Abstract
Dichlorodiphenyltrichloroethane (DDT) is still used in Africa for the indoor control of malaria and it may represent a potential hazard for wildlife. The littoral sediments of two alkaline-saline lakes, Natron (Tanzania) and Bogoria (Kenya), in the Eastern Rift Valley, supporting large populations of lesser flamingos (Phoeniconaias minor), were analysed for DDT residues. Physical–chemical analyses (temperature, conductivity, pH and dissolved oxygen) were also performed on the water of the two lakes and in the tributaries of Lake Natron, to evaluate the influence of the environmental variables on pollutant occurrence. At Lake Natron, around 1 km from the sediment collection sites, tree leaves of Acacia tortilis were also collected. The main metabolite found in all sediment samples was pp’DDE, whilst equal concentrations of pp’DDT and pp’DDE were measured in acacia leaves. The levels of DDTs measured in the sediments were within 5.9–30.9 ng g−1 d.w., reaching the maximum value in a tributary of Lake Natron. On the whole, the contamination of Lake Natron and Lake Bogoria basins seems to be quite moderate. Nevertheless, the pp’DDE/pp’DDT ratio equals 1 in the Acacia tortilis leaves, which makes one suppose that the input of the parent compound was rather recent and could have been from aerial transport or dust from relatively close-by old pesticides storage sites.
Keywords: Obsolete contaminant pollution, Soda lakes, Sediments, Acacia leaves, Tanzania, Kenya
Introduction
Alkaline–saline (“soda”) lakes in the East African Rift Valley (Fig. 1; latitude from 4°35′ N to 14°30′ S and a N–S distance of around 2,100 km; Spigel and Coulter 1996) have an outstanding biodiversity value, despite being hostile environments, because together they are home to approximately two million lesser flamingos (Phoeniconaias minor) a species considered “near-threatened” by the International Union for Conservation of Nature. Lesser flamingos feed preferentially on Arthrospira fusiformis, a planktonic cyanobacterium that is particularly abundant in these lakes (Mlingwa and Baker 2006). Whilst most of the soda lakes—e.g. Nakuru, Elmenteita and Bogoria in Kenya; Manyara in Tanzania—are all used extensively by Phoeniconaias minor, only one regular breeding site exists in East Africa, on soda islands in the middle of Lake Natron in Northern Tanzania (Brown 1973; Harper et al. 2003). Despite the high ecological value of this environment, few studies of the Lake Natron basin and its eco-hydrology exist and no data are reported in the literature on the contamination levels of Persistent Organic Pollutants (POPs).
Fig. 1.
Landscape in Northern Tanzania, inside the Rift Valley (Photo: Roberta Bettinetti 2009)
In Africa, pesticides have been used for combating agricultural pests and controlling disease vectors for more than 50 years (Mansour 2009) and the most common organochlorine compound used for pest control has been the insecticide pp’DDT (Dichloro Diphenyl Trichloroethane). In Tanzania, this compound has been restricted, banning it in agriculture since 1991 and in Kenya the last import was in 1985 (Wandiga 2001). At present, however, use is allowed only for Indoor Residual Spraying (IRS) for malaria vector control (Mandavilli 2006). Moreover, “hot spot” pollution areas have been found in a coastal region of Tanzania where obsolete pesticides stocked in a disused farm have been accidentally released since 1990 (Elfvendahl et al. 2004; Marco and Kishimba 2005, 2007).
Although there is no reason to expect a direct negative impact of DDT contamination in the most remote lakes of the Eastern Rift Valley, as these areas are sparsely populated and hundreds of kilometres distant from the recognised pesticide sources, an indirect influence of DDT cannot be excluded, due to long-range transport and local occasional IRS in tourist lodges.
Much of the knowledge of the African Rift Valley saline lakes has come from studies on their chemistry (e.g. Wood and Talling 1988), biodiversity (e.g. Jones et al. 1994; Harper et al. 2003), primary production (e.g. Melack 1981, 1988) or explanations for the deaths of large numbers of lesser flamingos (e.g. Harper et al. 2003; Krienitz et al. 2003; Ballot et al. 2004; Oaks et al. 2006). Little is known (Saoke 2005) about the degree of pollution in these environments by pp’DDT (e.g. Koeman et al. 1972; Gitahi et al. 2002; Kishimba et al. 2004; Mavura and Wangila 2004), however.
The main objective of this study was to evaluate the degree of DDT contamination of the river and lake environments by surface sediment analyses, in order to ascertain whether such remote environments were still contaminated. Physical and chemical water parameters were measured in situ in water where the sediments were collected, to evaluate the possible influences of pH, temperature, dissolved oxygen and alkalinity on the presence/proportion of DDT-related compounds recovered in the sediments. Leaves of Acaciatortilis were also sampled in the Lake Natron area as a bioindication of any recent atmospheric DDT pollution.
