Abstract
By mainly targeting larger predatory fish, commercial fisheries have indirectly promoted rapid increases in densities of their prey; smaller predatory fish like sprat, stickleback and gobies. This process, known as mesopredator release, has effectively transformed many marine offshore basins into mesopredator-dominated ecosystems. In this article, we discuss recent indications of trophic cascades on the Atlantic and Baltic coasts of Sweden, where increased abundances of mesopredatory fish are linked to increased nearshore production and biomass of ephemeral algae. Based on synthesis of monitoring data, we suggest that offshore exploitation of larger predatory fish has contributed to the increase in mesopredator fish also along the coasts, with indirect negative effects on important benthic habitats and coastal water quality. The results emphasize the need to rebuild offshore and coastal populations of larger predatory fish to levels where they regain their control over lower trophic levels and important links between offshore and coastal systems are restored.
Keywords: Mesopredator release, Human transformation, Commercial fisheries, Cod, Baltic Sea, Swedish coast
Introduction
Fishery induced declines in populations of larger predatory fish have generated dramatic changes in the food web composition in offshore and coastal seas (Pauly et al. 1998; Lotze et al. 2006). In particular, decreased stocks of predatory fish have generated strong increases in their prey, medium-sized or “meso-” predators (i.e., “mesopredator release”), changing the interactions between higher trophic levels considerably (e.g., Myers et al. 2007; Baum and Worm 2009). In some instances, there are documented cascading effects from such mesopredator increases on lower levels in the pelagic food web, including community-wide decreases of zooplankton, and increases in jellyfish and phytoplankton (e.g., Frank et al. 2005; Daskalov et al. 2007; Casini et al. 2008).
Effects of overfishing have traditionally been synonymous with effects on commercially important stocks, and on the consequences for either the market actors or coastal human societies that have experienced dramatic changes in their livelihood. There has also been a strong concern for many of the larger pelagic species, which have a high societal impact and cultural value. Management actions have therefore been centered on protecting the economic viability of commercial stocks and on restoring biological diversity of apex predators, such as whales, dolphins, seals, sharks, and tuna. Today, most management organizations promote ecosystem-based management (EBM). EBM is an adaptive management approach that focuses on the complexity of interactions within and between ecological and social systems, acknowledging that diversity of species and their traits are important for ecosystem performance and stability (Christensen et al. 1996). For coastal societies, EBM is a favorable long-term strategy because it considers multiple ecosystem services and manage the capacity of ecosystems to tolerate disturbances and stress, rather than focusing on one interest group by managing a single function or the production of one species (Christensen et al. 1996; Leslie and McLeod 2007).
Ecosystems are connected by flows of energy, materials and organisms. Spatial subsidies across ecosystem borders are important for population dynamics and community structure in many recipient ecosystems (Polis and Strong 1996). An important vector that transports resources between offshore and coastal ecosystems is constituted by migrating animals that utilize both systems during their life cycles (e.g., Varpe et al. 2005). The existence of such migrations implies that changes in offshore food webs may profoundly impact coastal ecosystems. For example, in the early 1990s, negative effects of increased predation from offshore populations of killer whales (Orca orca) were reported from the coast of Alaska (Estes et al. 1998). Killer whales increased their foraging along the coast and thereby limited the coastal populations of sea otters (Enhydra lutris). This released the main prey of sea otters—herbivorous sea urchins—from predation control, resulting in severe overgrazing of giant kelp; the habitat-founding species in the ecosystem. There is an increasing realization that major changes in offshore pelagic food webs might impact the functioning of coastal ecosystems, including a reduced production of the crucial ecosystem services they provide.
In this study, we suggest that observed effects of coastal mesopredators on lower trophic levels may in fact be triggered by fishery induced changes in offshore food webs. We base this hypothesis on analyses of fish monitoring data from two different areas: the marine Atlantic west coast of Sweden, and the brackish Baltic east coast of Sweden, combined with published information on concomitant food web changes, highlighting how changes in offshore food web composition appear to give rise to complex responses also in the coastal ecosystems.
Increase of Mesopredators on the Swedish Atlantic Coast
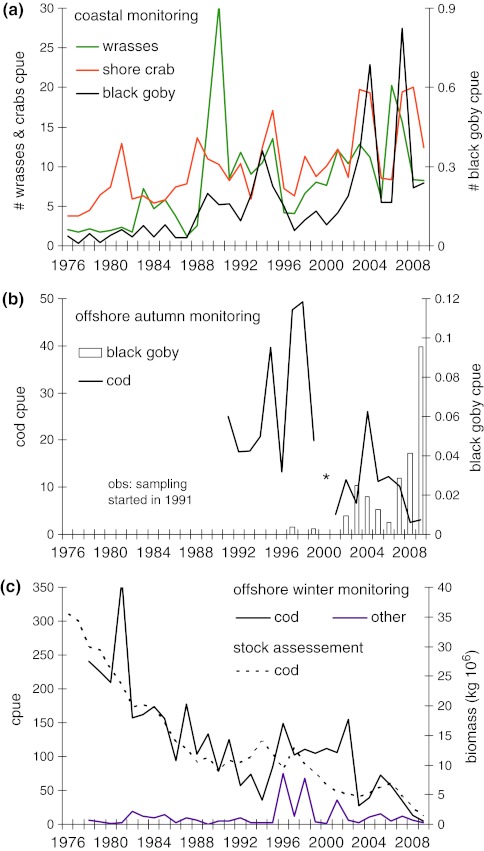
Declines in offshore predator populations may increase the abundance of nearshore mesopredators by direct decreases in predation rates, but also by complex, indirect food web interactions. On the Atlantic coast of Sweden (Skagerrak and Kattegat) the coastal mesopredator community is dominated by wrasses, gobiids and the common shore crab (Carcinus maenus) (Pihl and Wennhage 2002; Pihl et al. 2006; Swedish Board of Fisheries unpublished). Long-term monitoring data from a coastal area in the southern basin, Kattegat (Swedish Board of Fisheries, Vendelsö), suggests that these important mesopredators have all become increasingly abundant.
