Abstract
To mitigate global warming caused by burning fossil fuels, a renewable energy source available in large quantity is urgently required. We are proposing large-scale photobiological H2 production by mariculture-raised cyanobacteria where the microbes capture part of the huge amount of solar energy received on earth’s surface and use water as the source of electrons to reduce protons. The H2 production system is based on photosynthetic and nitrogenase activities of cyanobacteria, using uptake hydrogenase mutants that can accumulate H2 for extended periods even in the presence of evolved O2. This review summarizes our efforts to improve the rate of photobiological H2 production through genetic engineering. The challenges yet to be overcome to further increase the conversion efficiency of solar energy to H2 also are discussed.
Keywords: Cyanobacteria, Hydrogen, Hydrogenase, Nitrogenase, Photobiological H2 production
Introduction
Photobiological production of H2 by cyanobacteria and eukaryotic microalgae that use H2O as the electron donor has the potential to produce renewable clean energy on a scale sufficient to meet much of the world energy demand (Ghirardi et al. 2007, 2009; Sakurai and Masukawa 2007; Tamagnini et al. 2007; Bothe et al. 2010; Ghirardi and Mohanty 2010). In cyanobacteria, H2 gas is generated by either hydrogenase or nitrogenase (Tamagnini et al. 2002, 2007). Both enzymes are sensitive to inactivation by O2. In contrast to hydrogenase that catalyzes the reversible reduction of protons to H2, nitrogenase catalyzes the unidirectional production of H2 as an obligatory side reaction during the fixation of N2:
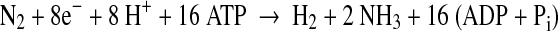 |
1 |
In the absence of N2 (e.g., under Ar), all electrons are allocated to H2 production:
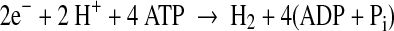 |
2 |
Although the reaction of nitrogenase requires a large investment of ATP (2 ATP per e−), the reaction is practically irreversible, thus allowing H2 to be accumulated as high as 20–30% of the total atmosphere, even with simultaneous O2 evolution (Sakurai and Masukawa 2007; Yoshino et al. 2007). Nitrogenase is restricted to bacteria and archaea, and nitrogen-fixing oxygenic phototrophs are limited to a subset of the cyanobacteria. Heterocyst-forming cyanobacteria are able to reconcile the two incompatible processes of O2-sensitive nitrogenase and of oxygenic photosynthesis by undergoing differentiation in which about 5–10% of the vegetative cells become heterocysts that provide a microaerobic environment, allowing nitrogenase to function in an aerobic environment (Wolk et al. 1994; Berman-Frank et al. 2003) (Fig. 1). Because of their ability to generate energy by oxygenic photosynthesis while forming a separate space for anaerobic reactions, we are taking advantage of heterocyst-forming cyanobacteria to improve nitrogenase-based H2 production through gene engineering. Such improvements will allow the development of large-scale photobiological H2 production on the sea surface.
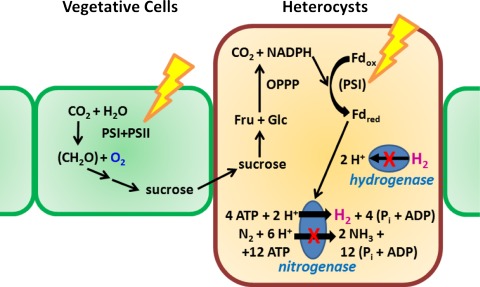
Fig. 1.