Materials and Methods
Study Sites
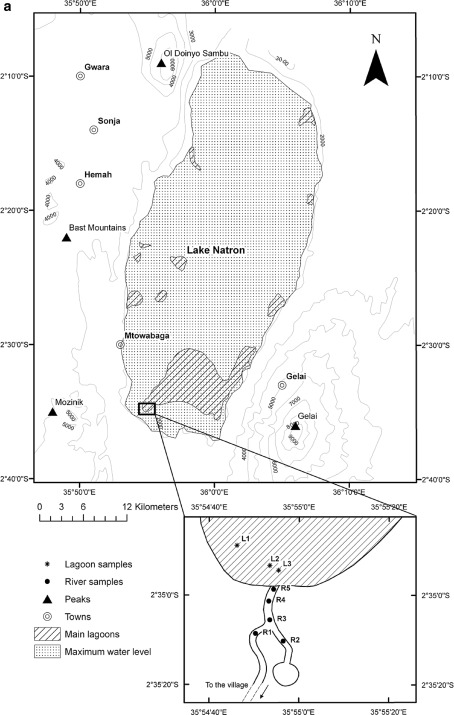
Lake Natron (Figs. 2, 3, 4a) lies in the Eastern Rift Valley lake of northern Tanzania just south of the Tanzania–Kenya border. Lying at an average height of 610 m a.s.l., the lake spans 75 km in length and 22–35 km in width (Dawson 2008). Four permanent rivers feed Lake Natron from Tanzania—the Engare Nyiro, the Peninj, the Moinik and the Engare Sero, plus a number of seasonal streams. These drain into the lake from the Ngorongoro Highlands in the south, Mount Lengai (2,942 m a.s.l.) in the southeast, and the Nkito Hills in the west (Hughes and Hughes 1992).
Fig. 2.
Lake Natron and its inlet river (Photo: Roberta Bettinetti 2009)
Fig. 3.
East tropical African Lakes (modified from Nyamweru 1983)
Fig. 4.
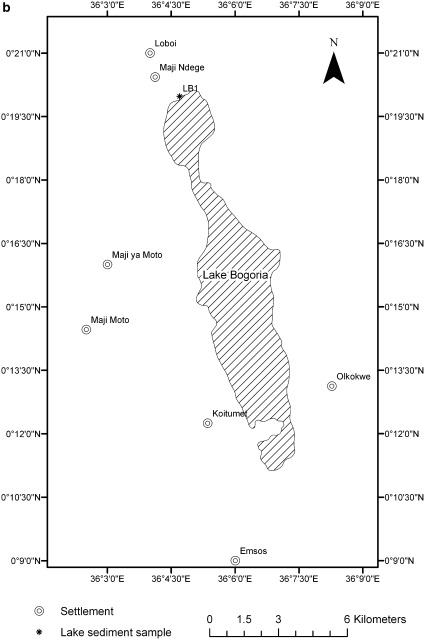
Detailed map of Lake Natron (a) and Lake Bogoria (b) with sampling sites
The perennial Engare Nyiro River (also known as the Ewaso Ngiro), the lake’s principal affluent, rises in Kenya’s Mau Forest, flows south through the Ngare Ngiro (or Shompole) Swamp before crossing the border and entering the lake in the north. Twenty eight springs, most saline or sub-saline, flow from the base of surrounding volcanoes or the Rift Escarpment into Lake Natron and in some cases sustain a number of isolated lagoons around the lake edge. The maximum depth of the lake is approximately 3–4 m and whilst the largest single input is derived from direct precipitation, high evaporation rates (up to 20 mm day−1) and low rainfall characterise the region. During periods of drought (from June to October) when the water level is low, a large portion of the lake’s bed is exposed, covered by a salt crust that dissolves during the rainy seasons (Wetlands International 2002). In this northern and eastern region of Tanzania, the rainfall is bimodal with two rainy seasons. The ‘short rains’ or Vuli last from October to December, and the ‘long rains’ or Masika last from March to May. As a consequence, the total surface area of Lake Natron fluctuates considerably, both seasonally and across larger temporal scales. Figure 4a shows the extent of the exposed salt crust and the distribution of the main lagoons identified from satellite imagery taken in 2009.
In addition to flamingos, in the area around Lake Natron there is a significant population of large mammals and 113 bird species (Yanda and Madulu 2005); the streams and lagoons also support two endemic fish species (Alcolapia alcalicus and A. grahammi). Permanent villages are located along freshwater inflows to the lake. In recent years, the human population has increased significantly, bringing with it an overexploitation of the local natural resources: irrigated small-scale farming has developed around the lake basin and adjacent areas, even where the soil is not particularly productive. Pastoralism by the Maasai, however, remains the dominant landuse in the area. The whole lake in Tanzania is a wetland of international importance under the Ramsar convention but only in 2009 any staff were appointed by the Wetlands Division of the Tanzanian Ministry of Natural Resources to begin to implement sustainable wetlands policies amongst the communities around the lake.