Vendelsö (latitude 57°18′, longitude 12°7′) is a reference area for the nuclear power plant of Ringhals. The discharge of heated cooling water from the power plant is not expected to affect the Vendelsö area (Bergström et al. 2009). In 1976, a standardized fyke net monitoring program was initiated in Vendelsö (HELCOM 2008). Since 1976, six stations have been fished at Vendelsö between 9 and 12 consecutive nights both in April and August every year, generating an effort of 108 to 144 fyke net nights per year. At each of the six stations, two fyke nets have been placed perpendicular to the shore, covering a transect from 2 to 5 m depth. At each sampling occasion, the abundance of all fish and larger crustaceans have been registered, as well as water temperature, sechi depth and salinity (for more info, see Thoresson 1996). The August sampling better represents changes in wrasse and shore crab populations (20 and 5 times higher catch in August compared to April, respectively). However, gobiids are as common in April as in August, and we therefore included both sampling periods in our analyses of coastal mesopredators. Data were square root or log10 transformed if necessary to improve linearity and temporal trends were analyzed with linear regression. We tested all analyzed time series for autocorrelation to the 15th lag using the autocorrelation function (STATISTICA, version 8.0; StatSoft, Inc. 2007). All coastal and later analyzed offshore time series, showed significant first-order autocorrelations (dependence on the first lag), which may cause an underestimation of the standard error and a higher risk of Type 1 error. We therefore adjusted sample sizes for first-order autocorrelation by calculating effective sample sizes: effective sample size (N*) = sample size (N) × (1 − rc)/(1 + rc), where rc is the first-order autocorrelation coefficient (Dawdy and Matalas 1964). In the results, F* and p* indicate that sample sizes are corrected for significant first-order autocorrelations.
Since 1976, the abundance of the dominating wrasses, corkwing wrasse, Symphodus melops, and goldsinny wrasse, Ctenolabrus rupestris has steadily increased in the catches (linear regression: N = 34, R = 0.49, 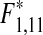 = 10.26, p* = 0.009), as has the common shore crab (linear regression: N = 34, R = 0.49,
= 10.26, p* = 0.009), as has the common shore crab (linear regression: N = 34, R = 0.49, 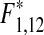 = 22.29, p* < 0.001) and black goby, Gobius niger (linear regression on square root transformed data: N = 34, R = 0.72,
= 22.29, p* < 0.001) and black goby, Gobius niger (linear regression on square root transformed data: N = 34, R = 0.72, 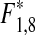 = 34.80, p* < 0.001; Fig. 1a). During the same time (1976–2009), the powerplant at Ringhals has also increased its effect and there is a significant increase in water temperatures in the area affected by cooling water (Bergström et al. 2009; www.fiskeriverket.se). At Vendelsö, there is a marginally significant trend towards increasing water temperatures in August (R = 0.33, F1,32 = 3.84, p = 0.059), but not in April (R = 0.28, F1,32 = 2.80, p = 0.103). However, the temperature increase at Vendelsö in August corresponds to ca a 0.5°C per decade, which is comparable to the general increase in surface water temperatures in the whole Kattegat area and significantly lower than the temperature increase measured at the nuclear power plant (Swedish Board of Fisheries 2008; Bergström et al. 2009; www.fiskeriverket.se). In all, this suggests that the increase in effect at the power plant at Ringhals has not had a major impact on the temperature increase at Vendelsö. Note that the densities of black goby are relatively low in this program, since the catchability of the species is low in fyke nets. However, increasing densities of black goby are also indicated by catches in an offshore autumn trawl survey (ICES International Bottom Trawl Survey of demersal fish in September, IBTS), where densities have increased 45 times between the 1990s and the 2000s in Kattegat (ICES subdivision 21; linear regression on square root transformed data: N = 18, R = 0.85,
= 34.80, p* < 0.001; Fig. 1a). During the same time (1976–2009), the powerplant at Ringhals has also increased its effect and there is a significant increase in water temperatures in the area affected by cooling water (Bergström et al. 2009; www.fiskeriverket.se). At Vendelsö, there is a marginally significant trend towards increasing water temperatures in August (R = 0.33, F1,32 = 3.84, p = 0.059), but not in April (R = 0.28, F1,32 = 2.80, p = 0.103). However, the temperature increase at Vendelsö in August corresponds to ca a 0.5°C per decade, which is comparable to the general increase in surface water temperatures in the whole Kattegat area and significantly lower than the temperature increase measured at the nuclear power plant (Swedish Board of Fisheries 2008; Bergström et al. 2009; www.fiskeriverket.se). In all, this suggests that the increase in effect at the power plant at Ringhals has not had a major impact on the temperature increase at Vendelsö. Note that the densities of black goby are relatively low in this program, since the catchability of the species is low in fyke nets. However, increasing densities of black goby are also indicated by catches in an offshore autumn trawl survey (ICES International Bottom Trawl Survey of demersal fish in September, IBTS), where densities have increased 45 times between the 1990s and the 2000s in Kattegat (ICES subdivision 21; linear regression on square root transformed data: N = 18, R = 0.85, 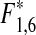 = 43.97, p* = 0.001; Fig. 1b).
= 43.97, p* = 0.001; Fig. 1b).
Fig. 1.