Photobiological H2 production by heterocyst-forming cyanobacteria. Vegetative cells of cyanobacteria produce carbohydrates (CH2O) by oxygenic photosynthesis (the Calvin cycle). Under nitrogen-limited conditions, a subset of cells forms developmentally specialized heterocysts that do not produce O2 because they lack photosystem II activity. Heterocysts are surrounded by peptidoglycan, glycolipid, and polysaccharide layers, which restrict the entry of O2, and a small amount of O2 that diffuses into heterocysts is removed by respiration; thus, the inside of heterocysts is kept microoxic. The microoxic environment protects the O2-labile nitrogenase from inactivation by O2, and the enzyme is utilized for the unidirectional H2 production. Electrons required for nitrogenase reaction depend on sucrose provided by vegetative cells. Sucrose is degraded by the metabolic pathway involving the oxidative pentose phosphate pathway (OPPP), generating NADPH used for the reduction of ferredoxin (Fd) by photosystem I (PSI) and for the formation of ATP by oxidative phosphorylation. ATP is also formed by photophosphorylation involving photosynthetic electron transport system and PSI. In wild-type cells, H2 produced is absorbed by the Hup. By inactivating the gene encoding Hup, the mutant cells can accumulate H2 even in the presence of O2 produced by photosynthesis. By creating selective mutations in the gene encoding nitrogenase, the mutated nitrogenases direct the electron flux through the enzyme selectively toward proton reduction in the presence of N2
Inactivation of Uptake Hydrogenase (Hup)
One of the major obstacles to efficient solar energy conversion to H2 is the presence of hydrogenases that reabsorb the H2 produced by nitrogenases, especially in the presence of O2 (Tamagnini et al. 2002, 2007; Sakurai and Masukawa 2007). Two distinct types of hydrogenases are known in cyanobacteria: the Hup and the bidirectional hydrogenase (Hox). Hup catalyzes a virtually unidirectional uptake of H2, and Hox catalyzes both uptake and production of this gas. Many heterocystous cyanobacteria contain both Hup and Hox, although a few have only Hup (Tamagnini et al. 2007; Masukawa et al. 2009). Anabaena sp. PCC 7120 has been chosen as a model strain to improve nitrogenase-based H2 production (Masukawa et al. 2002a) because it is amenable to genetic engineering (Elhai and Wolk 1988) and its complete genomic sequence is available (Kaneko et al. 2001). As this organism contains both types of hydrogenases, each individual and both hydrogenases were inactivated by targeted gene disruption, producing two single mutants, ΔHup and ΔHox, and a double mutant, ΔHupΔHox (Masukawa et al. 2002a). Elimination of Hup activity resulted in a 4- to 7-fold increase in the rates of H2 production in an Ar atmosphere compared with wild-type cells, while the effects of inactivation of Hox activity on H2 production were not evident under the conditions tested. Hup-disrupted mutants also were shown to be effective in enhancing H2 production by several other Anabaena and Nostoc strains of cyanobacteria (Happe et al. 2000; Lindberg et al. 2002; Schütz et al. 2004; Carrasco et al. 2005; Yoshino et al. 2007).
A promising approach to further improve photobiological H2 production in the presence of O2 is to initially select parental strains with high nitrogenase activity and inactivate their Hup activities. Out of 13 heterocystous strains tested, Nostoc sp. strain PCC 7422 exhibited the highest nitrogenase activity as measured by the acetylene reduction assay (Yoshino et al. 2007). After determining the nucleotide sequences of Hup-encoding genes of Nostoc sp. PCC 7422, the Hup-minus mutant (ΔHup) was constructed by insertional disruption of hupL. When the ΔHup mutant cultures were grown in an initial headspace gas of Ar + 5% CO2 under continuous illumination, they accumulated H2 up to 20–30% (v/v), concomitant with oxygen evolution. The presence of 20% O2 in the initial headspace gas of the ΔHup cultures inhibited H2 accumulation by <20%, suggesting a low susceptibility of the nitrogenase of this mutant to O2. A high conversion efficiency of light energy to H2 of 1.8% versus total solar radiation (averaged over 6 days) was obtained for the ΔHup mutant at an incident light energy of 70 μmol photons m−2 s−1 of photosynthetically active radiation. Under laboratory (optimal) conditions, efficiencies exceeding 1% versus total solar radiation are sometimes reported (Kumazawa and Mitsui 1994; Sakurai and Masukawa 2007); however, these high efficiencies are only attained at low light intensities of about one twenty-fifth of full sunlight received on the equator, and the efficiencies greatly decline with increasing light intensities as shown in the PCC 7120 ΔHup mutant (Masukawa et al. 2002b). Under full sunlight, the highest efficiencies reported were about 0.1% (Tsygankov et al. 2002). Possible strategies to overcome the problem of low light saturation in cyanobacteria include decreasing the number of light-harvesting antenna (Melis 2009; Kosourov et al. 2011) and/or reaction center concentrations by targeted mutagenesis and selection of wild-type strains with better tolerance to high light intensity.