Lake Bogoria (Figs. 3, 4b) is a deeper, saline and alkaline lake with three basins which lie in a volcanic region in a half-graben basin in the south of Kenya, a little north of the equator. The southern basin, a relict volcanic crater, is the deepest part (14 m), joined to the rest of the lake by a narrow isthmus. With a mean surface area of 34 km2 and a mean depth of 5.4 m, it is sustained by two semi-permanent surface water river inflows, two small permanent spring-fed freshwater streams and numerous semi-saline hot springs, most of which emerge from fissures along the western and south-eastern shores of the lake (Harper et al. 2003). The principal source of freshwater is the Sandai-Wasenges River, which drains the Subukia and Iguamiti highlands to the South-East. The river is ephemeral, drying up at the Sandai swamps during the dry season (December–February), and transports huge volumes of suspended solids during the wet season. The lake has no outlet and the intense evaporation has led to high levels of salt and mineral accumulation; however, the water level fluctuations are minimal because of its high volume/area (Wetlands International 2002).
Lake Bogoria is an important conservation area in Kenya holding regionally and nationally endangered species; it was designated as a national reserve in 1974 and in 2001 it was listed as a wetland of international importance under the Ramsar convention. Despite this fact, the land and lake of the National Reserve are at risk because of excessive grazing and erosion (land) and over-stocking and unsustainable arable agriculture in its catchment. The root causes of these problems are poverty and the absence of any effective land use advisory service.
Samples Collection and Chemical Analysis
Surface sediments (the first layer of deposition, 30 g w.w. at each sampling site) were collected by hand using a steel spatula in March 2009 (the end of the dry season), in three different sites in southern area of the Lake Natron and in five sites along the course of two inlet rivers to the lake, (Fig. 4a). One sediment sample was also collected in April 2009 in Lake Bogoria (LB1, Fig. 4b). A sample of Acacia tortilis leaves (n = 6, from one tree, approximately 2.5 m above the ground) was also collected at 1 km to the south from the sediment sampling sites of Lake Natron at the same time of sediment sampling. This species was the only tree present in the neighbourhood of the lake. After the collection, leaves and sediments were stored at about 10°C for 5 days and then they were kept frozen until lyophilisation.
At the same sites and times, temperature, conductivity, pH and oxygen concentrations in the water column above the sediment were measured in situ with portable instruments. The organic matter content of the sediments was determined by weight Loss-On-Ignition (LOI) at 550°C (Dean 1974) in laboratory.
Extraction of freeze-dried (Edwards Pirani 1001 freeze-dryer) homogenised sediments and leaves (1 g) was performed in glass microfibre thimbles (19-mm internal diameter × 90-mm external length, Whatman, England) for 2 h with 60 ml of n-hexane (Carlo Erba, Italy, pesticide analysis grade) using a modified Soxhlet apparatus (Velp Scientifica-ECO 6 thermoreactor). For the leaf sample only, organic matter was destroyed with H2SO4 (98%, Carlo Erba, Italy) and chlorinated compounds were then recovered by several n-hexane washings. Next, n-hexane extracts were concentrated down to about 2 ml and passed through a Florisil column (4 × 0.7 cm), in the case of the sediment samples, with Cu powder (0.1 g) on the top. Cu powder was previously activated by HCl (18%, Carlo Erba, Italy) and washed with water, acetone and n-hexane. The Florisil column was eluted with 25 mL of n-hexane-dichloromethane (Carlo Erba, Italy, pesticide analysis grade) 85:15 (v/v) mixture and the eluate was concentrated to exactly 0.5 mL. The purified extracts were analysed by gas-chromatography (GC Carlo Erba, Top 8000) coupled with 63Ni electron capture detector (Carlo Erba ECD 80) using an on-column injection system (volume injected: 1 μl). The column was a WCOT fused silica CP-Sil-8 CB (50 m × 0.25 mm I.D., film thickness 0.25 μm, Varian, USA). The temperature programme used was from 60 to 180°C at 20°C min−1, followed by a run from 180 to 200°C at 1.5°C min−1. A further run from 200 to 270°C at 3°C min−1; an isothermal condition at 270°C was maintained for 20 min, with helium as carrier gas (1 ml min−1) and nitrogen as auxiliary gas (30 ml min−1) was implemented. Sample quantification was performed using external reference standards containing a mixture of HCB (Hexachlorobenzene), HCHs (Hexachlorocyclohexanes), pp’DDT, (pp’Dichlorodiphenyltrichloroethane), pp’DDE (pp’Dichlorodiphenyldichloroethylene) and pp’DDD (pp’Dichlorodiphenyldichloroethane) (Pestanal, Sigma-Aldrich, Germany) in iso-octane (Carlo Erba, Italy, pesticide analysis grade). The detection limit for each chlorinated pesticide was 0.1 ng g−1 d.w. Recovery efficiency was tested on reference sediment and it was within 80–100% for the three DDT congeners.