Trends in larger predatory fish and mesopredators in Kattegat, ICES subdivision 21, the North Sea. a Abundances of mesopredators in the coastal monitoring at Vendelsö by the Swedish Board of Fisheries: black goby (Gobius niger—black line), wrasses (Symphodus melops and Ctenolabrus rupestris—green line) and shore crab (Carcinus maenus—orange line). Cpue denote catch (numbers) per night per fyke net. Note that Symphodus melops was five times more common than Ctenolabrus rupestris and therefore dominated the trend for wrasses. b Autumn abundances of black goby (white bars) and cod (Gadus morhua—black line) in offshore trawls by the International Bottom Trawl Survey (IBTS). All autumn trawls are from September. The early autumn is the main season when cod predates on black goby in nearshore habitats. Regular autumn trawls are only available within IBTS from 1991. Cpue denote catch in kg per trawl hour. Note that this survey is aimed at offshore demersal fish, and the black goby is therefore only represented by a fraction of its true abundance. In 2006, corresponding nearshore abundances of black goby averaged 92 individuals per beach-seine haul in vegetated shallow bays along the Swedish Atlantic coast (Pihl et al. 2006). Asterisk indicates that there is no data from the autumn of 2000. c Winter abundances of cod (black line) and other larger demersal predators (haddock: Melanogrammusaeglefinus; ling: Molva molva; and pollack: Pollachiuspollachius) in offshore trawls (IBTS), and stock assessment of the total biomass of cod in the Kattegat over time. Scientific winter trawls for demersal fish have been performed in February since 1978. Cpue denote catch in kg per trawl hour
The increase in coastal mesopredators coincided with a long-term decreasing trend of cod populations in the international offshore bottom trawl survey in Kattegat (Gadus morhua; IBTS average catch weight kg per trawl hour in September: linear regression on square root transformed data, N = 18, R = −0.65,  = 11.70, p* = 0.001, Fig. 1b; IBTS in February: linear regression, N = 18, R = −0.77,
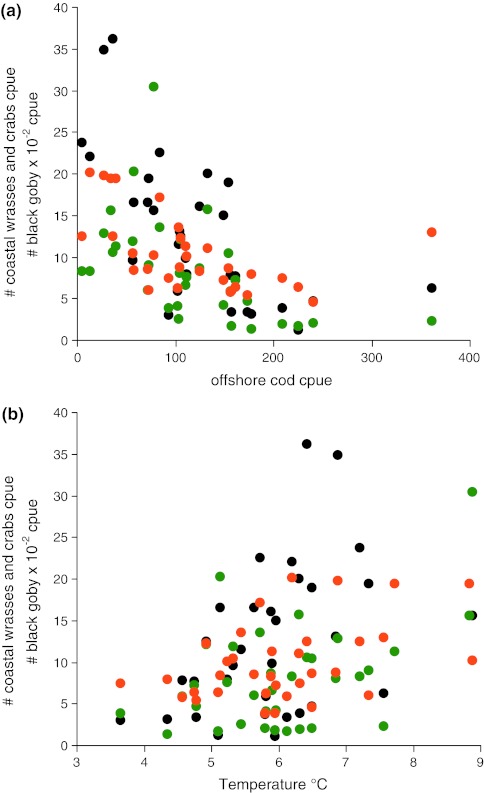
= 11.70, p* = 0.001, Fig. 1b; IBTS in February: linear regression, N = 18, R = −0.77, 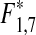 = 43.26, p* < 0.001, Fig. 1c). Cod is the dominant larger demersal predator in the area and was subjected to a strong commercial fishery during the decline from 1970s to the 2000s. Juveniles of offshore populations of cod settle in nearshore habitats and the larger individuals predate significantly on gobiids, shore crabs and wrasses (Pihl 1982; Pihl and Ulmestrand 1993; Salvanes and Nordeide 1993). Notably, the catch of cod in the offshore bottom trawl survey was negatively correlated with the catch of mesopredators in the coastal fyke net monitoring program (Pearson’s product moment correlation; wrasses [sqrt transformed data] r = −0.61, N = 32, t = 4.24, p < 0.001; shore crab: r = −0.51, N = 32, t = 3.22, p = 0.003; black goby [sqrt transformed data]: r = −0.64, N = 31, t = 4.58, p < 0.001; Fig. 2a), suggesting a link between declining cod populations and the increase in mesopredators. However, general changes in climatic variability have been proposed to have generated changes in the composition of fish communities during the same time period in the North Sea, with increasing temperatures as a main driver (Alheit et al. 2005). Temperature is important for the year-class strength of many fish species. To compare possible drivers of mesopredator abundances, we therefore constructed multiple regression models for the coastal mesopredator groups at Vendelsö; including offshore cod (catch weight kg per trawl hour in February) and average temperatures during the fyke net sampling in April and August as explanatory variables. The temperature in August did not significantly explain any variation in mesopredator abundances and was deleted from all models. Instead, offshore biomass of cod together with water temperatures in April contributed significantly to all models (Fig. 2; Table 1). The results indicate that spring temperatures are probably important for the abundance of mesopredators in the area. However, in contrast to cod biomass local spring temperatures did not change over time. Thus, the results suggest that decreasing predation pressure by juvenile cod, alongside with changed climatic variability may have contributed significantly to the increased abundances of mesopredators along the coast.
= 43.26, p* < 0.001, Fig. 1c). Cod is the dominant larger demersal predator in the area and was subjected to a strong commercial fishery during the decline from 1970s to the 2000s. Juveniles of offshore populations of cod settle in nearshore habitats and the larger individuals predate significantly on gobiids, shore crabs and wrasses (Pihl 1982; Pihl and Ulmestrand 1993; Salvanes and Nordeide 1993). Notably, the catch of cod in the offshore bottom trawl survey was negatively correlated with the catch of mesopredators in the coastal fyke net monitoring program (Pearson’s product moment correlation; wrasses [sqrt transformed data] r = −0.61, N = 32, t = 4.24, p < 0.001; shore crab: r = −0.51, N = 32, t = 3.22, p = 0.003; black goby [sqrt transformed data]: r = −0.64, N = 31, t = 4.58, p < 0.001; Fig. 2a), suggesting a link between declining cod populations and the increase in mesopredators. However, general changes in climatic variability have been proposed to have generated changes in the composition of fish communities during the same time period in the North Sea, with increasing temperatures as a main driver (Alheit et al. 2005). Temperature is important for the year-class strength of many fish species. To compare possible drivers of mesopredator abundances, we therefore constructed multiple regression models for the coastal mesopredator groups at Vendelsö; including offshore cod (catch weight kg per trawl hour in February) and average temperatures during the fyke net sampling in April and August as explanatory variables. The temperature in August did not significantly explain any variation in mesopredator abundances and was deleted from all models. Instead, offshore biomass of cod together with water temperatures in April contributed significantly to all models (Fig. 2; Table 1). The results indicate that spring temperatures are probably important for the abundance of mesopredators in the area. However, in contrast to cod biomass local spring temperatures did not change over time. Thus, the results suggest that decreasing predation pressure by juvenile cod, alongside with changed climatic variability may have contributed significantly to the increased abundances of mesopredators along the coast.