Modification of the Catalytic Active Center of Nitrogenase
According to Eqs. 1 and 2, one expects to be able to increase the H2 production activity of nitrogenase by decreasing the electron allocation to N2 fixation. Although replacement of N2 by Ar is effective for increasing H2 production, this approach increases the operational cost for large-scale generation of H2. Mutagenesis of nitrogenase offers an alternative mechanism to redirect electron flow and overcome this N2 competition.
The well-characterized molybdenum-containing nitrogenase consists of an Fe protein (dinitrogenase reductase) and a MoFe protein (dinitrogenase). The Fe protein contains a single [4Fe–4S] cluster and supplies electrons from reduced ferredoxin or flavodoxin to the MoFe protein. The latter contains two unique metal clusters, the [8Fe–7S] P-cluster and the [1Mo–7Fe–9S–1X–homocitrate] FeMo cofactor, where the FeMo-co is believed to be the active site that binds and reduces substrates. Homocitrate is required for efficient nitrogen fixation. The crystal structure of the purified MoFe protein from the homocitrate synthase gene (nifV) disruption mutant of Klebsiella pneumoniae revealed an altered enzyme that contains citrate, instead of homocitrate, in its FeMo-co (Mayer et al. 2002). Citrate-containing nitrogenase was shown to catalyze the reduction of N2 poorly, but this enzyme was able to reduce protons effectively in an N2 atmosphere. The cyanobacterium Anabaena sp. PCC 7120 has two homocitrate synthase genes, nifV1 and nifV2, in its chromosome (Kaneko et al. 2001). With the ΔHup strain as the parental strain, two single gene disruption mutants, ΔHupΔNifV1 and ΔHupΔNifV2, and a double gene disruption mutant, ΔHupΔNifV1ΔNifV2, were constructed (Masukawa et al. 2007). N2-fixing growth rates of the two nifV single mutants and the double mutant were decreased moderately and severely, respectively, compared with those of the parental ΔHup strain. For the ΔHupΔNifV1 cells, both the rate of H2 production and the heterocyst frequency were sustained at higher levels than those for the parental ΔHup strain, leading to significantly increased rates of H2 production by the former culture compared with those by the latter culture in the presence of N2. Although the presence of N2 inhibited H2 production by the ΔHupΔNifV1ΔNifV2 mutant less strongly than that by the parental ΔHup strain and the other nifV mutants, H2 production activity of the former mutant was low. With Anabaena sp. PCC 7120, the inactivation of nifV1 has proven effective in improving H2 production in the presence of N2.
Not only the FeMo-co itself but also the amino acid residues in the vicinity of the FeMo-co are important in substrate reduction. Substitutions of selected amino acids in the vicinity of the FeMo-co active site within Azotobacter vinelandii nitrogenase were shown to eliminate or greatly diminish N2 fixation while, in some cases, allowing for effective proton reduction (Seefeldt et al. 2009). Therefore, certain amino acid exchanges near FeMo-co in cyanobacterial nitrogenase might produce variant MoFe proteins that redirect the electron flux through the enzyme preferentially to proton reduction, producing more H2 in the presence of N2 in an aerobic environment. Based on the crystal structure of A. vinelandii MoFe protein (Einsle et al. 2002), portions of 19 amino acid residues, all highly conserved, are predicted to reside within 5 Å of FeMo-co. Out of this set, six residues (Q193, H197, Y236, R284, S285, and F388) in the NifD subunit of Anabaena MoFe protein (equivalent to residues Q191, H195, Y229, R277, S278, and F381 in A. vinelandii) were targeted for mutagenesis in an attempt to direct electron flow selectively toward proton reduction in the presence of N2. Each of the selected six residues was replaced by nonpolar, polar, or charged residues by using a parental Anabaena strain with ΔNif and ΔHup mutations; in total, 49 NifD variants were constructed (Masukawa et al. 2010). Several variants examined in an N2 atmosphere significantly increased their in vivo rates of H2 production, approximating rates equivalent to those in an Ar atmosphere when measured on a chlorophyll a basis, and these cultures accumulated high levels of H2 compared to the reference strains including the ΔHup strain. The R284H culture exhibited the most dramatically increased levels of accumulated H2 compared to the reference strain cultures when grown under N2. The H2 accumulation by this mutant under N2 after 1 week was 87% of that observed for the reference strains under Ar. This variant has the potential of being used as the parental strain for further engineering of Anabaena in efforts to attain even greater levels of photobiological H2 production.
