, Olmos-Oropeza G. Birds and fish as bioindicators of tourist disturbance in springs in semi-arid regions in Mexico: a basis for management. Animal Biodiversity and Conservation. 2007;30:29–41. [Google Scholar]
- Palomino D, Carrascal LM. Threshold distances to nearby cities and roads influence the bird community of a mosaic landscape. Biological Conservation. 2007;140:100–109. doi: 10.1016/j.biocon.2007.07.029. [DOI] [Google Scholar]
- PC NWCA. National wetland conservation action plan for China. Beijing: State Forestry Administration; 2000. [Google Scholar]
- Prins, H.H.T., and Olff, H. (1998). Species richness of African grazer assemblages: towards a functional explanation. In Dynamics of tropical communities, eds. Newbery, D.M., H.H.T. Prins, and N.D. Brown. British Ecological Society Symposium, vol 37, 449–490. Oxford: Blackwell Science.
- Rattner BA, Eisenreich KM, Golden NH, McKernan MA, Hothem RL, Custer TW. Retrospective ecotoxicological data and current information needs for terrestrial vertebrates residing in coastal habitat of the United States. Archives of Environmental Contamination and Toxicology. 2005;49:257–265. doi: 10.1007/s00244-004-0193-y. [DOI] [PubMed] [Google Scholar]
- Rees EC, Bruce JH, White GT. Factors affecting the behavioural responses of whooper swans (Cygnus c cygnus) to various human activities. Biological Conservation. 2005;121:369–382. doi: 10.1016/j.biocon.2004.05.009. [DOI] [Google Scholar]
- Ren BP. Neo-type industrialization: the innovation on China’s economic strategy. Economist. 2003;3:4–11. [Google Scholar]
- Schmutz JA, Ely CR. Survival of greater white-fronted geese: effects of year, season, sex, and body condition. Journal of Wildlife Management. 1999;63:1239–1249. doi: 10.2307/3802841. [DOI] [Google Scholar]
- Shindo J, Okamoto K, Kawashima H. Prediction of the environmental effects of excess nitrogen caused by increasing food demand with rapid economic growth in eastern Asian countries, 1961–2020. Ecological Modelling. 2006;193:703–720. doi: 10.1016/j.ecolmodel.2005.09.010. [DOI] [Google Scholar]
- Syroechkovekiy, E.E. 2006. Long-term declines in arctic goose populations in eastern Asia. In: Waterbirds around the world, eds. G.C. Boere, C.A. Galbraith, and D.A. Stroud, 649–662. Edinburgh: The Stationary Office.
- Teel TL, Manfredo MJ, Stinchfield HM. The need and theoretical basis for exploring wildlife value orientations cross-culturally. Human Dimensions of Wildlife. 2007;12:297–305. doi: 10.1080/10871200701555857. [DOI] [Google Scholar]
- Thiollay JM. Raptor declines in West Africa: comparisons between protected, buffer and cultivated areas. Oryx. 2007;41:322–329. doi: 10.1017/S0030605307000809. [DOI] [Google Scholar]
- Tolvanen, P., Øien, I.J., and Ruokolainen, K. 2000. Fennoscandian lesser white-fronted goose conservation project. Annual Report 1999. WWF Finland Report 12 and Norwegian Ornithological Society, NOF Rapportserie Report no. 1-2000.
- Eerden MR, Drent RH, Stahl J, Bakker JP. Connecting seas: Western Palaearctic continental flyway for water birds in the perspective of changing land use and climate. Global Change Biology. 2005;11:894–908. doi: 10.1111/j.1365-2486.2005.00940.x. [DOI] [Google Scholar]
- Wang B. Cultural eutrophication in the Changjiang (Yangtze River) plume: history and perspective. Estuarine, Coastal and Shelf Science. 2006;69:471–477. doi: 10.1016/j.ecss.2006.05.010. [DOI] [Google Scholar]
- Warwick RM, Clarke KR. Relearning the ABC: taxonomic changes and abundance/biomass relationships in disturbed benthic communities. Marine Biology. 1994;118:739–744. doi: 10.1007/BF00347523. [DOI] [Google Scholar]
- Waterbird research in China. Shanghai: East China Normal University Press; 1994. [Google Scholar]
- Wu G, Leeuw J, Skidmore AK, Prins HHT, Liu Y. Concurrent monitoring of vessels and water turbidity enhances the strength of evidence in remotely sensed dredging impact assessment. Water Research. 2007;41:3271–3280. doi: 10.1016/j.watres.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yu Z, Li X, Zhou W, Zhang D. Land use change and land degradation in China from 1991 to 2001. Land Degradation and Development. 2007;18:209–219. doi: 10.1002/ldr.757. [DOI] [Google Scholar]
- Zydelis R, Kontautas A. Piscivorous birds as top predators and fishery competitors in the lagoon ecosystem. Hydrobiologia. 2008;611:45–54. doi: 10.1007/s10750-008-9460-7. [DOI] [Google Scholar]