Fig. 2.
Relation between coastal abundances of the mesopredators black goby (Gobius niger—black dots), wrasses (group of small bodies fish—green dots), and shore crabs (Carcinus meneas—orange dots) on the Kattegat coast of Sweden, and a offshore abundances of cod (Gadus morhua) in winter (February), and b coastal temperatures in spring (April). Offshore cod was sampled by the International Bottom Trawl Survey (IBTS). Cpue for cod denote catch per trawl hour. Coastal abundances of mesopredators and temperatures are from the coastal monitoring at Vendelsö by the Swedish Board of Fisheries (fish data includes April and August samplings). Cpue for mesopredators denote catch per night per fyke net
Table 1.
Multiple regression results for coastal mesopredatory fish at Vendelsö (Kattegat), including offshore cod biomass and local temperatures in spring (April) and late summer (August) as additative explanatory variables
| Full model | Univariate results: spring biomass offshore cod | Univariate results: coastal spring temperatures | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R | F2,29 | P | Partial correlation | F1,29 | P | Partial correlation | F1,29 | P | |
| Wrasses (sqrt transformed data) | 0.69 | 13.40 | <0.001 | −0.60 | 16.61 | <0.001 | 0.41 | 5.87 | 0.022 |
| Common shore crab | 0.63 | 9.35 | 0.001 | −0.49 | 9.00 | 0.005 | 0.43 | 6.43 | 0.017 |
| Black goby (sqrt transformed data) | 0.83 | 32.33 | <0.001 | −0.70 | 27.14 | <0.001 | 0.69 | 26.12 | <0.001 |
August temperatures never contributed significantly to the models and were therefore deleted
Black goby, shore crabs and wrasses all have the potential to regulate the abundance of crustacean and gastropod herbivores (“mesograzers”), potentially resulting in cascading effects on vegetation (Norderhaug et al. 2005, Newcombe and Taylor 2010). Concomitant with the observed changes in food web composition, beds of eelgrass (Zostera marina L.)—the dominating foundation species on shallow soft-bottoms—declined with 60% on the northern Swedish Atlantic coast since the 1980s and up to 85% in northern Kattegat (Kungälv; Baden et al. 2003; Nyqvist et al. 2009). These losses have been attributed to blooms of mat-forming filamentous algae (e.g., Cladophora spp., Ectocarpales and Ulva spp.) generated by a combination of increased nutrient supply (via coastal eutrophication) and low grazing pressure, mediated by high predation pressure on functionally important grazers from high densities of mesopredators (Fig. 3; Moksnes et al. 2008; Baden et al. 2010). Field experiments using cages show that predation by local mesopredators decrease the biomass of potential mesograzers by more than 95% in this system (Moksnes et al. 2008; Baden et al. 2010). Cage experiments also demonstrate that the black goby indirectly increases the biomass accumulation of ephemeral algae in seagrass patches up to five times by controlling the most efficient grazers: adult (>9 mm) individuals of the amphipod Gammarus locusta (Moksnes et al. 2008). Today, gammarid and isopod mesograsers occur in very low abundances in eelgrass beds, where they were abundant in the 1980s (Jephson et al. 2008; Moksnes et al. 2008; Baden et al. 2010). Seagrass beds decrease turbidity by stabilizing sediments, and act as nursery ground for a number of commercially important fishes in the area (such as cod; Pihl et al. 2006). Thus, the decreases in offshore populations of larger demersal fish and the following dramatic increase in populations of mesopredators described here for Kattegat, might have impacted crucial components of these nearshore ecosystems, including their nursery function for the top predators already impacted by fishing. However, even though these results indicate that altered cross ecosystem interactions have fundamentally impacted the coastal ecosystem, there is still a strong need to clearly link such time-series of higher trophic levels with the experimental results from lower trophic levels in seagrass meadows.
Fig. 3.