Concluding Comments
We have demonstrated several promising strategies for enhancing photobiological production of H2 in an aerobic, nitrogen-containing environment. More than 1% conversion efficiencies of light energy to H2 have been attained with the ΔHup mutant under laboratory conditions. To further increase the conversion efficiency under outdoor conditions, there are many challenges to be overcome. These hurdles involve the factors limiting light utilization efficiency at high light intensity, inhibitory effects of fixed nitrogen on nitrogenase activity, the low turnover rate of nitrogenase (6.4 s−1), etc. By combining several effective improvements through genetic engineering, high-H2-producing cyanobacterial strains suitable for large-scale production could be created.
Acknowledgments
This study was supported by the Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494) and by the JST PRESTO.
Biographies
Hajime Masukawa
is a researcher at JST, PRESTO and a visiting researcher at Kanagawa University. His research interests include photobiological hydrogen production by nitrogenase of cyanobacteria and regulation of heterocyst differentiation.
Masaharu Kitashima
is a visiting researcher at Kanagawa University. His research interest includes photobiological hydrogen production by nitrogenase of cyanobacteria.
Kazuhito Inoue
is a Professor at Kanagawa University. His research interests include photobiological hydrogen production by cyanobacteria, Photosystem I reaction center of green sulfur bacteria and heliobacteria.
Hidehiro Sakurai
is a visiting Professor at Kanagawa University and emeritus professor at Waseda University. His research interests include photobiological hydrogen production by cyanobacteria, Photosystem I reaction center of green sulfur bacteria and heliobacteria.
Robert P. Hausinger
is a Professor at Michigan State University. His research interests include mechanisms of catalysis by metalloenzymes and biosynthesis of protein metallocenters.
References
- Berman-Frank I, Lundgren P, Falkowski P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Research in Microbiology. 2003;154:157–164. doi: 10.1016/S0923-2508(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Bothe H, Schmitz O, Yates MG, Newton NE. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiology and Molecular Biology Reviews. 2010;74:529–551. doi: 10.1128/MMBR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco CD, Holliday SD, Hansel A, Lindblad P, Golden JW. Heterocyst-specific excision of the Anabaena sp. strain PCC 7120 hupL element requires xisC. Journal of Bacteriology. 2005;187:6031–6038. doi: 10.1128/JB.187.17.6031-6038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsle O, Tezcan FA, Andrade SL, Schmid B, Yoshida M, Howard JB, Rees DC. Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- Elhai J, Wolk CP. Conjugal transfer of DNA to cyanobacteria. Methods in Enzymology. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, Mohanty P. Oxygenic hydrogen photoproduction—current status of the technology. Current Science. 2010;98:499–507. [Google Scholar]
- Ghirardi ML, Posewitz MC, Maness PC, Dubini A, Yu J, Seibert M. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annual Review of Plant Physiology. 2007;58:71–91. doi: 10.1146/annurev.arplant.58.032806.103848. [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, Dubini A, Yu J, Maness PC. Photobiological hydrogen-producing systems. Chemical Society Reviews. 2009;38:52–61. doi: 10.1039/b718939g. [DOI] [PubMed] [Google Scholar]
- Happe T, Schütz K, Böhme H. Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Journal of Bacteriology. 2000;182:1624–1631. doi: 10.1128/JB.182.6.1624-1631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, Iriguchi M, Ishikawa A, et al. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Research. 2001;8:205–213. doi: 10.1093/dnares/8.5.205. [DOI] [PubMed] [Google Scholar]
- Kosourov SN, Ghirardi ML, Seibert M. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. International Journal of Hydrogen Energy. 2011;36:2044–2048. doi: 10.1016/j.ijhydene.2010.10.041. [DOI] [Google Scholar]