Statistical Analysis
Principal component analysis (PCA) was applied to the correlation matrix of the measured data of sediments at each sampling sites of Lake Natron and rivers (pp’DDT, pp’DDD, pp’DDE and LOI%). In order to treat all variables as if they were of equal importance regardless of their scale of measurement, data were standardised to 0 mean and to 1 standard deviation. PCA calculates a number of linear combinations of the observed variables that explain as much as possible their original variation. Each principal component (PC) results from a linear combination of the original variables multiplied by coefficients (factor scores) whose numerical values are proportional to the contribution of the variable to the component. As a result of an effective ordination process, the first PC accounts for the greatest proportion of the original variance, whilst the second, as well as the following PCs, progressively explain smaller amounts of data variation. PCA was processed using Addinsoft, XLSTAT© ver. 7.5.3.
Results
The conductivities of the inflowing streams to the Natron lagoon show considerable differences according to their source (Fig 4a; Table 1). The lowest conductivity was measured at site R2, a tributary stream fed by underground spring waters, indicated by its slightly lower surface water temperature. Closeby a second stream, R1, also fed by springs further back from the lake, had 20 times higher conductivity, and it was diluted into the lagoon through R4 and R5. Once in the lagoon, however, evaporation at the high temperatures elevated the conductivity to conditions which must be typical of the main lake (it could not be reached because of very flocculent deep mud). At Lake Bogoria where there is little spatial change in concentrations as the lake is deep, conductivity was 68,650 μS cm−1.
Table 1.
Physical–chemical parameters of the water column, LOI and DDT residues in sediments at different sites of Lake Natron and Lake Bogoria (to identify the location of the stations see Fig. 4a, b)
| Water column | Sediments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sites | St. | DO (mg l−1) | pH | T (°C) | Cond. (μS cm−1) | LOI (%) | pp’DDT (ng g−1 d.w.) | pp’DDE (ng g−1 d.w.) | pp’DDD (ng g−1 d.w.) | pp’DDE/pp’DDT |
| Lake Natron | L1 | 0.6 | 9.93 | 33.7 | 106650 | 20.5 | 0.6 | 15.9 | 1.5 | 26.5 |
| L2 | 17.2 | 10.01 | 33.1 | 14500 | 15.4 | 0.6 | 7.9 | 1.5 | 13.2 | |
| L3 | 19.4 | 10.32 | 32.5 | 12800 | 13.2 | 0.4 | 4.6 | 0.9 | 11.5 | |
| Natron inlet | R1 | 2.8 | 9.50 | 33.1 | 39500 | 15.8 | 1.6 | 27.2 | 2.1 | 17.0 |
| R2 | 3.5 | 8.43 | 31.0 | 1950 | 10.9 | 0.7 | 12.6 | 0.6 | 18.0 | |
| R3 | 4.1 | 9.18 | 33.0 | 21500 | 6.3 | 0.9 | 16.1 | 1.2 | 17.9 | |
| R4 | 12.1 | 9.21 | 33.2 | 12500 | 8.7 | 0.8 | 6.5 | 0.7 | 8.1 | |
| R5 | 13.5 | 10.20 | 34.5 | 13500 | 11.8 | 0.8 | 10.6 | 1.5 | 13.2 | |
| Lake Bogoria | LB1 | >20.0 | 10.14 | 31.3 | 68650 | 2.7 | 0.4 | 10.8 | 0.7 | 27.0 |
The measurements of dissolved oxygen concentrations (DO) reflect the degree of productivity of the lake, recorded during the day so they reflect photosynthesis. The highest DO values at Lake Natron were at sites L2 and L3 and at R4 and R5 where less alkaline water was lying over shallow epipelic (at the surface of mud) cyanobacteria and algae, photosynthesizing highly and providing food for thousands of lesser flamingos (pers. obs.). Low productivity was characteristic of the upstream sites where the oxygen concentration was lower. The particularly high levels of DO at Lake Bogoria reflect the extremely high photosynthesis of planktonic Arthrospira fusiformis in this lake, a major lesser flamingo feeding environment.