Shallow seagrass (Zostera marina) bed on the Swedish Atlantic coast (Fiskebäckskil), with heavy load of filamentous macroalgae (Ectocarpales and Ulva spp.). Field experiments in the area show that the summer/autumn algal accumulation may partly be caused by an intense predation from highly abundant mesopredators on the most effective herbivore; adult Gammarus locusta amphipods (e.g., Moksnes et al. 2008). Photo: Johan Eklöf
Increase of Mesopredators on the Swedish Baltic Sea Coast
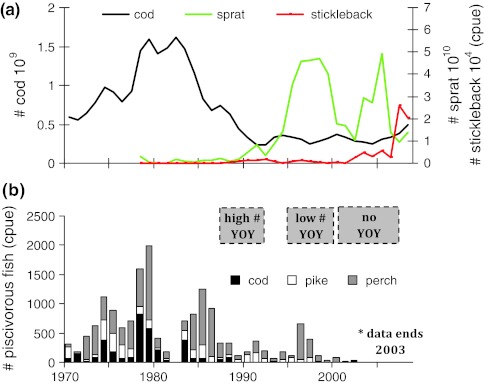
There are also indications from the coast of the central Baltic Sea that offshore fisheries on cod may have cascading effects on coastal food webs. Interestingly, these cascading effects seem dependent on offshore-inshore migrations of mesopredators, combined with changes in interspecific competition, as well as release in predation pressure (Ljunggren et al. 2010). On the Swedish coast of the central Baltic Sea, juvenile three-spined stickleback (Gasterosteus aculeatus)—a smaller predatory fish feeding on crustacean and gastropod mesograzers (Eriksson et al. 2009; Sieben et al. 2011b)—completely dominate many sheltered coastal communities in early summer (Eriksson et al. 2009; Ljunggren et al. 2010). Stickleback changes distribution over the ontogeny, and after their first summer the majority migrate offshore (unpublished data). Since the 1980s, the offshore food web in the central Baltic Sea has changed dramatically, indicating a strong basin-wide mesopredator release phase (Alheit et al. 2005; Österblom et al. 2007; Casini et al. 2008; Möllmann et al. 2008). Initially, the offshore Baltic Sea cod populations declined by 75% during the 1980s, due to climate induced poor recruitment conditions combined with high fishing pressure (Fig. 4a; ICES 2010a). This generated a trophic cascade in the open sea, including a four-fold increase in the dominating pelagic mesopredator sprat (Sprattus sprattus balticus), a 50% decrease in summer zooplankton biomass, and a doubling in phytoplankton biomass (Casini et al. 2008). Recently, offshore abundances of stickleback have increased exponentially (Fig. 4a; Ljunggren et al. 2010), suggesting a dramatic increase of this mesopredator also in the coastal habitat. Coastal monitoring of migrating fish is poor, but a combination of unique data from an open archipelago of the Swedish Baltic coast (the Kalmar sound) indicates that the changes in fish community structure in the open Baltic Sea have also affected nearshore areas. In the 1970–1980s, high abundances of cod were commonly registered near the shore, but from the early 1990 cod has vanished (Fig. 4b) concomitant with the overall collapse of the Baltic cod stock (Fig. 4a). This suggests that the general decline in Baltic cod may have limited the distribution to offshore areas, excluding the coastal zone. From 1990, there has also been a continuous decline in the densities of the dominating larger nearshore predators—European perch (Perca fluviatilis) and northern pike (Esox lucius)—in the same area (Fig. 4b). Thus, the recent strong increase in stickleback may have been enabled by release from predation both from coastal and offshore predators: the overwintering stickleback population may have gained from the declines in offshore cod, whereas the spawning and juvenile stickleback populations may have gained from declines of both stationary coastal predators (perch and pike) and a decreased distribution of Baltic cod. This emphasizes that changed distributions and simultaneous migrations of both larger and mesopredatory fish may be important pathways linking human impacts on offshore food webs with coastal ecosystems (Fig. 5).
Fig. 4.
Trends in a offshore abundances of cod (stock assessment estimates, ICES 2010a), sprat (acoustic estimates from the ordinary international acoustic survey, ICES 2010b) and stickleback (trawl hauls made during the ordinary international acoustic survey), and b coastal abundances of larger predators: cod, perch and pike together with local recruitment of perch and pike (in boxes: the number of young of the year fish YOY). a Sprat and stickleback data are from ICES subdivision 27, cod data are from subdivisions 25–32. Cod and sprat are estimated total numbers, while stickleback cpue is number per trawl hour. b Trap-net monitoring data (catch per night per trap) of cod, perch and pike between 1970 and 2002 from Gåsö (Mönsterås, county of Kalmar) on the southeast coast of Sweden. The Gåsö data is part of a larger time-series from four sites around a paper plant (Mönsterås bruk) which stops in 2003. Here, we present data exclusively from Gåsö, the site most affected by the open sea, because it is a good representation of fish both from the fresh-water and marine communities. Full pike data is presented in Sect. 1 in the electronic supplement to Ljunggren et al. (2010). Estimates of high, low and no recruitment of perch and pike are also based on Ljunggren et al. (2010)
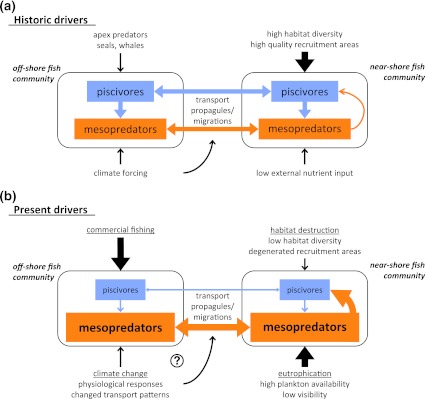
Fig. 5.
Suggested human-driven shifts in the structure of higher trophic levels in marine food webs and resulting consequences for the relation between offshore and nearshore fish communities (see also Eriksson et al. 2010). a Historic drivers: diverse communities of apex predators and natural climate forcing influenced offshore fish communities. Coastal areas had a strong function as recruitment areas for offshore fish from all trophic levels, which resulted in top-down control of the near shore fish communities. b Present drivers: commercial fishing has skewed the offshore communities toward dominance by mesopredators. Such mesopredator release events increase the abundance of planktivorous and/or facultative planktivorous fish (fish that eat both plankton and benthic mesograzers) in nearshore habitats, which cause food competition and egg predation, and thereby may limit the recruitment of stationary fish. Declining populations of stationary piscivores and/or limited migrations of offshore piscivorous fish to nearshore areas in combination with increasing resource loads, may enhance bottom-up control of the nearshore ecosystem. Specific effects of climate change are not well understood (Harley et al. 2006)
On the Swedish coast of the central Baltic Sea, there is evidence that the high densities of three-spined stickleback indirectly increase the load of bloom forming filamentous algae in shallow bays by controlling mesograzers (Eriksson et al. 2009; Sieben et al. 2011b). Today, fish communities in areas with low abundances of perch and pike can be dominated by abundant three-spined stickleback: an average haul with a beach seine may contain up to 3,000 individuals (standardized area 100 m2, Fig. 6). In stickleback-dominated areas, habitat quality is also impacted: almost 50% of the bays are overgrown by heavy thickets of filamentous algae (Eriksson et al. 2009; Fig. 6). Notably, large-scale exclusions of sticklebacks (thousands of individuals removed using beach seines from 20 × 30 m enclosures) in an often overgrown bay decreased the recruitment of filamentous algae by 60% (Sieben et al. 2011a). Meanwhile, stickleback abundances are much lower in areas where perch and pike still are abundant (average haul up to 60 individuals), and only 10% of the bays are overgrown by algae. This indicates that declines in larger predators may allow for massive increases in sticklebacks and thereby cause cascading negative effects to lower trophic levels. This is confirmed by small-scale experimental exclusion of larger fish and grazers, showing that declines in larger predators—by inducing a four level trophic cascade—increase filamentous algal growth with rates comparable to those caused by nutrient enrichment alone (Eriksson et al. 2009; Sieben et al. 2011b). Food competition and egg predation by the high abundances of stickleback may now even contribute to decreased recruitment success of perch and pike (Nilsson 2006; Ljunggren et al. 2010), and potentially “lock” the coastal food web in an alternative, mesopredator-dominated regime. Thus, overfishing of offshore cod populations may have contributed to a shift in nearshore food web structure.