- Kumazawa S, Mitsui A. Efficient hydrogen photoproduction by synchronously grown cells of a marine cyanobacterium, Synechococcus sp. Miami BG-043511, under high cell-density conditions. Biotechnology and Bioengineering. 1994;44:854–858. doi: 10.1002/bit.260440711. [DOI] [PubMed] [Google Scholar]
- Lindberg P, Schütz K, Happe T, Lindblad P. A hydrogen producing, hydrogenase-free mutant strain of Nostoc punctiforme ATCC 29133. International Journal of Hydrogen Energy. 2002;27:1291–1296. doi: 10.1016/S0360-3199(02)00121-0. [DOI] [Google Scholar]
- Masukawa H, Mochimaru M, Sakurai H. Disruption of the uptake hydrogenase gene, but not of the bidirectional hydrogenase gene, leads to enhanced photobiological hydrogen production by the nitrogen fixing cyanobacterium Anabaena sp. PCC 7120. Applied Microbiology and Biotechnology. 2002;58:618–624. doi: 10.1007/s00253-002-0934-7. [DOI] [PubMed] [Google Scholar]
- Masukawa H, Mochimaru M, Sakurai H. Hydrogenases and photobiological hydrogen production utilizing nitrogenase system in cyanobacteria. International Journal of Hydrogen Energy. 2002;27:1471–1474. doi: 10.1016/S0360-3199(02)00125-8. [DOI] [Google Scholar]
- Masukawa H, Inoue K, Sakurai H. Effects of disruption of homocitrate synthase genes on Nostoc sp. strain PCC 7120 photobiological hydrogen production and nitrogenase. Applied and Environmental Microbiology. 2007;73:7562–7570. doi: 10.1128/AEM.01160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa H, Zhang X, Yamazaki E, Iwata S, Nakamura K, Mochimaru M, Inoue K, Sakurai H. Survey of the distribution of different types of nitrogenases and hydrogenases in heterocyst-forming cyanobacteria. Marine Biotechnology. 2009;11:397–409. doi: 10.1007/s10126-008-9156-z. [DOI] [PubMed] [Google Scholar]
- Masukawa H, Inoue K, Sakurai H, Wolk CP, Hausinger RP. Site-directed mutagenesis of the Anabaena sp. strain PCC 7120 nitrogenase active site to increase photobiological hydrogen production. Applied and Environmental Microbiology. 2010;76:6741–6750. doi: 10.1128/AEM.01056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SM, Gormal CA, Smith BE, Lawson DM. Crystallographic analysis of the MoFe protein of nitrogenase from a nifV mutant of Klebsiella pneumoniae identifies citrate as a ligand to the molybdenum of iron molybdenum cofactor (FeMoco) Journal of Biological Chemistry. 2002;277:35263–35266. doi: 10.1074/jbc.M205888200. [DOI] [PubMed] [Google Scholar]
- Melis A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Science. 2009;177:272–280. doi: 10.1016/j.plantsci.2009.06.005. [DOI] [Google Scholar]
- Sakurai H, Masukawa H. Promoting R & D in photobiological hydrogen production utilizing mariculture-raised cyanobacteria. Marine Biotechnology. 2007;9:128–145. doi: 10.1007/s10126-006-6073-x. [DOI] [PubMed] [Google Scholar]
- Seefeldt LC, Hoffman BM, Dean DR. Mechanism of Mo-dependent nitrogenase. Annual Review of Biochemistry. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz K, Happe T, Troshina O, Lindblad P, Leitao E, Oliveira P, Tamagnini P. Cyanobacterial H2 production—a comparative analysis. Planta. 2004;218:350–359. doi: 10.1007/s00425-003-1113-5. [DOI] [PubMed] [Google Scholar]
- Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wunschiers R, Lindblad P. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiology and Molecular Biology Reviews. 2002;66:1–20. doi: 10.1128/MMBR.66.1.1-20.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnini P, Leitao E, Oliveira P, Ferreira D, Pinto F, Harris DJ, Heidorn T, Lindblad P. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiology Reviews. 2007;31:692–720. doi: 10.1111/j.1574-6976.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- Tsygankov AA, Fedorov AS, Kosourov SN, Rao KK. Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnology and Bioengineering. 2002;80:777–783. doi: 10.1002/bit.10431. [DOI] [PubMed] [Google Scholar]
- Wolk CP, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant DA, editor. The molecular biology of cyanobacteria. Dordrecht: Kluwer Academic Publishers; 1994. pp. 769–823. [Google Scholar]
- Yoshino F, Ikeda H, Masukawa H, Sakurai H. High photobiological hydrogen production activity of a Nostoc sp. PCC 7422 uptake hydrogenase-deficient mutant with high nitrogenase activity. Marine Biotechnology. 2007;9:101–112. doi: 10.1007/s10126-006-6035-3. [DOI] [PubMed] [Google Scholar]