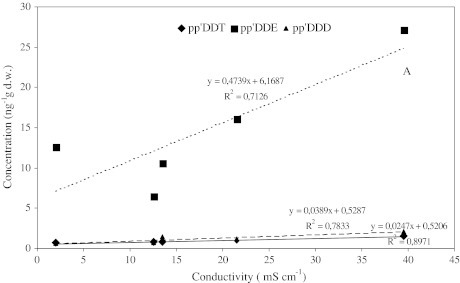
HCB and HCH isomers in the sediments were always below the detection limit. The maximum value of Total DDT was detected in sediments of site R1 of the river coming from the west (Fig. 4a) which crosses a Maasai village, whilst the river fed by the underground spring (R2) was less contaminated. A significant correlation was found between conductivity and DDTs for river sediments (Fig. 5), which reflects a pollutant dilution effect along the rivers to the lake due to the mixing of spring waters, which should have lower DDT residues, with the river which crosses a Maasai village (R1) and drains a larger basin. The maximum contamination in lagoon sediments was measured farthest site from the river inlet. Comparable levels of DDT residues were found in Lake Bogoria sediments.
Fig. 5.
Correlation between DDTs and conductivity (dotted line pp’DDE; dashed line pp’DDD; full line pp’DDT)
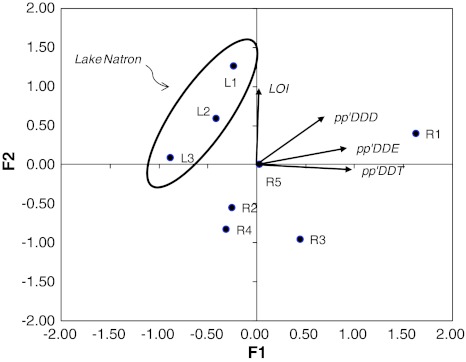
Multivariate analysis amongst LOI% content and DDTs for Lake Natron sediments (Lake Bogoria was not considered in this analysis since it was supposed to be affected by DDT pollution sources in a different way, being located in a different watershed) showed that the first two axes of the PCA accounted for 92% of the data variance (Fig. 6). The first principal component axis (57% of variance) describes a gradient of pp’DDT and pp’DDE from site L3 up to site R1, which presents the highest compounds’ concentrations. The second axis (35% of variance) illustrates the increasing percentage of organic matter (LOI) from river samples up to site L1 in the lagoon. pp’DDD does not contribute in the described data variation. It can be observed in the biplot that the Lake Natron samples form a cluster separated by the rivers along a North-East direction, indicating that the organic matter, pp’DDT and pp’DDE differentiate the lagoon samples from those of the rivers. The inputs of organic matter to the rivers and into lake probably have a different origin; in the first case it should come from the humans and their livestock farming, whilst in the second case from the degradation of phytoplankton and faeces of the birds.
Fig. 6.
Bivariate PCA scores plot (F1 vs. F2) for the analytical data. LOI organic matter percentage determined by weight loss-of-ignition. The arrows show the direction of increasing values of the analysed variables
Discussion
Recent data of DDT contamination of the Rift Valley lakes are quite scanty. Similar levels of DDTs found in the present work were found in the sediments of Lake Nakuru (Table 2; Mavura and Wangila 2004), another Kenyan soda lake in the Rift Valley (Fig. 3). In this case, the parent compound pp’DDT was only found in the rainy season at concentrations higher than its metabolites. In the Tanzanian side of Lake Victoria (Fig. 3), the concentrations of DDTs ranged between the detection limit and 12 ng g−1 d.w. (Table 2; Kishimba et al. 2004) during the dry season, whilst higher concentrations were measured during the rainy season. In light of these observations, in the near future the investigation of the contamination by DDT even during the rainy season for lakes Bogoria and Natron seems to be important.
Table 2.
DDT residues in sediments of Rift Valley lakes (Fig. 3) in ng g−1 d.w.