Fig. 6.
Bloom of filamentous algae in shallow bays with high densities of three-spined stickleback (Gasterosteus aculeatus) on the Swedish coast of the central Baltic Sea. Top From above the surface of a bay dominated by Fucus vesiculosus overgrown with predominantly unbranched green microalgae (e.g., Ulotrix spp., Urospora spp.) and colonial diatoms (e.g. Melosira spp.). Photo: Gustav Johansson. Bottom Under the surface in a bay dominated by Myriophyllum sp. and Potamogeton sp. overgrown with filamentous macroalgae (mainly Cladophora glomerata, Ectocarpus siliquosus and Pylaiella littoralis). Photo: Ulf Bergström
Managing Interactions between Nearshore Communities and Offshore Food Webs
The examples provided above makes it increasingly clear that traditional management of marine resources has severe limitations, since it often ignores interactions between the status of coastal habitats and fisheries, cross-system fluxes, predator–prey interactions as well as other ecosystem components (Pikitch et al. 2004). EBM may provide a better platform for coastal management, where one of the main objectives should be the incorporation of spatial considerations, as shown by our synthesis. Offshore and coastal resources are used at different spatial scales with the potential for cascading detrimental effects both within and across ecosystems, e.g., cascading effects from offshore exploitation of top predators on nearshore biotopes. Since cross-ecosystem management also crosses geographical and sectorial management borders and academic disciplines (e.g., coastal vs. offshore, benthic vs. pelagic, fisheries vs. water quality, or zoology vs. botany), a shift within management organization structure may be a crucial first step (Olsson et al. 2008; Österblom et al. 2010). With this we mean that measures for protecting or restoring coastal ecosystems need a broad approach addressing cumulative impacts that traditionally are managed by separate management sectors: including restoration of offshore food webs, improvements in water quality and increased habitat protection through implementation of marine protected areas (Lotze et al. 2006). Furthermore, changes in offshore and coastal food webs co-occur with major changes in large-scale hydrodynamics and transport through human engineering and climate change (Harley et al. 2006; Eriksson et al. 2010), external nutrient loading by eutrophication (Cloern 2001) and habitat destruction through coastal development and dredging (Airoldi and Beck 2007). Therefore, cascading effects of increases in mesopredator abundances will most likely interact with other human-driven changes in environmental conditions and abiotic resources, eventually altering the functions of coastal communities (Fig. 5; Olff et al. 2009; Eriksson et al. 2010). A crucial development of EBM is to acknowledge and jointly approach these multiple and potentially interacting drivers of cross-ecosystem changes (e.g., fisheries, eutrophication, and habitat destruction), instead of—as in the past—dealing with them in isolation.
For example, overexploitation of offshore fish populations has triggered governmental actions to rebuild commercially important stocks and ensure sustainable fisheries. However, single-species management of fish stocks does usually not account for the complexity of food web interactions, especially those that link different ecosystems. Our results emphasize that to meet management goals for coastal areas we need to rebuild predator populations not only to maximize production of the target species, but to levels at which their ecological function is restored, both in offshore and coastal food webs. This includes increasing the abundance of offshore populations of larger predatory fish to restore their control over lower trophic levels and to restore significant migrations between offshore and coastal habitats (Fig. 5). It also means rebuilding nearshore populations of larger predatory fish to restore their ecological function along the coast. In our case-studies, this implies that to improve the quality of coastal habitats, we will need to combine nutrient reductions and habitat restoration with fisheries specific targets. On the Atlantic coast of Sweden, we need to strengthen offshore populations of cod to promote significant coastal migrations, as well as rebuilding local nearshore populations of cod. In the Baltic Sea, we need to strengthen the cod population until its distribution area expands to include coastal habitats, and we need to rebuild local populations of pike and perch by protecting and restoring their spawning areas. These are specific case-studies that span two different types of coast: one in an enclosed brackish water sea and one bordering the highly exploited North Sea continental shelf. However, offshore fisheries and coastal exploitation are global forces that have had significant effects on continental seas world-wide (Lotze et al. 2006, Worm et al. 2006). Examples from both the Pacific and the western Atlantic also support that offshore trophic cascades or changes in migration patterns of offshore predators, can have cascading effects on coastal food-webs (reviewed in Baum and Worm 2009). Thus, the offshore coastal linkages described in our study systems may be relevant for a wide range of developed coasts that border highly exploited offshore systems.
Acknowledgments
Johan S. Eklöf was funded by FORMAS (grants no. 2008-839 and 2009-1086). The contribution of Jens Olsson, Michele Casini, and Ulf Bergström was made within the PLAN FISH project funded by the Swedish Environmental Protection Agency. Thanks to Han Olff and Helmut Hillebrand for inspiring discussions about cross ecosystem and predator removal effects, to Gustav Johansson for artwork and to Per Olov Moksnes and an anonymous reviewer for constructive comments on an earlier version of the manuscript.
Biographies
Britas Klemens Eriksson
is an assistant professor at the Department of Marine Benthic Ecology and Evolution, the Centre for Ecological & Evolutionary Studies, University of Groningen. He holds a PhD in Plant Ecology from Uppsala University. His research deals with effects of resource exploitation and biodiversity loss on the function of coastal ecosystems.
Katrin Sieben
is a doctoral candidate at the Department of Marine Benthic Ecology and Evolution, the Centre for Ecological & Evolutionary Studies, University of Groningen. Her research focuses on testing cascading effects of changing coastal fish communities on lower trophic levels in coastal food-webs.
Johan Eklöf
is a post-doc at the Department of Marine Ecology at Gothenburg University, and has a part-time position as researcher at the Department of Systems Ecology, Stockholm University. He holds a PhD in marine ecotoxicology from the Stockholm University. He works with food-web ecology, ecosystem dynamics and management of shallow benthic ecosystems.
Lars Ljunggren
is a researcher associated to the Institute of Coastal Research at the Swedish University of Agricultural Sciences. He holds a PhD in Fisheries Biology from the Swedish University of Agricultural Sciences. He works with fisheries and water quality assessment.
Jens Olsson
is a researcher at the Institute of Coastal Research at the Swedish University of Agricultural Sciences. He holds a PhD in limnology from the Uppsala University. He works with integrated ecosystem analysis of coastal systems, coastal fish ecology and population genetics.
Michele Casini
is a researcher at the Institute of Marine Research at the Swedish University of Agricultural Sciences. He holds a PhD in marine ecology from the Gothenburg University. He works with fish stock assessment and management, food-web ecology and ecosystem dynamics.
Ulf Bergström
is a researcher at the Institute of Coastal Research at the Swedish University of Agricultural Sciences. He holds a PhD in marine ecology from the Umeå University. His work concerns habitat and food web ecology, and applications within management of coastal ecosystems.
References
- Airoldi L, Beck MW. Loss, status and trends for coastal marine habitats of Europe. Oceanography and Marine Biology. 2007;45:345–405. [Google Scholar]
- Alheit J, Mömann C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N. Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. Ices Journal of Marine Science. 2005;62:1205–1215. doi: 10.1016/j.icesjms.2005.04.024. [DOI] [Google Scholar]
- Baden S, Gullstrom M, Lunden B, Pihl L, Rosenberg R. Vanishing seagrass (Zostera marina, L.) in Swedish coastal waters. AMBIO. 2003;32:374–377. doi: 10.1579/0044-7447-32.5.374. [DOI] [PubMed] [Google Scholar]
- Baden S, Bostrom C, Tobiasson S, Arponen H, Moksnes PO. Relative importance of trophic interactions and nutrient enrichment in seagrass ecosystems: A broad-scale field experiment in the Baltic-Skagerrak area. Limnology and Oceanography. 2010;55:1435–1448. doi: 10.4319/lo.2010.55.3.1435. [DOI] [Google Scholar]
- Baum JK, Worm B. Cascading top-down effects of changing oceanic predator abundances. Journal of Animal Ecology. 2009;78:699–714. doi: 10.1111/j.1365-2656.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- Bergström L, Jansson M, Sundqvist F, Andersson J. Biologiska undersökningar vid Ringhals kärnkraftverk 1979–2007. Fiskeriverket Informerar. 2009;2:1–37. [Google Scholar]
- Casini M, Lovgren J, Hjelm J, Cardinale M, Molinero JC, Kornilovs G. Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proceedings of the Royal Society B Biological Sciences. 2008;275:1793–1801. doi: 10.1098/rspb.2007.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, N.L., A.M. Bartuska, J.H. Brown, S. Carpenter, C. Dantonio, R. Francis, J.F. Franklin, J.A. MacMahon, et al. 1996. The report of the ecological society of America committee on the scientific basis for ecosystem management. Ecological Applications 6: 665–691.
- Cloern JE. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series. 2001;210:223–253. doi: 10.3354/meps210223. [DOI] [Google Scholar]
- Daskalov GM, Grishin AN, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10518–10523. doi: 10.1073/pnas.0701100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawdy DR, Matalas NC, editors. Statistical and probability analysis of hydrological data, part III: Analysis of variance, covariance and time series. New York: McGraw-Hill Book Company; 1964. [Google Scholar]
- Eriksson BK, Ljunggren L, Sandström A, Johansson G, Mattila J, Rubach A, Råberg S, Snickars M. Declines in predatory fish promote bloom-forming macroalgae. Ecological Applications. 2009;19:1975–1988. doi: 10.1890/08-0964.1. [DOI] [PubMed] [Google Scholar]
- Eriksson BK, van der Heide T, Koppel J, Piersma T, van der Veer HW, Olff H. Major changes in the ecology of the Wadden Sea: Human impacts, ecosystem engineering and sediment dynamics. Ecosystems. 2010;13:752–764. doi: 10.1007/s10021-010-9352-3. [DOI] [Google Scholar]
- Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- Harley, C.D.G., A.R. Hughes, K.M. Hultgren, B.G. Miner, C.J.B. Sorte, C.S. Thornber, L.F. Rodriguez, L. Tomanek, et al. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9: 228–241. [DOI] [PubMed]
- ICES. 2010a. Report of the Baltic Fisheries Assessment Working Group (WGBFAS). ICES CM 2010/ACOM:10.
- ICES. 2010b. Report of the International Fish Survey Working Group (WGBIFS). ICES CM 2010/SSGESST:07.
- Jephson T, Nystrom P, Moksnes PO, Baden SP. Trophic interactions in Zostera marina beds along the Swedish coast. Marine Ecology Progress Series. 2008;369:63–76. doi: 10.3354/meps07646. [DOI] [Google Scholar]
- Leslie HM, McLeod KL. Confronting the challenges of implementing marine ecosystem-based management. Frontiers in Ecology and the Environment. 2007;5:540–548. doi: 10.1890/060093. [DOI] [Google Scholar]
- Ljunggren, L., A. Sandström, U. Bergström, J. Mattila, A. Lappalainen, G. Johansson, G. Sundblad, M. Casini, et al. 2010. Recruitment failure of coastal predatory fish in the Baltic Sea is related to an offshore system shift. ICES Journal of Marine Sciences 67: 1587–1595.
- Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, et al. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809. [DOI] [PubMed]
- Moksnes P-O, Gullstrom M, Tryman K, Baden S. Trophic cascades in a temperate seagrass community. Oikos. 2008;117:763–777. doi: 10.1111/j.0030-1299.2008.16521.x. [DOI] [Google Scholar]
- Möllmann C, Muller-Karulis B, Kornilovs G, St John MA. Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: regime shifts, trophic cascade, and feedback coops in a simple ecosystem. Ices Journal of Marine Science. 2008;65:302–310. doi: 10.1093/icesjms/fsm197. [DOI] [Google Scholar]
- Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- Newcombe EM, Taylor RB. Trophic cascade in a seaweed-epifauna-fish food chain. Marine Ecology Progress Series. 2010;408:161–167. doi: 10.3354/meps08589. [DOI] [Google Scholar]
- Nilsson J. Predation of northern pike (Esox lucius L.) eggs: A possible cause of regionally poor recruitment in the Baltic Sea. Hydrobiologia. 2006;553:161–169. doi: 10.1007/s10750-005-1949-8. [DOI] [Google Scholar]
- Norderhaug KN, Christie H, Fossa JH, Fredriksen S. Fish-macrofauna interactions in a kelp (Laminaria hyperborea) forest. Journal of the Marine Biological Association of the United Kingdom. 2005;85:1279–1286. doi: 10.1017/S0025315405012439. [DOI] [Google Scholar]
- Nyqvist A, Andre C, Gullstrom M, Baden SP, Aberg P. Dynamics of seagrass meadows on the Swedish Skagerrak Coast. AMBIO. 2009;38:85–88. doi: 10.1579/0044-7447-38.2.85. [DOI] [PubMed] [Google Scholar]
- Olff H, Alonso DA, Berg MP, Eriksson BK, Loreau M, Piersma T, Rooney N. Parallel interaction webs in ecosystems. Philosophical Transactions of the Royal Society B. 2009;364:1755–1779. doi: 10.1098/rstb.2008.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson P, Folke C, Hughes TP. Navigating the transition to ecosystem-based management of the Great Barrier Reef, Australia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9489–9494. doi: 10.1073/pnas.0706905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österblom, H., A. Gårdmark, L. Bergström, B. Muller-Karulis, C. Folke, M. Lindegren, M. Casini, P. Olsson, et al. 2010. Making the ecosystem approach operational-Can regime shifts in ecological- and governance systems facilitate the transition? Marine Policy 34: 1290–1299.
- Österblom H, Hansson S, Hjerne O, Larsson U, Wulff F, Elmgren R, Folke C. Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems. 2007;10:887–889. doi: 10.1007/s10021-007-9069-0. [DOI] [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F. Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Pihl L. Food-intake of young cod and flounder in a shallow bay on the Swedish west-coast. Netherlands Journal of Sea Research. 1982;15:419–432. doi: 10.1016/0077-7579(82)90068-0. [DOI] [Google Scholar]
- Pihl L, Ulmestrand M. Migration pattern of juvenile cod (Gadus morhua) on the Swedish west coast. ICES Journal of Marine Science. 1993;50:63–70. doi: 10.1006/jmsc.1993.1007. [DOI] [Google Scholar]
- Pihl L, Wennhage H. Structure and diversity of fish assemblages on rocky and soft bottom shores on the Swedish west coast. Journal of Fish Biology. 2002;61:148–166. doi: 10.1111/j.1095-8649.2002.tb01768.x. [DOI] [Google Scholar]
- Pihl L, Baden S, Kautsky N, Ronnback P, Soderqvist T, Troell M, Wennhage H. Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science. 2006;67:123–132. doi: 10.1016/j.ecss.2005.10.016. [DOI] [Google Scholar]
- Pikitch, E.K., C. Santora, E.A. Babcock, A. Bakun, R. Bonfil, D.O. Conover, P. Dayton, P. Doukakis, et al. 2004. Ecosystem-based fishery management. Science 305: 346–347. [DOI] [PubMed]
- Polis GA, Strong DR. Food web complexity and community dynamics. American Naturalist. 1996;147:813–846. doi: 10.1086/285880. [DOI] [Google Scholar]
- Salvanes AGV, Nordeide JT. Dominating sublittoral fish species in a west Norwegian fjord and their trophic links to cod (Gadus morhua L.) Sarsia. 1993;78:221–234. [Google Scholar]
- Sieben K, Ljunggren L, Bergstrom U, Eriksson BK. A meso-predator release of stickleback promotes recruitment of macroalgae in the Baltic Sea. Journal of Experimental Marine Biology and Ecology. 2011;397:79–84. doi: 10.1016/j.jembe.2010.11.020. [DOI] [Google Scholar]
- Sieben K, Rippen AD, Eriksson BK. Cascading effects from predator removal depend on resource availability in a benthic food web. Marine Biology. 2011;158:391–400. doi: 10.1007/s00227-010-1567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Board of Fisheries. 2008. Fiskebestånd och miljö i hav och sötvatten. Resurs och Miljöoöversikt, 1–180.
- Thoresson G. Guidelines for coastal fish monitoring. Kustlaboratoriet: Fiskeriverket; 1996. [Google Scholar]
- Varpe O, Fiksen O, Slotte A. Meta-ecosystems and biological energy transport from ocean to coast: the ecological importance of herring migration. Oecologia. 2005;146:443–451. doi: 10.1007/s00442-005-0219-9. [DOI] [PubMed] [Google Scholar]
- Worm, B., E.B. Barbier, N. Beaumont, J.E. Duffy, C. Folke, B.S. Halpern, J.B.C. Jackson, H.K. Lotze, et al. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314: 787–790. [DOI] [PubMed]