| Sites | Season | pp’DDT (ng g−1 d.w.) | pp’DDD (ng g−1 d.w.) | pp’DDE (ng g−1 d.w.) | ΣDDT (ng g−1 d.w.) | References |
|---|---|---|---|---|---|---|
| Lake Nakuru | Dry | bdl | 2.3 | 7.5 | 9.8 | Mavura and Wangila (2004) |
| Rainy | 13.6 | bdl | 2.2 | 15.8 | ||
| Lake Victoria (Southern basin) | Dry | bdl-12 | Kishimba et al. (2004) | |||
| Rainy | bdl-131 | |||||
| Lake Natron | Dry | 0.4–0.6 | 0.9–1.5 | 4.6–15.9 | 5.9–18.0 | Present research |
| Lake Bogoria | Dry | 0.4 | 0.7 | 10.8 | 12.0 | Present research |
bdl Below detection limit
The peak concentrations found in Lake Victoria sediments were ascribed to an old pesticide stockpile at Vikuge (Elfvendahl et al. 2004; Kishimba et al. 2004; Marco and Kishimba 2005, 2007), which could also be a point source pollution for many kilometres around, even for lakes Natron and Bogoria at a distance of around 500 km. The hypothesis of the aerial transport from such a ‘hot spot’ pollution site seems to be confirmed by the analysis of the sample of Acacia tortilis leaves. Although Acacia sp. is not usually used as a micropollutant bioindicator (but it was the only tree species occurring in the area), and as a consequence cannot be used for quantitative comparisons, it can provide useful information for a qualitative evaluation. Only pp’DDT and pp’DDE were detected at comparable concentrations (about 0.5 ng g−1 d.w.), indicating that pp’DDD found in river and lake sediments could be formed in soil and/or sediments in anaerobic conditions (Zoro et al. 1974). Moreover, the pp’DDE/pp’DDT ratio equal to 1 in the leaves might indicate a recent local input of the parent compound through the atmospheric transport. The ratio pp’DDE/pp’DDT was particularly high for the lake and river sediments, indicating that most of the parent compound pp’DDT should have a low persistence in this tropical environment, as already demonstrated by in situ studies (Samuel and Pillai 1989). The occurrence of pp’DDT as dominant pollutant in Lake Nakuru sediments (Mavura and Wangila 2004) during the rainy season and its disappearance during the dry season, leads support to this hypothesis.
Conclusions
DDT pollution was found to be quite variable in the littoral sediments of the Rift Valley lakes. As the capacity of binding the pollutants in sediments seems to depend mainly on their organic matter content, the biotic components may play a role in the patchiness of contamination.
At present, low contamination levels were measured in the sediments of lakes Natron and Bogoria which should exclude any risk for DDT bioaccumulation in top predators and explain why lesser flamingo population is still abundant and healthy in these aquatic environments.
However, further investigation should be undertaken to verify if pollution increases during the rainy season as occur in many other lakes located in the same region. Moreover, as the lesser flamingo is a filter feeder species with a sieve-feeding mechanism to harvest phytoplankton, it ingests around 72 g d.w. day−1 of cyanobacteria and algae (Vareschi 1978), with consumption rates sometimes exceeding the primary production rates (Vareschi and Jacobs 1985), so its load of pesticide residues should be checked by other methods, such as eggs analysis. Finally, a new sampling campaign should be carefully organised, bypassing the difficulties linked to the logistic matters of these remote areas, addressing particular attention to the contamination of the different components of the food chain, such as the one of the phytoplankton communities.
Acknowledgments
This research was partially funded by the British Council DELPHE 291 ‘FIELD IT’ FOR EAST AFRICA Project (University of Nairobi, Kenya). It was conducted under research permits from the Tanzanian Wildlife Research Institute and the Kenyan National Council for Science & Technology to Dr David Harper. We thank the staff of his project ‘Rift Valley Lakes Exploration & Research’ (RiVLER) for maintaining the camp, food and accommodation. We would also like to thank AfriCover for providing some of the data used to make the maps.
Biographies
Roberta Bettinetti
is an Assistant Professor at the Chemical and Environmental Sciences Department, University of Insubria, Como, Italy. She is a biologist and holds a PhD in Invertebrate Biology at the University of Milan in 1999. Her research interests are mainly focused on fate and assessment of Persistent Organic Pollutants (POPs) in freshwater and brackish environments, with particular attention to the implications on the food webs.
Silvia Quadroni
is a biologist and holds a PhD (2010) in Environmental Sciences at the University of Insubria, Como, Italy. The main topics of her research are referred to the ecological risk assessment of pollution of lakes and rivers, with a particular experience in the analytical determination of contaminants.
Giuseppe Crosa
is Full Professor of Ecology at the Department of Structural and Functional Biology, University of Insubria Varese, Italy. He pursues research interests in applied freshwater ecology. The main body of his research is devoted to the environmental impact assessment and ecological management of regulated rivers and reservoirs with particular attention on the biological effects of water withdrawals and translocations.
David Harper
is a Senior Lecturer in Ecology and Conservation Biology in the University of Leicester UK. He has led research groups in the Rift Valley lakes of Kenya and Tanzania for over 25 years. The past 12 of these have been addressing the ecology and conservation of lesser flamingos in the soda lakes between Tanzania, Kenya and Ethiopia.
Jennifer Dickie
is a Research Associate in the Geography Department, University of Leicester. She has a background in Environmental Science, specialising in dryland geomorphology. Her work focuses on plant-soil interactions and their impact on land degradation. Her current interests also include mobile GIS and virtual reality with a particular focus on human/environmental applications.
Margaret Kyalo
is a master thesis student working at the University of Nairobi within a project on the feeding habits of the lesser flamingos inhabiting the area of the East Rift Valley, Africa
Kenneth Mavuti
is a Professor of Zoology (Hydrobiology) at the University of Nairobi, Kenya and holds a PhD in Aquatic Ecology. His main topics of interest are the hydrobiology and fisheries in marine and freshwater environments and the environmental impact assessment and management.
Silvana Galassi
is a biologist with a very long experience in the field of ecotoxicology. She is Full Professor of Ecology at the Biology Department of the University of Milan, Italy. Currently, her main research fields are the risk assessment of organic micropollutants making use of ecotoxicological test and of suitable predictive models.
Contributor Information
Roberta Bettinetti, Email: roberta.bettinetti@uninsubria.it.
Silvia Quadroni, Email: silvia.quadroni@uninsubria.it.
Giuseppe Crosa, Email: giuseppe.crosa@uninsubria.it.
David Harper, dmharper57@googlemail.com.
Jennifer Dickie, Email: jad145@hotmail.com.
Margaret Kyalo, Email: margaretkyalo@yahoo.com.
Kenneth Mavuti, Email: kmavuti@uonbi.ac.ke.
Silvana Galassi, Email: silvana.galassi@unimi.it.
References
- Ballot A, Krienitx L, Kotut K, Wiegand C, Metcalf JS, Codd GA, Pflugmacher S. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. Journal of Plankton Research. 2004;26:925–935. doi: 10.1093/plankt/fbh084. [DOI] [Google Scholar]
- Brown LH. The mystery of the flamingos. London: Country Life Ltd; 1973. [Google Scholar]
- Dawson, J.B. 2008. The Gregory Rift Valley and Neogene—Recent volcanoes of Northern Tanzania. Geological Society Memoir No. 33.
- Dean WE., Jr Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. Journal of Sedimentary Petrology. 1974;44:242–248. [Google Scholar]
- Elfvendahl S, Mihale M, Kishimba MA, Kylin H. Pesticide pollution remains severe after cleanup of a stockpile of obsolete pesticides at Vikuge, Tanzania. AMBIO: A Journal of the Human Environment. 2004;33(8):503–508. doi: 10.1579/0044-7447-33.8.503. [DOI] [PubMed] [Google Scholar]
- Gitahi SM, Harper D, Muchiri SM, Tole MP, Ng’ang’a RN. Organochlorine and organophosphorus pesticide concentrations in water, sediment, and selected organisms in Lake Naivasha (Kenya) Hydrobiologia. 2002;488:123–128. doi: 10.1023/A:1023386732731. [DOI] [Google Scholar]
- Harper D, Childress RB, Harper MM, Boar RR, Hickley P, Mills SC, Otieno N, Drane T, Vareschim E, Nasirwa O, Mwatha W, Darlington JPEC, Escutè-Gasulla X. Aquatic biodiversity and saline lakes: Lake Bogoria National Reserve, Kenya. Hydrobiologia. 2003;500:259–276. doi: 10.1023/A:1024722821407. [DOI] [Google Scholar]
- Hughes RH, Hughes JS. A directory of African wetlands, Gland, Switzerland, Nairobi, Kenya, and Cambridge UK. Huston: IUCN, UNEP and WCMC; 1992. [Google Scholar]
- Jones, B.E., W.D. Grant, N.C. Colins, and W.E. Mwatha. 1994. Alkaliphiles: Diversity and identification. In Bacterial diversity and systematics, ed. F.G. Priest, A. Ramos-Cormenzana, and B.J. Tindall, 195–229. New York: Plenum Press.
- Kishimba MA, Henry L, Mwevura H, Mmochi AJ, Mihale M, Hellar H. The status of pesticide pollution in Tanzania. Talanta. 2004;64:48–53. doi: 10.1016/j.talanta.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Koeman JH, Pennings JH, Goeij JJ, Tjoe PS, Olindo PM, Hopcrafts J. A preliminary report on possible contamination of Lake Nakuru in Kenya with some metals and chlorinated hydrocarbon pesticides. Journal of Applied Ecology. 1972;9:411–416. doi: 10.2307/2402441. [DOI] [Google Scholar]
- Krienitz L, Ballot A, Kotut K, Wiegand C, Pűtz S, Metcalf JS, Codd GA, Pflugmacher S. Contribution of hot spring cyanobacteria to the mysterious deaths of lesser flamingos at Lake Bogoria, Kenya. FEMS Microbiology Ecology. 2003;43:141–148. doi: 10.1111/j.1574-6941.2003.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Mandavilli A. DDT returns. Nature Medicine. 2006;12:870–871. doi: 10.1038/nm0806-870. [DOI] [PubMed] [Google Scholar]
- Mansour SA. Persistent organic pollutants (POPs) in Africa: Egyptian scenario. Human Experimental Toxicology. 2009;28(9):531–566. doi: 10.1177/0960327109347048. [DOI] [PubMed] [Google Scholar]
- Marco JAM, Kishimba MA. Concentrations of pesticide residues in grasses and sedges due to point source contamination and the indications for public health risks, Vikuge, Tanzania. Chemosphere. 2005;61(9):1293–1298. doi: 10.1016/j.chemosphere.2005.03.059. [DOI] [PubMed] [Google Scholar]
- Marco JAM, Kishimba MA. Organochlorine pesticides and metabolites in young leaves of Mangifera indica from sites near a point source in Coast region, Tanzania. Chemosphere. 2007;68:832–837. doi: 10.1016/j.chemosphere.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Mavura, M.J., and P.T. Wangila. 2004. Distribution of pesticide residues in various lake matices: Water, sediment, fish, algae, the case of Lake Nakuru, Kenya. In Proceedings of the African Network for Chemical Analysis of Pesticides (ANCAP), Arusha, 8–11 August 2004.
- Melack LM. Photosynthetic activity of phytoplankton in tropical African soda lakes. Hydrobiologia. 1981;81:71–85. doi: 10.1007/BF00048707. [DOI] [Google Scholar]
- Melack LM. Primary producer dynamics associated with evaporative concentration in a shallow, equatorial lake (Lake Elmenteita, Kenya) Hydrobiologia. 1988;158:1–14. doi: 10.1007/BF00026264. [DOI] [Google Scholar]
- Mlingwa C, Baker N. Lesser Flamingo Phoenicopterus minor counts in Tanzanian soda lakes: Implications for conservation. In: Boere GC, Galbraith CA, Stroud DA, editors. Waterbirds around the World. Edinburgh, UK: The Stationery Office; 2006. pp. 230–233. [Google Scholar]
- Nyamweru CK. Rift and volcanoes: A study of the East African Rift system. Nairobi: Thomas Nelson and Sons; 1983. p. 128. [Google Scholar]
- Oaks JL, Bradway D, Davis M, Harper DM. Septic arthritis and disseminated infections caused by Mycobacterium avium in Lesser Flamingos, Lake Bogoria, Kenya. Flamingo. 2006;14:30–32. [Google Scholar]
- Samuel T, Pillai MKK. The effect of temperature and solar radiations on volatilization, mineralization and degradation of 14C-DDT in soil. Environmental Pollution. 1989;57:63–77. doi: 10.1016/0269-7491(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Saoke, P. 2005. Kenya POPs situation report: DDT, pesticides and Polychlorinated Biphenyls. The International POPs Elimination Project (IPEP). Physicians for social responsibility (PSR) - Kenya. http://www.ipen.org/ipepweb1/library/ipep_pdf_reports/1ken%20kenya%20country%20situation%20report.pdf.
- Spigel, R.H., and G.W. Coulter. 1996. Comparison of hydrology and physical limnology of the East African Great Lakes: Tanganyika, Malawi, Victoria, Kivu and Turkana (with reference to some North American Great Lakes). In The limnology, climatology and paleoclimatology of East African Lakes, ed. T.C. Johnson and E. Odada, 103–139. Toronto: Gordon and Breach.
- Vareschi E. The ecology of Lake Nakuru (Kenya) abundance and feeding of the lesser flamingo. Oecologia. 1978;32:11–35. doi: 10.1007/BF00344687. [DOI] [PubMed] [Google Scholar]
- Vareschi E, Jacobs J. The ecology of Lake Nakuru. Oecologia. 1985;65:412–424. doi: 10.1007/BF00378917. [DOI] [PubMed] [Google Scholar]
- Wandiga SO. Use and distribution of organochlorine pesticides. The future in Africa. Pure Applied Chemistry. 2001;73:1147–1155. doi: 10.1351/pac200173071147. [DOI] [Google Scholar]
- Wetlands International. 2002. Ramsar sites database: A directory of wetlands of international importance. www.wetlands.org/RDB/Ramsar_Dir/_COUNTRIES.htm.
- Wood RB, Talling JF. Chemical and algal relationships in a salinity series of Ethiopian inland waters. Hydrobiologia. 1988;158:29–67. doi: 10.1007/BF00026266. [DOI] [Google Scholar]
- Yanda PZ, Madulu NF. Water resource management and biodiversity conservation in the Eastern Rift Valley Lakes, Northern Tanzania. Physics and Chemistry of the Earth. 2005;30:717–725. [Google Scholar]
- Zoro JA, Hunter JM, Eglinton G. Degradation of p,p′-DDT in reducing environments. Nature. 1974;247:235–237. doi: 10.1038/247235a0. [DOI] [PubMed] [Google Scholar]